Identification of a Conserved Linear Epitope on the p54 Protein of African Swine Fever Virus
Abstract
1. Introduction
2. Materials and Methods
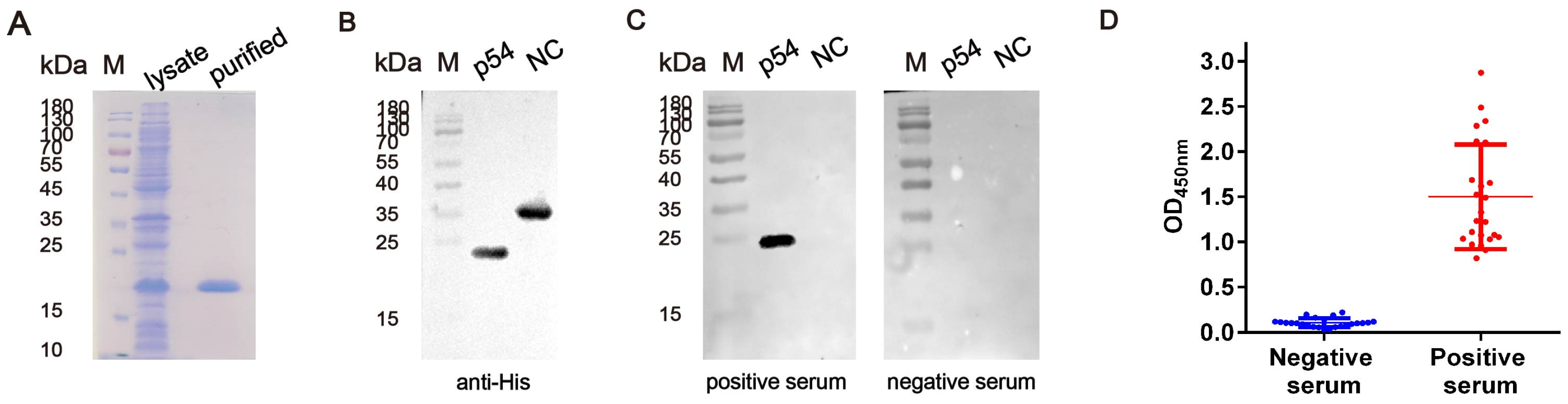
2.1. Expression of the p54 Protein
2.2. Animal Immunization of Strategy
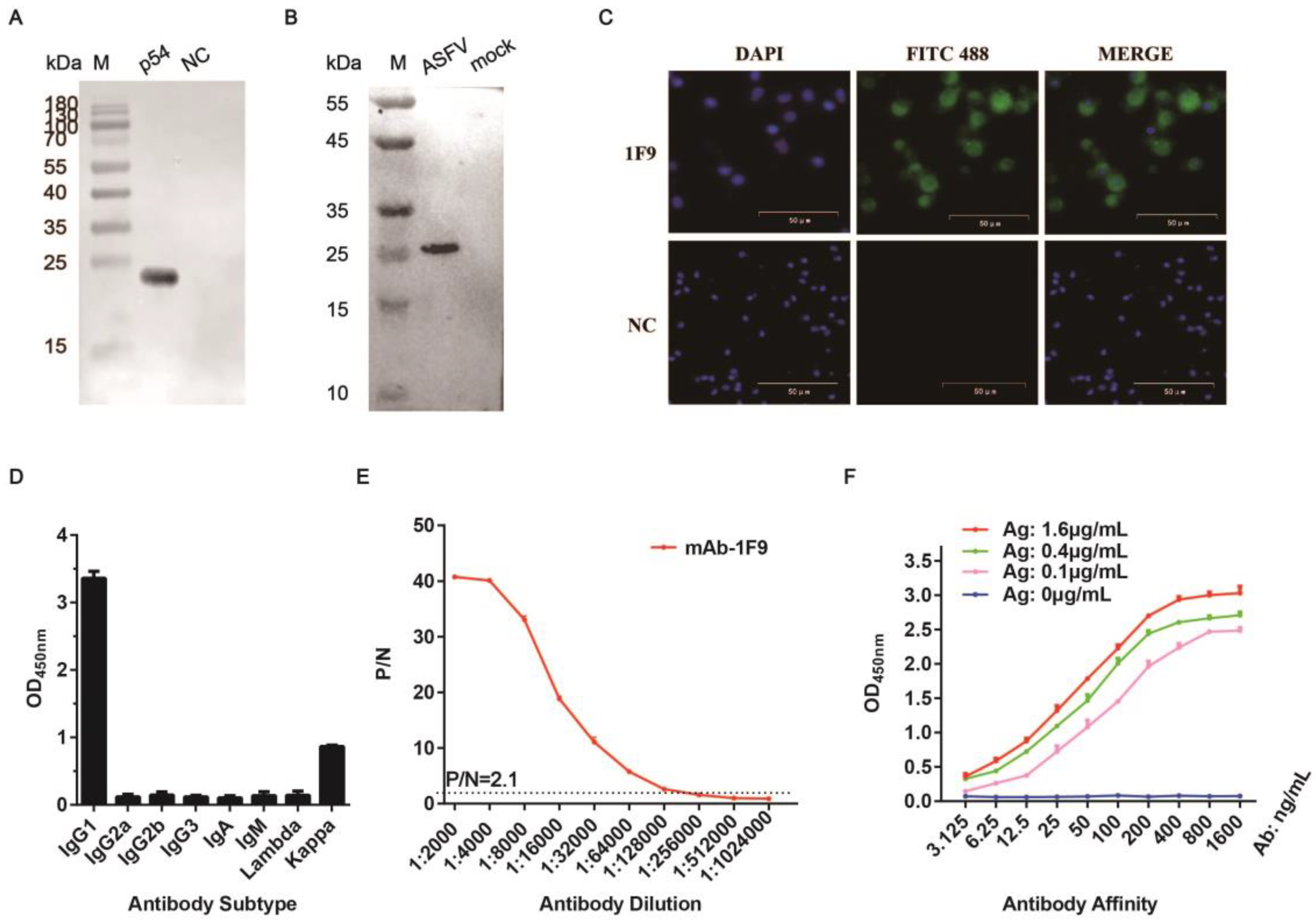
2.3. Preparation of mAbs
2.4. Western Blotting Analysis
2.5. Indirect Immunofluorescence Assay (IFA)
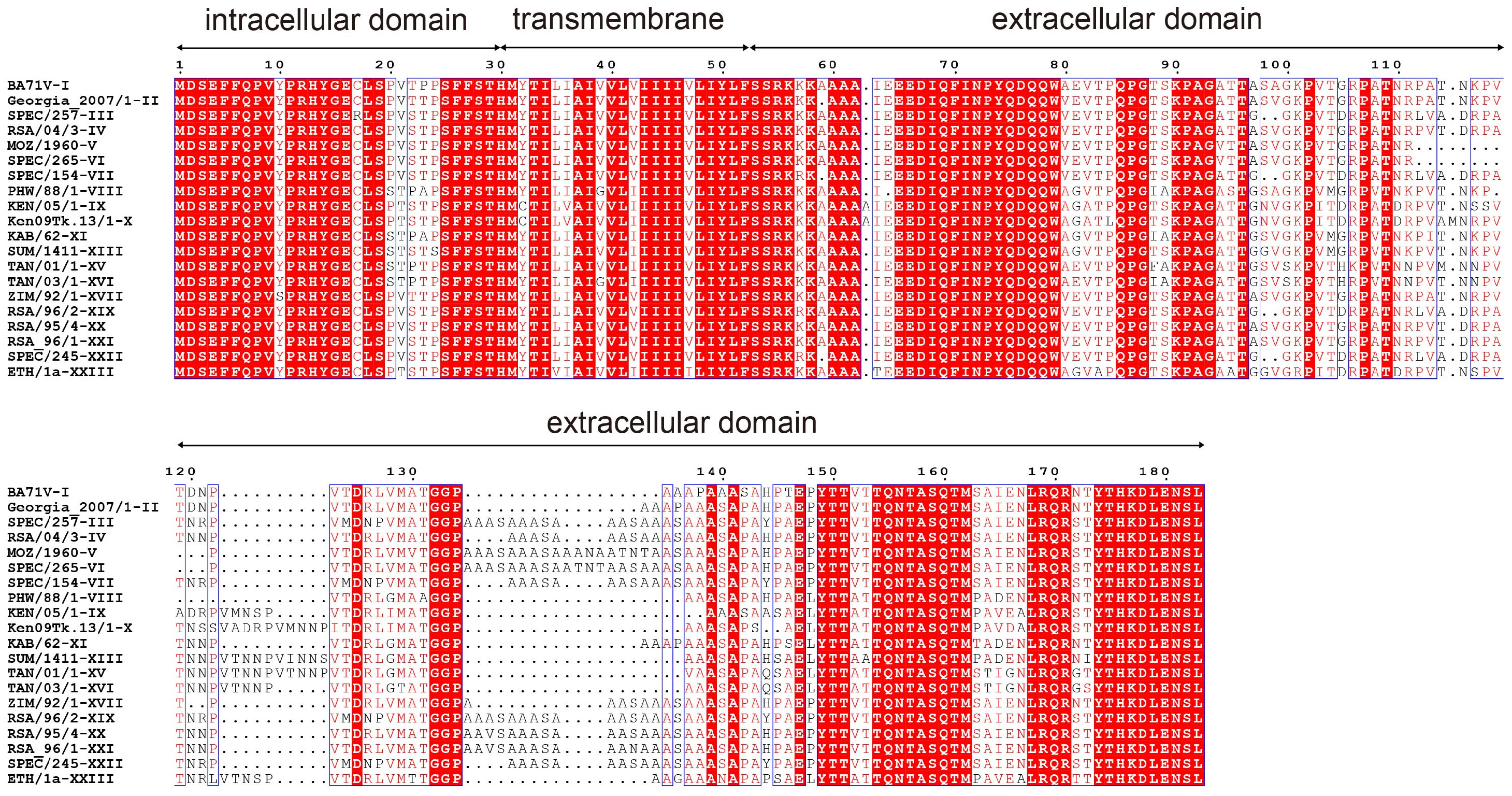
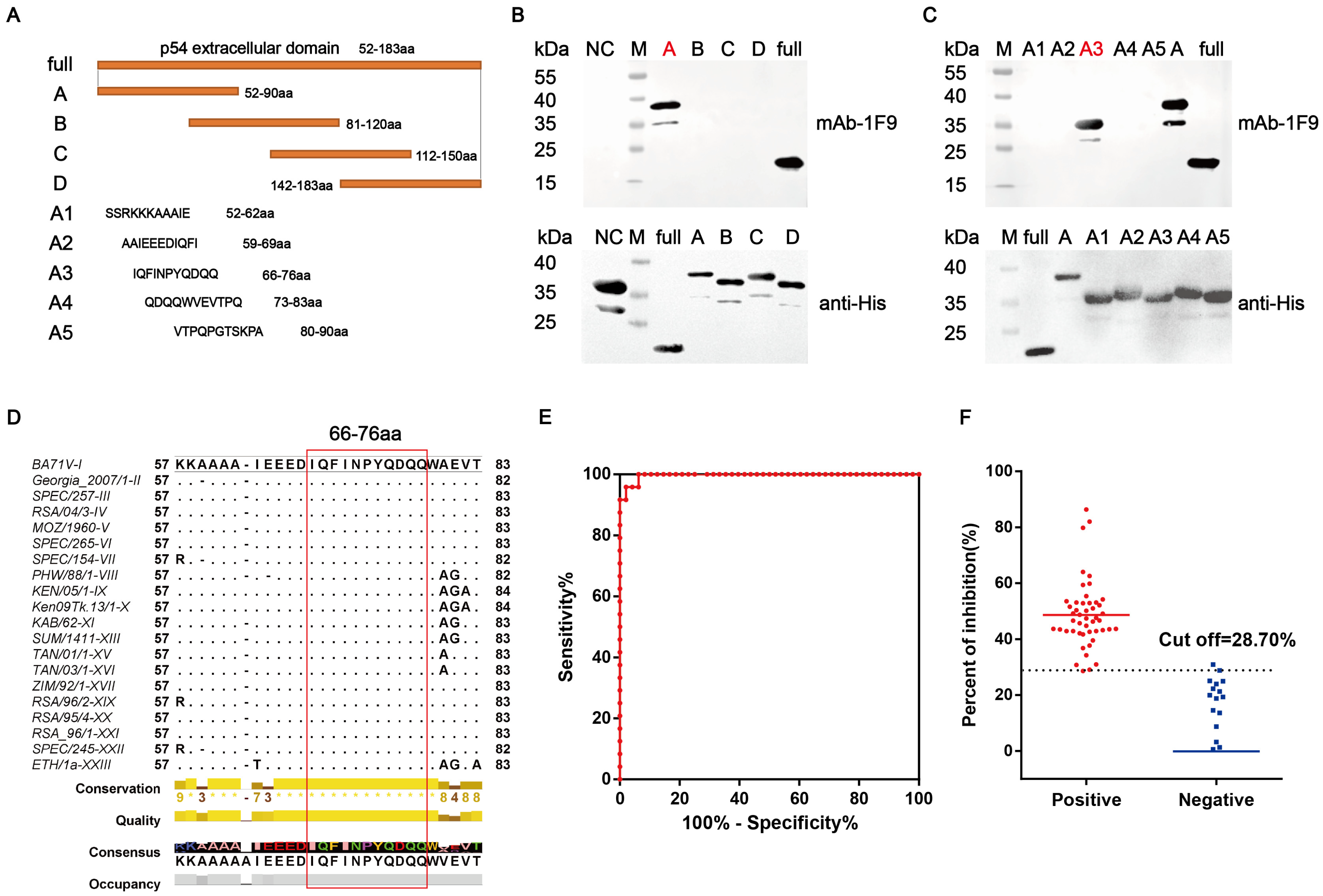
2.6. Identification of Linear B-Cell Epitopes
2.7. Blocking ELISA Analysis of ASFV-Positive Sera
2.8. Analysis of the Spatial Structure of Epitopes
3. Results
3.1. The Generation of the Recombinant p54 Protein
3.2. Generation and Characterization of Anti-p54 mAbs
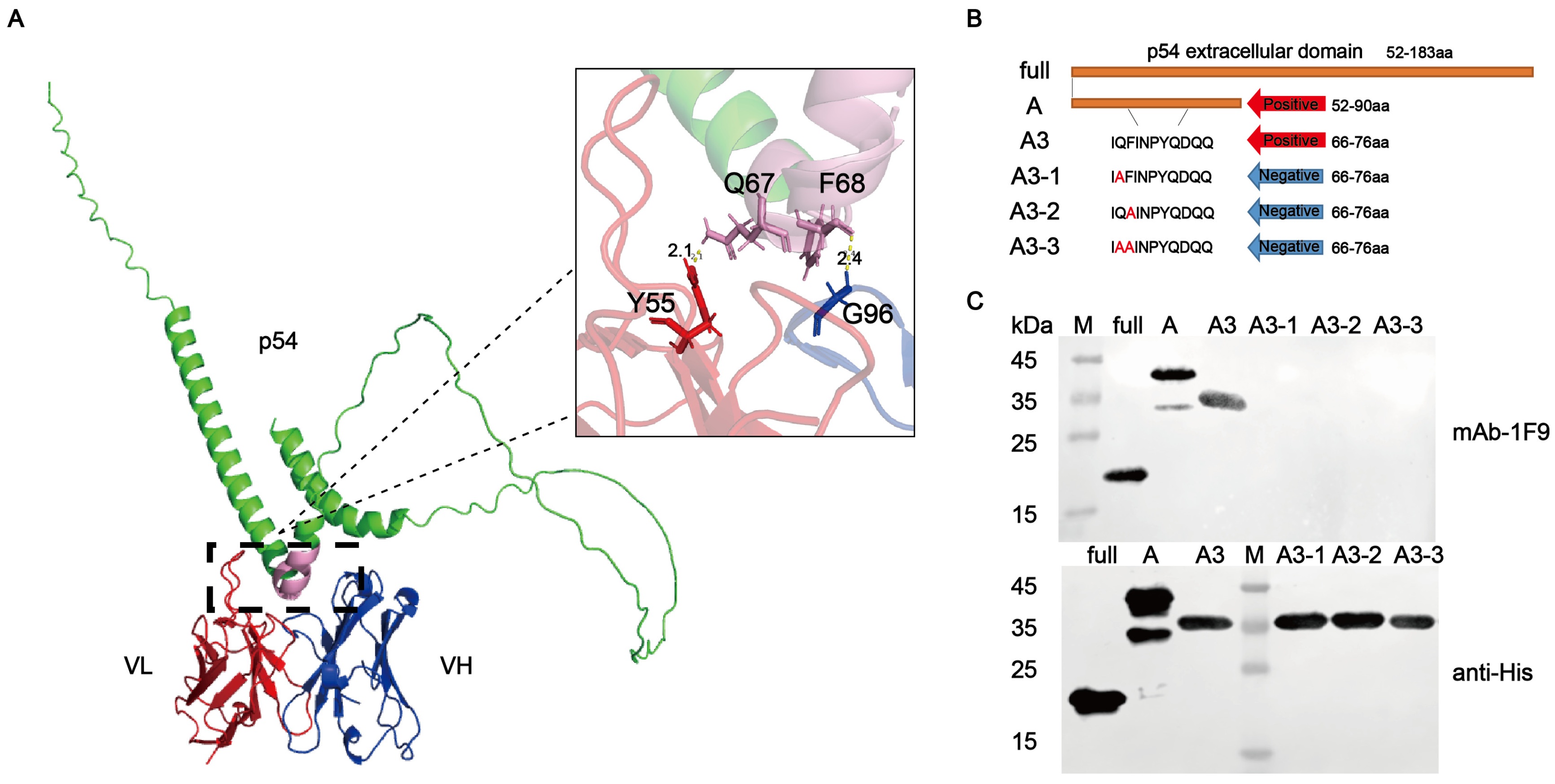
3.3. Identifying the Epitope Recognized by the p54 mAbs
3.4. Key Residues of Epitope for Recognition by p54 mAb
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penrith, M.L.; Vosloo, W. Review of African swine fever: Transmission, spread and control. