The Hidden Threat: Rodent-Borne Viruses and Their Impact on Public Health
Abstract
1. Introduction
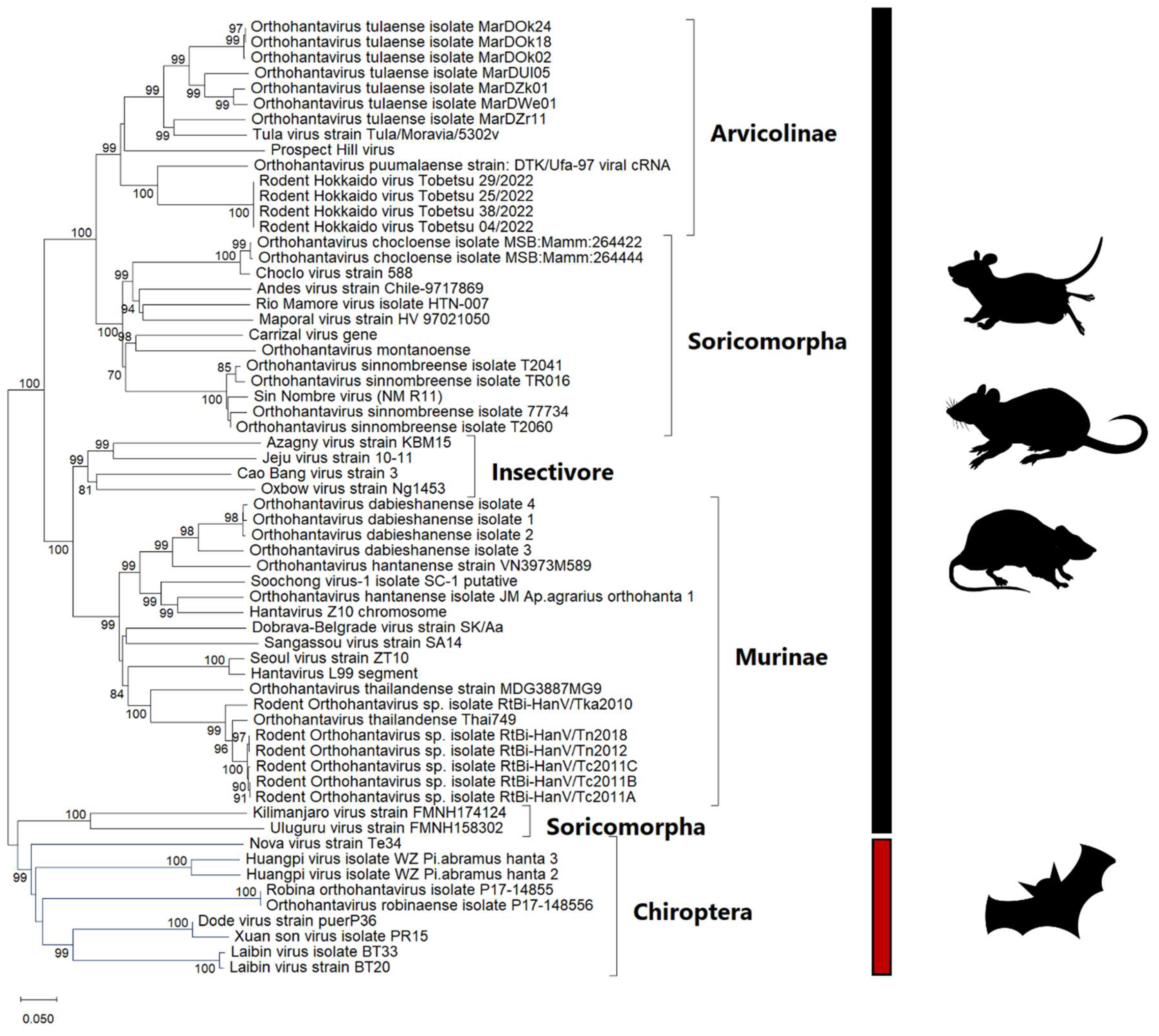
2. Hantavirus
2.1. Characteristics of Hantaviruses and Their Molecular Relationships
2.2. Epidemiology
2.3. Clinical Picture
2.4. Diagnosis and Control
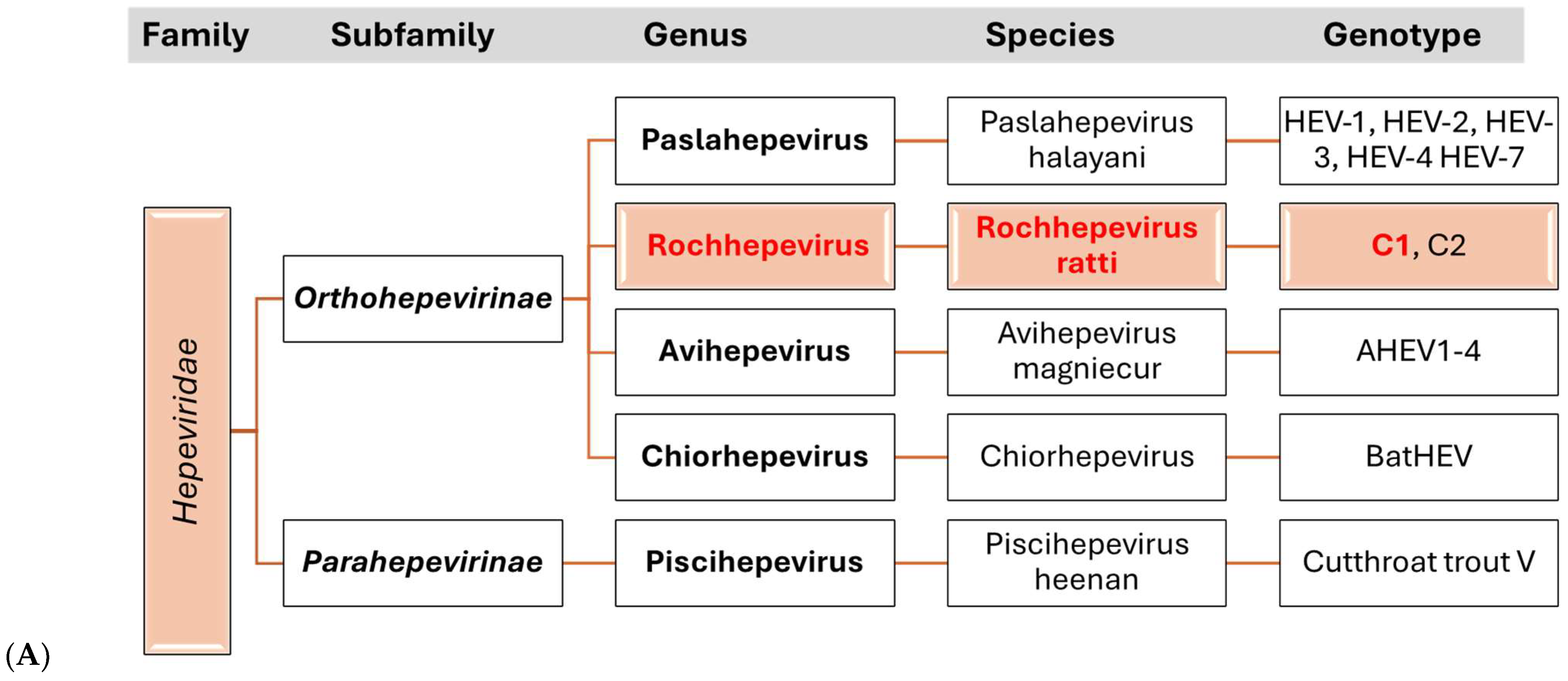
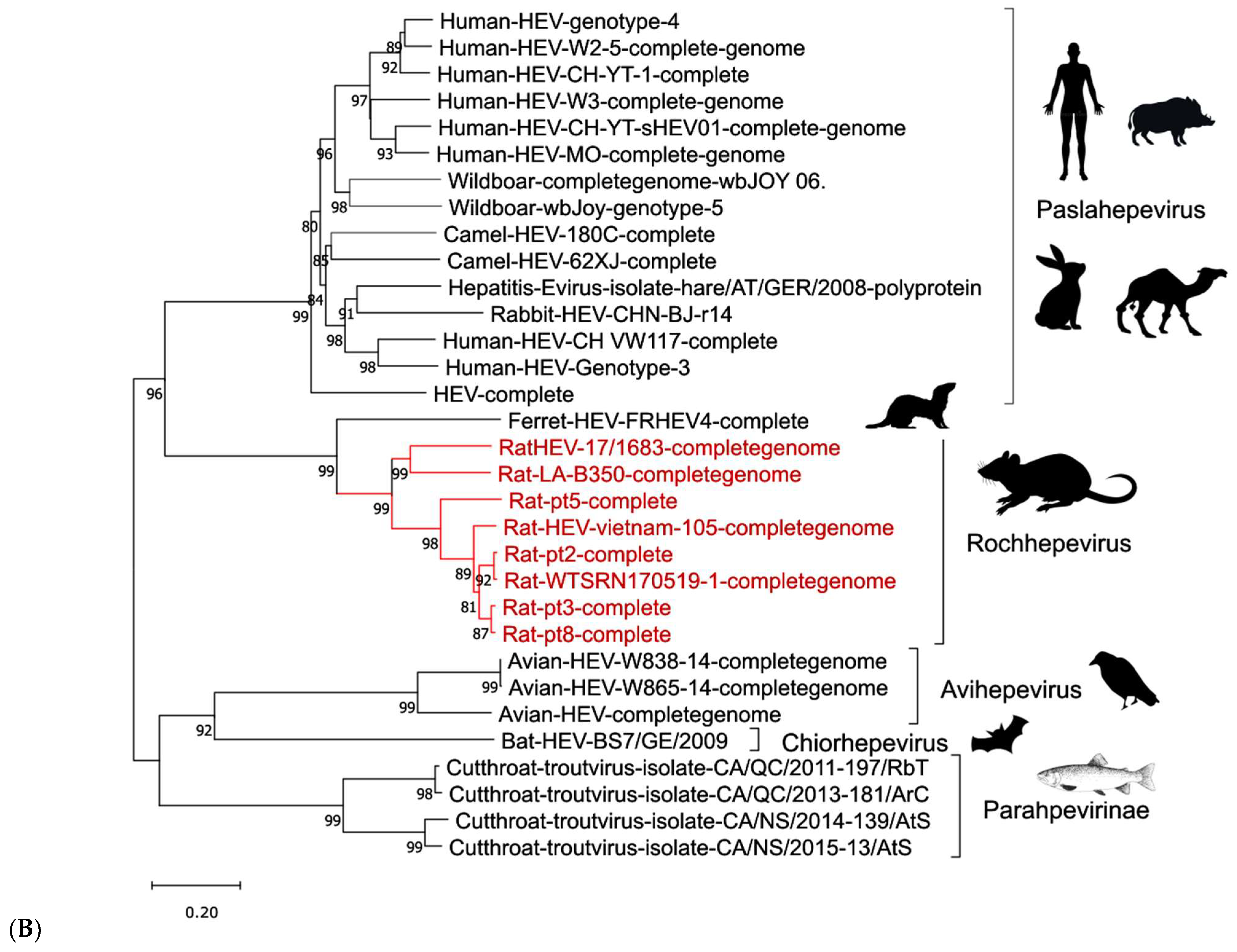
3. Hepatitis E Virus
3.1. Etiology
3.2. Epidemiology
3.3. Clinical Picture
3.4. Diagnosis and Control
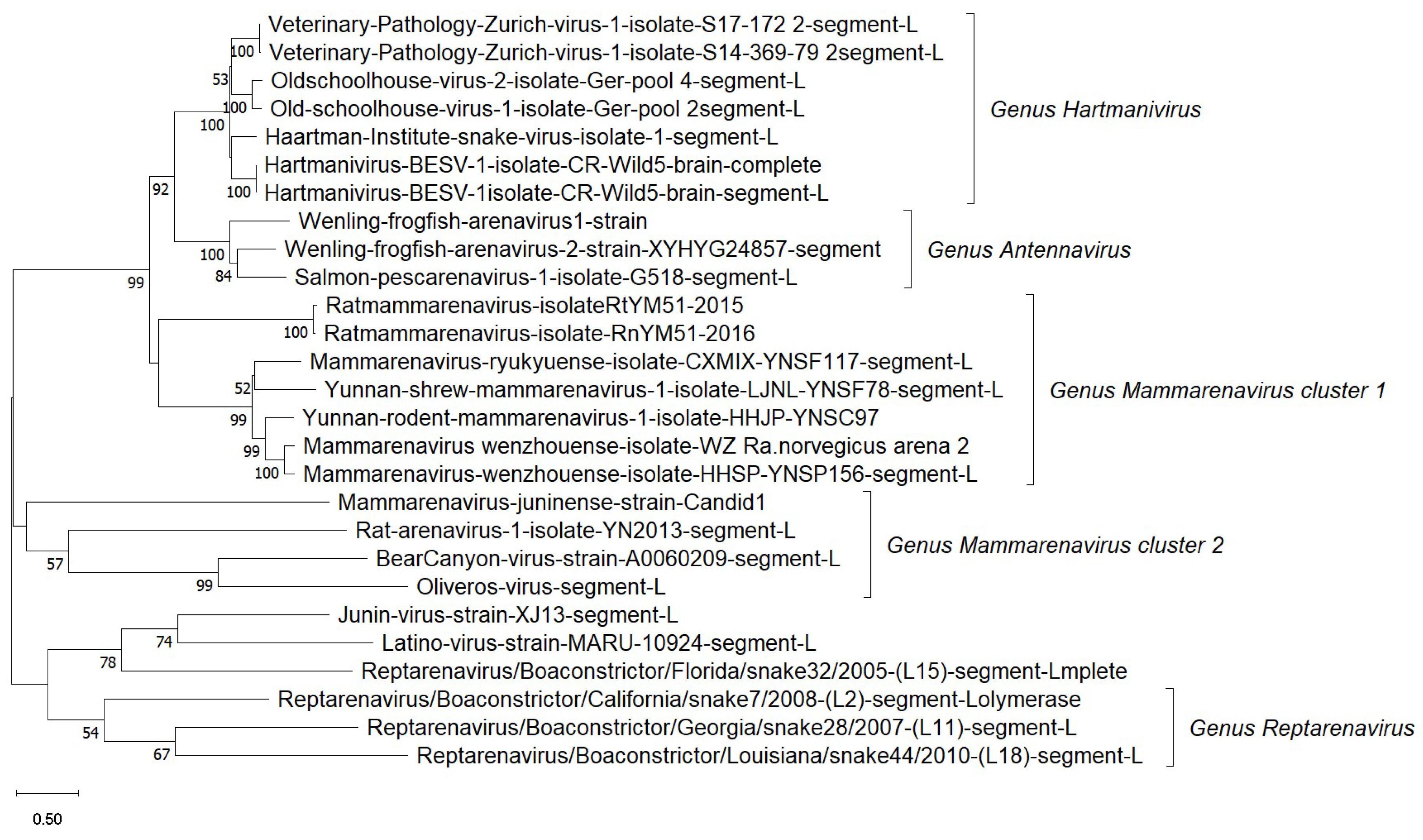
4. Arenaviruses
4.1. Etiology
4.2. Epidemiology
4.3. Clinical Picture
5. Ljungan Virus
6. Poxviruses
6.1. Monkeypox Virus (MPXV)
6.2. Cowpox Virus (CPXV)
7. Coronaviruses
8. The Role of Climate Change and Human Activity in the Emergence of Rodent-Borne Diseases
- (i)
