Steroid Pulse Therapy Leads to Secondary Infections and Poor Outcomes in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Intensive Care Units: A Retrospective Cohort Study
Abstract
1. Introduction
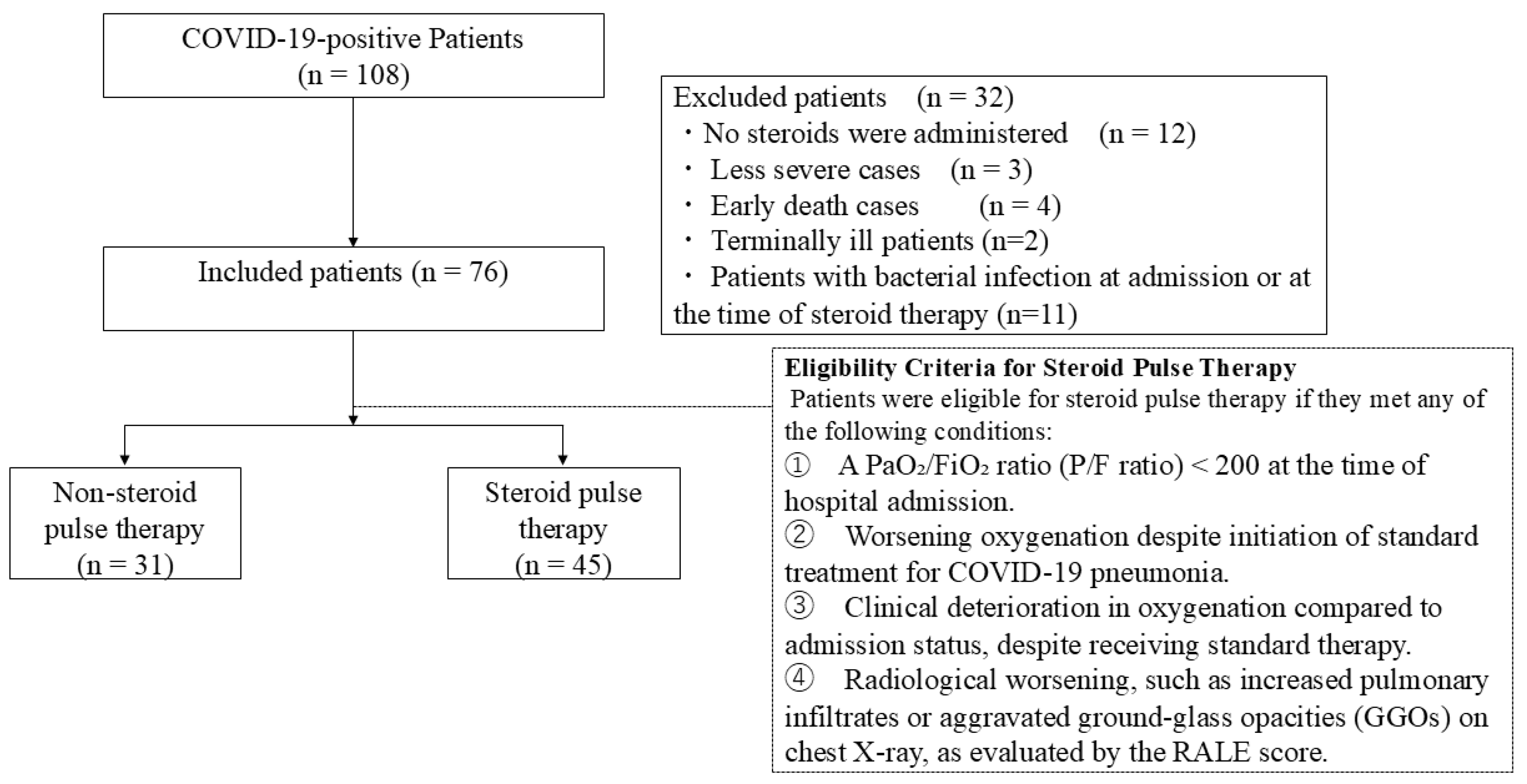
2. Materials and Methods
2.1. Steroid Pulse Therapy for Severe COVID-19
2.2. Criteria for Non-Response to Conventional Therapy
2.3. Secondary Infections
2.4. Ventilator-Free Days (VFD)
2.5. CONUT Score
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Changes in Patient Parameters over Time
4. Discussion
4.1. Pros and Cons of Steroid Pulse Therapy
4.2. Multiorgan Failure
4.3. Steroid Dosage
4.4. Duration of Treatment
4.5. Remdesivir Treatment
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease |
| HFNCO | High-flow nasal cannula oxygen therapy |
| P/F ratio | partial pressure of arterial oxygen/fraction of inspired oxygen ratio |
| SOFA | sequential organ failure assessment |
| WHO | World Health Organization |
| PCR | polymerase chain reaction |
| ICU | Intensive Care Unit |
| APACHE | acute physiology and chronic health evaluation |
| PEEP | positive end expiratory pressure |
| VFDs | ventilation-free days |
| CONUT | controlling nutritional status |
| BMI | body mass index |
| LDH | lactate dehydrogenase |
| KL-6 | sialylated carbohydrate antigen |
| HR | heart rate |
| CRF | chronic renal failure |
| CK | creatinine kinase |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| CRP | C-reactive protein |
| HbA1c | hemoglobin A 1c |
| NGSP | National Glycohemoglobin Standardization Program |
| IQR | interquartile range |
| ARDS | acute respiratory distress syndrome |
| RCT | randomized controlled trial |
References
- WHO. COVID-19 Epidemiological Update, 2024. 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---24-december-2024 (accessed on 20 April 2025).