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Lirola, L.G.; Mur, L.; Rivera, B.; Mogler, M.; Sun, Y.; Lizano, S.; Goodell, C.; Harris, D.L.; Rowland, R.R.; Gallardo, C.; et al. Detection of African Swine Fever Virus Antibodies in Serum and Oral Fluid Specimens Using a Recombinant Protein 30 (p30) Dual Matrix Indirect ELISA. PLoS ONE 2016, 11, e0161230. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiu, S.; Xiao, Y.; Yu, H.; Li, H.; Wu, S.; Feng, C.; Lin, X. Development of a Blocking ELISA Kit for Detection of ASFV Antibody Based on a Monoclonal Antibody Against Full-Length p72. J. AOAC Int. 2022, 105, 1428–1436. [Google Scholar]
- Liberti, R.; Colabella, C.; Anzalone, L.; Severi, G.; Di Paolo, A.; Casciari, C.; Casano, A.B.; Giammarioli, M.; Cagiola, M.; Feliziani, F.; et al. Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. Open Vet. J. 2023, 13, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Ley, V.; Gómez-Puertas, P.; García, R.; Rodriguez, J.F.; Escribano, J.M. The structural protein p54 is essential for African swine fever virus viability. Virus Res. 1996, 40, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.L.; Andrés, G. African swine fever virus morphogenesis. Virus Res. 2013, 173, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Filgueira, D.M.; González-Camacho, F.; Gallardo, C.; Resino-Talaván, P.; Blanco, E.; Gómez-Casado, E.; Alonso, C.; Escribano, J.M. Optimization and validation of recombinant serological tests for African Swine Fever diagnosis based on detection of the p30 protein produced in Trichoplusia ni larvae. J. Clin. Microbiol. 2006, 44, 3114–3121. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Puertas, P.; Rodríguez, F.; Oviedo, J.M.; Ramiro-Ibáñez, F.; Ruiz-Gonzalvo, F.; Alonso, C.; Escribano, J.M. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 1996, 70, 5689–5694. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, C.; Yang, Z.; Jia, W. Identification of a conservative site in the African swine fever virus p54 protein and its preliminary application in a serological assay. J. Vet. Sci. 2022, 23, e55. [Google Scholar] [CrossRef] [PubMed]