- Warmer climates can expand the geographical range of rodent species, enabling them to thrive in new environments and increasing the risk of pathogen spillovers. Changes in seasonal rainfall can impact food availability, driving rodents into urban and peri-urban areas where contact with humans becomes more frequent, facilitating disease transmission [4,178,179]. Indeed, zoonotic transmission can arise in both livestock and human populations. Climate change significantly alters rodent habitats and behaviors, raising the likelihood of zoonotic disease spread. Rising temperatures and shifting precipitation patterns modify ecosystems, forcing rodents to adapt by migrating to new areas, including human settlements.
- (ii)
- Climate changes alter pathogen dynamics by influencing the survival, replication, and transmission of pathogens transmitted by rodents. Rising temperatures and humidity levels can enhance the persistence of bacterial, viral, and parasitic pathogens in the environment, increasing their transmission potential. Additionally, altered rodent immunity and stress from environmental changes can lead to higher pathogen-shedding rates. These shifts contribute to the emergence and re-emergence of rodent-borne zoonoses, necessitating improved monitoring and adaptive public health interventions [180,181,182].
- (iii)
- Extreme weather events, such as hurricanes, floods, droughts, and wildfires, have a significant influence on the emergence of rodent-borne zoonotic diseases [183,184,185]. Flooding can displace rodent populations, forcing them into human-inhabited areas, thereby increasing direct contact and contamination of water and food sources with pathogens, such as Leptospira spp. Droughts reduce natural food availability, driving rodents to forage in urban environments and heightening the potential for disease spillover. Wildfires destroy habitats, prompting rodent migration and altering predator–prey dynamics, which can potentially increase rodent densities [186]. These disturbances exacerbate the dissemination of pathogens, underscoring the need for proactive disease surveillance and environmental management in disaster-prone regions [187,188,189]. Changes in food availability due to altered vegetation cycles can lead to population surges, intensifying competition, and aggressive behaviors that enhance pathogen spread.