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Remmington, C.; Barrett, N.A.; Agarwal, S.; Lams, B.; Collins, P.; Camarda, V.; Meadows, C.; Hanks, F.; Sanderson, B.; Retter, A.; et al. Steroid exposure and outcome in COVID-19 pneumonia. BJA Open 2023, 5, 100128. [Google Scholar] [CrossRef] [PubMed]
- Moromizato, T.; Sakaniwa, R.; Tokuda, Y.; Taniguchi, K.; Shibuya, K. Intravenous methylprednisolone pulse therapy and the risk of in-hospital mortality among acute COVID-19 patients: Nationwide clinical cohort study. Crit. Care 2023, 27, 53. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Fujii, K.; Yoshifuji, A.; Kondo, Y.; Itoh, K.; Sekine, K.; Kikuchi, T.; Ryuzaki, M. Clinical efficacy and safety of combination therapy of tocilizumab and steroid pulse therapy for critical COVID-19 in HD patients. Clin. Exp. Nephrol. 2022, 26, 75–85. [Google Scholar] [CrossRef]
- Salvarani, C.; Massari, M.; Costantini, M.; Merlo, D.F.; Mariani, G.L.; Viale, P.; Nava, S.; Guaraldi, G.; Dolci, G.; Boni, L.; et al. Intravenous methylprednisolone pulses in 14andomized14d patients with severe COVID-19 pneumonia: A double-blind, 14andomized, placebo-controlled trial. Eur. Respir. J. 2022, 60, 2200025. [Google Scholar] [CrossRef]
- Kooistra, E.J.; Dahm, K.; van Herwaarden, A.E.; Gerretsen, J.; Nuesch Germano, M.; Mauer, K.; Smeets, R.L.; van der Velde, S.; van den Berg, M.J.W.; van der Hoeven, J.G.; et al. Molecular mechanisms and treatment responses of pulmonary fibrosis in severe COVID-19. Respir. Res. 2023, 24, 196. [Google Scholar] [CrossRef]
- Ministry of Health. LaW. The Criteria for Severe in the Japanese Guidelines. Available online: https://www.mhlw.go.jp/content/001248424.pdf (accessed on 20 April 2025).