- Petrovan, V.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R.R. Epitope mapping of African swine fever virus (ASFV) structural protein, p54. Virus Res. 2020, 279, 197871. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Li, C.; Hou, H.; Chen, Y.; Zhang, A.; Han, S.; Wan, B.; Wu, Y.; He, H.; Wang, N.; et al. A Novel Linear B-Cell Epitope on the P54 Protein of African Swine Fever Virus Identified Using Monoclonal Antibodies. Viruses 2023, 15, 867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, G.; Dong, H.; Wu, S.; Du, Y.; Wan, B.; Ji, P.; Wu, Y.; Jiang, D.; Zhuang, G.; et al. Identification of a Linear B Cell Epitope on p54 of African Swine Fever Virus Using Nanobodies as a Novel Tool. Microbiol. Spectr. 2023, 11, e0336222. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Reis, A.L.; Kalema-Zikusoka, G.; Malta, J.; Soler, A.; Blanco, E.; Parkhouse, R.M.; Leitão, A. Recombinant antigen targets for serodiagnosis of African swine fever. Clin. Vaccine Immunol. 2009, 16, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Barderas, M.G.; Rodríguez, F.; Gómez-Puertas, P.; Avilés, M.; Beitia, F.; Alonso, C.; Escribano, J.M. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch. Virol. 2001, 146, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Han, D.; Zhang, Y.; Zhang, K.; Du, N.; Tong, W.; Li, G.; Zheng, H.; Liu, C.; Gao, F.; et al. Identification of one novel epitope targeting p54 protein of African swine fever virus using monoclonal antibody and development of a capable ELISA. Res. Vet. Sci. 2021, 141, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jiang, M.; Liu, H.; Liu, Y.; Zhou, J.; Chen, Y.; Ding, P.; Wang, Y.; Pang, W.; Qi, Y.; et al. Development and characterization of monoclonal antibodies against the N-terminal domain of African swine fever virus structural protein, p54. Int. J. Biol. Macromol. 2021, 180, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lun, Y.; Liu, B.; Dong, W.; Sun, J.; Pan, L. Comparative analysis of structural characteristics and epitopes in S proteins between SARS-CoV-2 and SARS-CoV. J. Zhejiang University. Med. Sci. 2020, 49, 315–323. [Google Scholar]