- (iv)
- Anthropogenic activities, such as deforestation, agricultural expansion, and urbanization, exacerbate the risks associated with rodent-borne zoonoses. Habitat destruction forces rodents to migrate, often bringing them closer to human settlements, as well as animals. Climate-induced shifts in vector populations, including insects, further compound this risk by altering transmission cycles [13]. To mitigate the negative impacts of climate change and anthropogenic activities on the emergence of rodent-borne zoonotic diseases, it is crucial to implement coordinated efforts that include surveillance, environmental management, and control of rodent populations [180,190,191].
9. Conclusions and Recommendations
- (i)
- Addressing the root causes of disease emergence: this involves multidisciplinary efforts, including surveillance, rodent control, and community education.
- (ii)
- Enhancing surveillance and early warning systems: rodents are key reservoirs for many emerging viruses; therefore, integrating rodent population monitoring with molecular diagnostics and ecological monitoring, alongside climate and land use information, will allow for timely interventions.
- (iii)
- Adapting to and mitigating climate change: Climate shifts influence rodent habitats, breeding cycles, and migration patterns, often expanding their range and increasing human exposure. Efforts should focus on reducing greenhouse gas emissions, conserving ecosystems, and promoting sustainable agriculture to minimize habitat disruption and help naturally regulate rodent populations.
- (iv)
- Promoting community education campaigns: These campaigns should emphasize hygiene and food safety while highlighting the role of rodents in disease transmission and the importance of proper food storage and waste management to ensure long-term disease prevention. Finally, collaboration among governments, scientists, and public health agencies is essential for developing and implementing evidence-based policies to combat the increasing threat of rodent-borne zoonoses effectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Meerburg, B.G.; Singleton, G.R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, D.; Bertolino, S.; Mortelliti, A. Rating the rat: Global patterns and research priorities in impacts and management of rodent pests. Mammal Rev. 2014, 44, 148–162. [Google Scholar] [CrossRef]
- Ecke, F.; Han, B.A.; Hörnfeldt, B.; Khalil, H.; Magnusson, M.; Singh, N.J.; Ostfeld, R.S. Population fluctuations and synanthropy explain transmission risk in rodent-borne zoonoses. Nat. Commun. 2022, 13, 7532. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, Á.; Pankovics, P. Review of Hepatitis E Virus in Rats: Evident Risk of Species Orthohepevirus C to Human Zoonotic Infection and Disease. Viruses 2020, 12, 1148. [Google Scholar] [CrossRef]
- Andreychev, A.; Boyarova, E.; Brandler, O.; Tukhbatullin, A.; Kapustina, S. Terrestrial and Subterranean Mammals as Reservoirs of Zoonotic Diseases in the Central Part of European Russia. Diversity 2022, 15, 39. [Google Scholar] [CrossRef]
- Cucchi, T.; Papayianni, K.; Cersoy, S.; Aznar-Cormano, L.; Zazzo, A.; Debruyne, R.; Berthon, R.; Bălășescu, A.; Simmons, A.; Valla, F.; et al. Tracking the Near Eastern origins and European dispersal of the western house mouse. Sci. Rep. 2020, 10, 8276. [Google Scholar] [CrossRef]
- Taylor, P.J.; Arntzen, L.; Hayter, M.; Iles, M.; Frean, J.; Belmain, S. Understanding and managing sanitary risks due to rodent zoonoses in an African city: Beyond the Boston Model. Integr. Zool. 2008, 3, 38–50. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.J.; Choi, I.; Kang, Y.M.; Kwak, D.; Seo, M.G. Prevalence and zoonotic potential of pathogens in micromammals (rodents and insectivores) in the Republic of Korea. Acta Trop. 2025, 266, 107649. [Google Scholar] [CrossRef]