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Okano, H.; Sakurai, R.; Yamazaki, T. Steroid pulse therapy as a treatment for patients with COVID-19 pneumonia at an intensive care unit: A single-center retrospective observational study. Cureus 2023, 15, e36386. [Google Scholar] [CrossRef]
- Villar, J.; Añón, J.M.; Ferrando, C.; Aguilar, G.; Muñoz, T.; Ferreres, J.; Ambrós, A.; Aldecoa, C.; Suárez-Sipmann, F.; Thorpe, K.E.; et al. Efficacy of dexamethasone treatment for patients with the acute respiratory distress syndrome caused by COVID-19: Study protocol for a randomized controlled superiority trial. Trials 2020, 21, 717. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Warren, M.A.; Zhao, Z.; Koyama, T.; Bastarache, J.A.; Shaver, C.M.; Semler, M.W.; Rice, T.W.; Matthay, M.A.; Calfee, C.S.; Ware, L.B. Severity scoring of lung edema on the chest radiograph is associated with clinical outcomes in ARDS. Am. J. Respir. Crit. Care Med. 2018, 198, 820–829. [Google Scholar] [CrossRef]
- Murray, J.F.; Matthay, M.A.; Luce, J.M.; Flick, M.R. An expanded definition of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988, 138, 720–723. [Google Scholar] [CrossRef]
- Girard, T.D.; Kress, J.P.; Fuchs, B.D.; Thomason, J.W.W.; Schweickert, W.D.; Pun, B.T.; Taichman, D.B.; Dunn, J.G.; Pohlman, A.S.; Kinniry, P.A.; et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomized controlled trial. Lancet 2008, 371, 126–134. [Google Scholar] [CrossRef] [PubMed]
- González-Madroño, A.; Mancha, A.; Rodríguez, F.J.; Culebras, J.; de Ulibarri, J.I. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: Comparison with two logistic regression models developed using SGA as the gold standard. Nutr. Hosp. 2012, 27, 564–571. [Google Scholar] [CrossRef]
- Meduri, G.U.; Headley, S.; Kohler, G.; Stentz, F.; Tolley, E.; Umberger, R.; Leeper, K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995, 107, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.M.S.S.; Freire, R.S.; Frota, E.; Rezende Santos, A.G.; Farias, M.E.L.; Rodrigues, M.G.A.; Silva, B.M.; Prado Jeronimo, C.M.; Netto, R.L.A.; Silva Borba, M.G.; et al. Short-Course of methylprednisolone improves respiratory functional parameters after 120 days in hospitalized COVID-19 patients (Metcovid trial): A randomized clinical trial. Front. Med. 2021, 8, 758405. [Google Scholar] [CrossRef]
- Rafiullah, M.; Siddiqui, K. Corticosteroid use in viral pneumonia: Experience so far and the dexamethasone breakthrough in coronavirus disease-2019. J. Comp. Eff. Res. 2020, 9, 1247–1254. [Google Scholar] [CrossRef]
- Tasaka, S.; Tatsumi, K.; Assembly of Pulmonary Circulation and Lung Injury, the Japanese Respiratory Society. Clinical practice of acute respiratory distress syndrome in Japan: A nationwide survey and scientific evidences. Respir. Investig. 2017, 55, 257–263. [Google Scholar] [CrossRef]
- Fauci, A.S.; Dale, D.C.; Balow, J.E. Glucocorticosteroid therapy: Mechanisms of action and clinical considerations. Ann. Intern. Med. 1976, 84, 304–315. [Google Scholar] [CrossRef]