- Lee, J.H.; Jung, Y.; Lee, S.K.; Kim, J.; Lee, C.S.; Kim, S.; Lee, J.S.; Kim, N.H.; Kim, H.G. Rapid Biosensor of SARS-CoV-2 Using Specific Monoclonal Antibodies Recognizing Conserved Nucleocapsid Protein Epitopes. Viruses 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Wu, Y.; Su, Z.; Zeng, Y.; Ye, G.; Wu, Q.; Li, L.; Zhang, A. Identification of a Conserved Linear Epitope on the p54 Protein of African Swine Fever Virus. Viruses 2025, 17, 823. https://doi.org/10.3390/v17060823
He K, Wu Y, Su Z, Zeng Y, Ye G, Wu Q, Li L, Zhang A. Identification of a Conserved Linear Epitope on the p54 Protein of African Swine Fever Virus. Viruses. 2025; 17(6):823. https://doi.org/10.3390/v17060823
Chicago/Turabian StyleHe, Kuijing, Yue Wu, Zhipeng Su, Yue Zeng, Guishan Ye, Qi Wu, Long Li, and Anding Zhang. 2025. "Identification of a Conserved Linear Epitope on the p54 Protein of African Swine Fever Virus" Viruses 17, no. 6: 823. https://doi.org/10.3390/v17060823
APA StyleHe, K., Wu, Y., Su, Z., Zeng, Y., Ye, G., Wu, Q., Li, L., & Zhang, A. (2025). Identification of a Conserved Linear Epitope on the p54 Protein of African Swine Fever Virus. Viruses, 17(6), 823. https://doi.org/10.3390/v17060823