- Keesing, F.; Ostfeld, R.S. Emerging patterns in rodent-borne zoonotic diseases. Science 2024, 385, 1305–1310. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Chomel, B.B.; Martínez-López, B. Climate change and zoonoses: A review of the current status, knowledge gaps, and future trends. Acta Trop. 2022, 226, 106225. [Google Scholar] [CrossRef] [PubMed]
- Tazerji, S.S.; Nardini, R.; Safdar, M.; Shehata, A.A.; Duarte, P.M. An Overview of Anthropogenic Actions as Drivers for Emerging and Re-Emerging Zoonotic Diseases. Pathogens 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Prins, C.; Sreekumar, D.; Sejian, V. Climate change and wildlife biodiversity: Impact and mitigation strategies. Arch. Life Sci. Res. 2025, 1, 6–22. [Google Scholar] [CrossRef]
- Dobigny, G.; Garba, M.; Tatard, C.; Loiseau, A.; Galan, M.; Kadaouré, I.; Rossi, J.-P.; Picardeau, M.; Bertherat, E. Urban Market Gardening and Rodent-Borne Pathogenic Leptospira in Arid Zones: A Case Study in Niamey, Niger. PLoS Negl. Trop. Dis. 2015, 9, e0004097. [Google Scholar] [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife-Livestock-Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef]
- Muyangwa, M.; Martynova, E.V.; Khaiboullina, S.F.; Morzunov, S.P.; Rizvanov, A.A. Hantaviral Proteins: Structure, Functions, and Role in Hantavirus Infection. Front. Microbiol. 2015, 6, 1326. [Google Scholar] [CrossRef]
- Parvate, A.; Williams, E.P.; Taylor, M.K.; Chu, Y.-K.; Lanman, J.; Saphire, E.O.; Jonsson, C.B. Diverse Morphology and Structural Features of Old and New World Hantaviruses. Viruses 2019, 11, 862. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Carey, D.E.; Reuben, R.; Panicker, K.N.; Shope, R.E.; Myers, R.M. Thottapalayam virus: A presumptive arbovirus isolated from a shrew in India. Indian J. Med. Res. 1971, 59, 1758–1760. [Google Scholar]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current Classification and Future Perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Zhang, Y.-Z. The evolution and emergence of hantaviruses. Curr. Opin. Virol. 2015, 10, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-X.; Chen, M.-J.; Sun, L. Haemorrhagic fever with renal syndrome: Literature review and distribution analysis in China. Int. J. Infect. Dis. 2016, 43, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Avšič-Županc, T.; Saksida, A.; Korva, M. Hantavirus infections. Clin. Microbiol. Infect. 2019, 21, e6–e16. [Google Scholar] [CrossRef]
- Klempa, B.; Avsic-Zupanc, T.; Clement, J.; Dzagurova, T.K.; Henttonen, H.; Heyman, P.; Jakab, F.; Kruger, D.H.; Maes, P.; Papa, A.; et al. Complex evolution and epidemiology of Dobrava-Belgrade hantavirus: Definition of genotypes and their characteristics. Arch. Virol. 2013, 158, 521–529. [Google Scholar] [CrossRef]
- Chen, J.-T.; Qin, J.; Li, K.; Xu, Q.-Y.; Wang, X.-P.; Plyusnin, A.; Hou, W.; Zhang, Y.-Z. Identification and characterization of a novel subtype of Tula virus in Microtus arvalis obscurus voles sampled from Xinjiang, China. Infect. Genet. Evol. 2019, 75, 104012. [Google Scholar] [CrossRef]
- Vaheri, A.; Henttonen, H.; Mustonen, J. Hantavirus Research in Finland: Highlights and Perspectives. Viruses 2021, 13, 1452. [Google Scholar] [CrossRef]
- Bi, Z.; Formenty, P.B.H.; Roth, C.E. Hantavirus Infection: A review and global update. J. Infect. Dev. Ctries. 2008, 2, 3–23. [Google Scholar] [CrossRef]
- Chaparro, J. Assessment of person-to-person transmission of hantavirus pulmonary syndrome in a Chilean hospital setting. J. Hosp. Infect. 1998, 40, 281–285. [Google Scholar] [CrossRef]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.L.; López, N.M.; Rossi, C.M.; Rabinovich, R.D. Hantavirus Pulmonary Syndrome Outbreak in Argentina: Molecular Evidence for Person-to-Person Transmission of Andes Virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef]