- Edalatifard, M.; Akhtari, M.; Salehi, M.; Naderi, Z.; Jamshidi, A.; Mostafaei, S.; Najafizadeh, S.R.; Farhadi, E.; Jalili, N.; Esfahani, M.; et al. Intravenous methylprednisolone pulse as a treatment for 15andomized15d severe COVID-19 patients: Results from a 15andomized controlled clinical trial. Eur. Respir. J. 2020, 56, 2002808. [Google Scholar] [CrossRef]
- Deinhardt-Emmer, S.; Wittschieber, D.; Sanft, J.; Kleemann, S.; Elschner, S.; Haupt, K.F.; Vau, V.; Häring, C.; Rödel, J.; Henke, A.; et al. Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and the correlation with tissue damage. Elife 2021, 10, e60361. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-associated pneumonia in patients with COVID-19: A systematic review and meta-analysis. Antibiotics 2021, 10, 545. [Google Scholar] [CrossRef]
- Ippolito, M.; Simone, B.; Filisina, C.; Catalanotto, F.R.; Catalisano, G.; Marino, C.; Misseri, G.; Giarratano, A.; Cortegiani, A. Bloodstream infections in hospitalized patients with COVID-19: A systematic review and meta-analysis. Microorganisms 2021, 9, 2016. [Google Scholar] [CrossRef]
- Buetti, N.; Ruckly, S.; de Montmollin, E.; Reignier, J.; Terzi, N.; Cohen, Y.; Siami, S.; Dupuis, C.; Timsit, J.F. COVID-19 increased the risk of ICU-acquired bloodstream infections: A case-cohort study from the multicentric outcomerea network. Intensiv. Care Med. 2021, 47, 180–187. [Google Scholar] [CrossRef]
- Ro, S.; Nishimura, N.; Imai, R.; Tomishima, Y.; So, C.; Murakami, M.; Okafuji, K.; Kitamura, A.; Jinta, T.; Tamura, T. Identification of patients with COVID-19 who are optimal for methylprednisolone pulse therapy. Multidiscip. Respir. Med. 2021, 16, 781. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; De Maio, F.; Carelli, S.; De Angelis, G.; Cacaci, M.; Montini, L.; Bello, G.; Cutuli, S.L.; Pintaudi, G.; Tanzarella, E.S.; et al. Staphylococcus aureus ventilator-associated pneumonia in patients with COVID-19: Clinical features and potential inference with lung dysbiosis. Crit. Care 2021, 25, 197. [Google Scholar] [CrossRef]
- Pappas, A.G.; Chaliasou, A.L.; Panagopoulos, A.; Dede, K.; Daskalopoulou, S.; Moniem, E.; Polydora, E.; Grigoriou, E.; Psarra, K.; Tsirogianni, A.; et al. Kinetics of Immune Subsets in COVID-19 Patients Treated with Corticosteroids. Viruses 2022, 15, 51. [Google Scholar] [CrossRef]
- Batırel, A.; Demirhan, R.; Eser, N.; Körlü, E.; Tezcan, M.E. Pulse steroid treatment for hospitalized adults with COVID-19. Turk. J. Med. Sci. 2021, 51, 2248–2255. [Google Scholar] [CrossRef]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Metzendorf, M.I.; Fischer, A.L.; Stegemann, M.; Spagl, M.; Nair, A.A.; Daniel, J.; Fichtner, F.; et al. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst. Rev. 2022, 11, CD014963. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.A.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Obata, R.; Maeda, T.; Rizk, D.; Kuno, T. Increased secondary infection in COVID-19 patients treated with steroids in New York City. Jpn. J. Infect. Dis. 2021, 74, 307–315. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium. Remdesivir and three other drugs for randomized patients with COVID-19: Final results of the WHO Solidarity 16andomized trial and updated meta-analyses. Lancet 2022, 399, 1941–1953. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of Covid-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Huang, W.; Berube, J.; McNamara, M.; Saksena, S.; Hartman, M.; Arshad, T.; Bornheimer, S.J.; O’Gorman, M. Lymphocyte subset counts in covid-19 patients: A meta-analysis. Cytom. Part A 2020, 97, 772–776. [Google Scholar] [CrossRef]

| Factor | All (n = 76) | Steroid Pulse Therapy (n = 45) | Non-Steroid Pulse therapy (n = 31) | p Value * |
|---|---|---|---|---|
| (A) Parameters | ||||
| Age (years) | 61.0 (54.8–75.0) | 60.0 (54.0–75.0) | 71.0 (57.0–76.5) | 0.1443 |
| Male, n (%) | 58 (76.3) | 36 (80) | 22 (71) | 0.3627 |
| BMI (kg/m2) | 25.7 (22.2–27.7) | 26.3 (24.0–27.7) | 23.1 (20.8–27.0) | 0.043 |
| sBP (mmHg) | 130 (117–143) | 130 (86–171) | 132 (70–176) | 0.959 |
| HR (bpm) | 82.5 (73.5–97.3) | 84.0 ± 18.8 | 87.4 ± 17.8 | 0.408 |
| RR | 24.6 ± 6.9 (12–57) | 24.2 ± 6.93 (16–57) | 25.0 ± 6.86 (12–40) | 0.521 |
| P/F ratio | 150.0 (90.9–271.4) | 200.0 (76.0–316.7) | 130.0 (97.0–220.0) | 0.377 |
| Comorbidities | ||||
| Diabetes (n, %) | 28 (36.8) | 18 (40.0) | 10 (32.2) | 0.492 |
| Cancer (n,%) | 6 (7.9) | 3 (6.7) | 3 (9.7) | 0.632 |
| Hypertension (n,%) | 37 (48.7) | 22 (48.9) | 15 (48.4) | 0.966 |
| CRF (n, %) | 2 (2.6) | 0 (0) | 2 (6.5) | 0.084 |
| Smoking (n,%) | 40 (52.6) | 25 (55.6) | 15 (48.4) | 0.539 |
| Duration from onset to hospitalization (day) | 6.0 (3.0–8.0) | 6.0 (4.0–7.0) | 6.0 (2.5–8.0) | 0.924 |
| Duration from onset to steroid administration (days) | 6.0 (4.0–8.0) | 6 (0–14) | 6 (0–30) | 0.836 |
| (B) Blood biochemical examination | ||||
| WBC (×103/μL) | 6.9 (5.1–9.4) | 6.4 (4.6–8.6) | 8.1 (5.4–10.3) | 0.158 |
| Lymphocyte (×103/μL) | 0.7 (0.4–0.9) | 0.6 (0.4–0.9) | 0.8 (0.6–1.0) | 0.045 |
| Neutrophil (103/μL) | 5.9 (4.1–8.4) | 5.6 (3.5–7.0) | 7.0 (4.9–9.3) | 0.021 |
| Hgb, g/dL | 13.7 (12–15.3) | 13.8 (12.1–15.6) | 12.9 (12.0–14.3) | 0.100 |
| Ht (%) | 39.7 ± 7.48 (1.0–51.9) | 41.0 (1–51.9) | 39.3 (26.4–50.1) | 0.139 |
| Plt (×103/μL) | 183.0 ± 65.5 (38–410) | 190.1 ± 68.1 | 190.6 ± 62.7 | 0.604 |
| D-dimer (μg/mL) | 1.2 (1.0–3.0) | 1.2 (1.0–2.3) | 1.4 (1.0–4.0) | 0.377 |
| LDH (U/L) | 451.5 (338–596.5) | 499.0 (338.0–611.0) | 398.0 (327.0–573.5) | 0.288 |
| CK (U/L) | 127.5 (55.8–435.8) | 131 (61–410) | 121 (38–639) | 0.583 |
| AST (U/L) | 47.5 (31.8–76) | 49 (36–76) | 44 (31–69) | 0.479 |
| ALT (U/L) | 31.5 (18.8–52.8) | 36.0 (20.0–56.0) | 25.0 (17.0–47.5) | 0.267 |
| CRP (mg/dL) | 10.0 (5.4–17.7) | 9.6 (5.8–15.9) | 11.5 (4.9–21.6) | 0.623 |
| HbA1c (NGSP (%)) | 6.6 (6.1–7.8) | 6.6 (6.1–7.8) | 6.5 (6.1–8.0) | 0.928 |
| Ferritin (ng /mL) | 697.5 (339.1–1397.3) | 554.0 (326.0–1232.5) | 882.0 (363.0–1421.0) | 0.462 |