- Alonso, D.O.; Pérez-Sautu, U.; Bellomo, C.M.; Prieto, K.; Iglesias, A.; Coelho, R.; Periolo, N.; Domenech, I.; Talmon, G.; Hansen, R.; et al. Person-to-Person Transmission of Andes Virus in Hantavirus Pulmonary Syndrome, Argentina, 2014. Emerg. Infect. Dis. 2020, 26, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the Etiologic Agent of Korean Hemorrhagic Fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Krüger, D.H.; Auste, B.; Stark, K.; Hofmann, J.; Weiss, S. Molecular and epidemiological characteristics of human Puumala and Dobrava-Belgrade hantavirus infections, Germany, 2001 to 2017. Eurosurveillance 2019, 24, 1800675. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.; Ullah, S.; Wei, D.-Q. Hantavirus: The Next Pandemic We Are Waiting For? Interdiscip. Sci. 2021, 13, 147–152. [Google Scholar] [CrossRef]
- Akram, S.M.; Mangat, R.; Huang, B. Hantavirus Cardiopulmonary Syndrome (Archived). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Essbauer, S.; Schmidt, J.; Conraths, F.J.; Friedrich, R.; Koch, J.; Hautmann, W.; Pfeffer, M.; Wölfel, R.; Finke, J.; Dobler, G.; et al. A new Puumala hantavirus subtype in rodents associated with an outbreak of Nephropathia epidemica in South-East Germany in 2004. Epidemiol. Infect. 2006, 134, 1333–1344. [Google Scholar] [CrossRef]
- Heyman, P.; Vaheri, A.; Lundkvist, Å.; Avsic-Zupanc, T. Hantavirus infections in Europe: From virus carriers to a major public-health problem. Expert Rev. Anti Infect. Ther. 2009, 7, 205–217. [Google Scholar] [CrossRef]
- Yan, L.; Fang, L.-Q.; Huang, H.-G.; Zhang, L.-Q.; Feng, D.; Zhao, W.-J.; Zhang, W.-Y.; Li, X.-W.; Cao, W.-C. Landscape elements and Hantaan virus-related hemorrhagic fever with renal syndrome, People’s Republic of China. Emerg. Infect. Dis. 2007, 13, 1301–1306. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Mu, D.; Xu, Z.; Qian, Q.; Chen, G.; Wen, L.; Yin, W.; Li, S.; Zhang, W.; et al. The Impacts of Climatic Factors and Vegetation on Hemorrhagic Fever with Renal Syndrome Transmission in China: A Study of 109 Counties. Int. J. Environ. Res. Public. Health 2019, 16, 3434. [Google Scholar] [CrossRef]
- Kariwa, H.; Yoshikawa, K.; Tanikawa, Y.; Seto, T.; Sanada, T.; Saasa, N.; Ivanov, L.I.; Slonova, R.; Zakharycheva, T.A.; Nakamura, I.; et al. Isolation and Characterization of Hantaviruses in Far East Russia and Etiology of Hemorrhagic Fever with Renal Syndrome in the Region. Am. Soc. Trop. Med. Hyg. 2012, 86, 545–553. [Google Scholar] [CrossRef]
- Padula, P.J.; Rossi, C.M.; Valle, M.O.D.; Martínez, P.V.; Colavecchia, S.B.; Edelstein, A.; Miguel, S.D.L.; Rabinovich, R.D.; Segura, E.L. Development and evaluation of a solid-phase enzyme immunoassay based on Andes hantavirus recombinant nucleoprotein. J. Med. Microbiol. 2000, 49, 149–155. [Google Scholar] [CrossRef]
- Krautkrämer, E.; Zeier, M.; Plyusnin, A. Hantavirus infection: An emerging infectious disease causing acute renal failure. Kidney Int. 2013, 83, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.M.; De Figueiredo, G.G.; Sabino Dos Santos Jr, G.; Figueiredo, L.T.M. Laboratory Diagnosis of Human Hantavirus Infection: Novel Insights and Future Potential. Future Virol. 2009, 4, 383–389. [Google Scholar] [CrossRef]
- Park, K.; Lee, S.-H.; Kim, J.; Lee, J.; Lee, G.-Y.; Cho, S.; Lee, S.H.; Park, K.; No, J.S.; Budhathoki, S.; et al. Multiplex PCR-Based Nanopore Sequencing and Epidemiological Surveillance of Hantaan orthohantavirus in Apodemus agrarius, Republic of Korea. Viruses 2021, 13, 847. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Factsheets Hepatitis E. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 10 February 2025).