| KL-6 (U/mL) | 380 (219–667) | 637.0 (344.5–1288.5) | 511.5 (365.3–820.0) | 0.728 |
| Lactate (mmoL/L) | 1.3 (1.0–1.6) | 1.3 (1.1–2.1) | 1.2 (0.8–1.6) | 0.161 |
| SOFA score | 4 (3–6) | 4.0 (3.0–5.0) | 4.0 (3.0–6.5) | 0.416 |
| CONUT score | 7 (5–9) | 7.0 (5.0–9.0) | 6.0 (5.5–9.0) | 0.819 |
| (C) Treatment | ||||
| Favipiravir, n (%) | 24/76 (31.6) | 11/45 (24.4) | 13/31 (42.0) | 0.107 |
| Remdesivir, n (%) | 547/7 (71.1) | 36 (80.0) | 18 (58.1) | 0.038 |
| Tocilizumab, n (%) | 53/76 (69.7) | 33/45 (73.3) | 20/31 (64.5) | 0.411 |
| Baricitinib, n (%) | 11/76 (14.5) | 10/45 (22.2) | 1/31 (3.22) | 0.021 |
| Anticoagulant therapy, n (%) | 74/76 (97.4) | 45/45 (100) | 29/31 (93.5) | 0.084 |
| Steroid therapy, n (%) | 68/76 (89.5) | 37/45 (82.2) | 31/31 (100) | 0.013 |
| (D) Respiratory severity | ||||
| Intubation, n (%) | 40/76 (52.6) | 27/45 (60.0) | 13/31 (41.9) | 0.121 |
| VFD | 1.5 (0–25.0) | 0 (0–17) | 15 (3–22) | 0.0295 |
| (E) Secondary infection | ||||
| Secondary infection, n (%) *** | 55 (72.4) | 34 (75.6) | 17 (54.8) | 0.0589 |
| Factor | Correlation Co-Efficient | SE | Odds Ratio | p-Value | 95% CI |
|---|---|---|---|---|---|
| Age | −0.010 | 0.003 | 0.920 | 0.005 | 0.868–0.974 |
| Ferritin | - | ||||
| Lactate | - | ||||
| Smoking | - | ||||
| Fabipiravir | - | ||||
| Remdecivir | 0.286 | 0.105 | 8.202 | 0.016 | 1.479–49.495 |
| Tocilizumab | - | ||||
| Steroid pulse therapy | −0.412 | 0.094 | 0.032 | <0.001 | 0.004–0.240 |
| Factor | Correlation Coefficient | SE | Odds Ratio | p-Value | 95% CI |
|---|---|---|---|---|---|
| Age (years) | −0.209 | 0.074 | 0.811 | 0.005 | 0.702–0.938 |
| Remdesivir, n (%) | 3.650 | 1.729 | 38.49 | 0.035 | 1.30–1140.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, K.; Ihara, S.; Yamaguchi, J.; Kuwana, T.; Kinoshita, K. Steroid Pulse Therapy Leads to Secondary Infections and Poor Outcomes in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Intensive Care Units: A Retrospective Cohort Study. Viruses 2025, 17, 822. https://doi.org/10.3390/v17060822
Nakagawa K, Ihara S, Yamaguchi J, Kuwana T, Kinoshita K. Steroid Pulse Therapy Leads to Secondary Infections and Poor Outcomes in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Intensive Care Units: A Retrospective Cohort Study. Viruses. 2025; 17(6):822. https://doi.org/10.3390/v17060822
Chicago/Turabian StyleNakagawa, Katsuhiro, Shingo Ihara, Junko Yamaguchi, Tsukasa Kuwana, and Kosaku Kinoshita. 2025. "Steroid Pulse Therapy Leads to Secondary Infections and Poor Outcomes in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Intensive Care Units: A Retrospective Cohort Study" Viruses 17, no. 6: 822. https://doi.org/10.3390/v17060822
APA StyleNakagawa, K., Ihara, S., Yamaguchi, J., Kuwana, T., & Kinoshita, K. (2025). Steroid Pulse Therapy Leads to Secondary Infections and Poor Outcomes in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Intensive Care Units: A Retrospective Cohort Study. Viruses, 17(6), 822. https://doi.org/10.3390/v17060822