- Khuroo, M.S. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am. J. Med. 1980, 68, 818–824. [Google Scholar] [CrossRef]
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983, 20, 23–31. [Google Scholar] [CrossRef]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Kwo, P.Y.; Schlauder, G.G.; Carpenter, H.A.; Murphy, P.J.; Rosenblatt, J.E.; Dawson, G.J.; Mast, E.E.; Krawczynski, K.; Balan, V. Acute hepatitis E by a new isolate acquired in the United States. Mayo Clin. Proc. 1997, 72, 1133–1136. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, R.; Erker, J.C.; Zhang, H.; Li, H.; Desai, S.; Mushahwar, I.K.; Harrison, T.J. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J. Gen. Virol. 1999, 80(Pt. 1), 169–177. [Google Scholar] [CrossRef]
- Lee, G.-H.; Tan, B.-H.; Chi-Yuan Teo, E.; Lim, S.-G.; Dan, Y.-Y.; Wee, A.; Kim Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.-K.; et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357.e3. [Google Scholar] [CrossRef]
- Pallerla, S.R.; Harms, D.; Johne, R.; Todt, D.; Steinmann, E.; Schemmerer, M.; Wenzel, J.J.; Hofmann, J.; Shih, J.W.K.; Wedemeyer, H.; et al. Hepatitis E Virus Infection: Circulation, Molecular Epidemiology, and Impact on Global Health. Pathogens 2020, 9, 856. [Google Scholar] [CrossRef]
- Purdy, M.A.; Drexler, J.F.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Plenge-Bönig, A.; Hess, M.; Ulrich, R.G.; Reetz, J.; Schielke, A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J. Gen. Virol. 2010, 91, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Krog, J.S.; Breum, S.Ø.; Jensen, T.H.; Larsen, L.E. Hepatitis E virus variant in farmed mink, Denmark. Emerg. Infect. Dis. 2013, 19, 2028–2030. [Google Scholar] [CrossRef] [PubMed]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschambault, Y.; Murnaghan, K.; Varga, J.; Johnston, L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019, 220, 951–955. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.-X.; Leung, K.-H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.-M.; Teng, J.L.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef]
- Rodriguez, C.; Marchand, S.; Sessa, A.; Cappy, P.; Pawlotsky, J.-M. Orthohepevirus C hepatitis, an underdiagnosed disease? J. Hepatol. 2023, 79, e39–e41. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; St Claire, M.; Emerson, S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef]
- Li, W.; Guan, D.; Su, J.; Takeda, N.; Wakita, T.; Li, T.-C.; Ke, C.W. High prevalence of rat hepatitis E virus in wild rats in China. Vet. Microbiol. 2013, 165, 275–280. [Google Scholar] [CrossRef]
- Mulyanto; Depamede, S.N.; Sriasih, M.; Takahashi, M.; Nagashima, S.; Jirintai, S.; Nishizawa, T.; Okamoto, H. Frequent detection and characterization of hepatitis E virus variants in wild rats (Rattus rattus) in Indonesia. Arch. Virol. 2013, 158, 87–96. [Google Scholar] [CrossRef]
- Johne, R.; Dremsek, P.; Kindler, E.; Schielke, A.; Plenge-Bönig, A.; Gregersen, H.; Wessels, U.; Schmidt, K.; Rietschel, W.; Groschup, M.H.; et al. Rat hepatitis E virus: Geographical clustering within Germany and serological detection in wild Norway rats (Rattus norvegicus). Infect. Genet. Evol. 2012, 12, 947–956. [Google Scholar] [CrossRef]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Pallerla, S.R.; Johne, R.; Todt, D.; Steinmann, E.; Schemmerer, M.; Wenzel, J.J.; Hofmann, J.; Shih, J.W.K.; Wedemeyer, H.; et al. Hepatitis E: An update on One Health and clinical medicine. Liver Int. 2021, 41, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Bendall, R.; Ellis, V.; Ijaz, S.; Ali, R.; Dalton, H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J. Med. Virol. 2010, 82, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Tamisier, M.; Moal, V.; Gerolami, R.; Colson, P. Discrepancy between anti-hepatitis E virus immunoglobulin G prevalence assessed by two assays in kidney and liver transplant recipients. J. Clin. Virol. 2013, 56, 62–64. [Google Scholar] [CrossRef]
- Mirazo, S.; Ramos, N.; Mainardi, V.; Arbiza, J.; Gerona, S. Transmission, diagnosis, and management of hepatitis E: An update. Hepatic Med. Evid. Res. 2014, 2014, 45–59. [Google Scholar] [CrossRef]
- WHO Recommended Strategies for the Prevention and Control of Communicable Diseases. Available online: https://iris.who.int/bitstream/handle/10665/67088/WHO_CDS_CPE_SMT_2001.13.pdf (accessed on 14 May 2025).
- Oldstone, M.B.A. Arenaviruses. I. The epidemiology molecular and cell biology of arenaviruses. Introduction. Curr. Top. Microbiol. Immunol. 2002, 262, V–XII. [Google Scholar]
- Emonet, S.; Lemasson, J.-J.; Gonzalez, J.-P.; De Lamballerie, X.; Charrel, R.N. Phylogeny and evolution of old world arenaviruses. Virology 2006, 350, 251–257. [Google Scholar] [CrossRef]
- Albariño, C.G.; Palacios, G.; Khristova, M.L.; Erickson, B.R.; Carroll, S.A.; Comer, J.A.; Hui, J.; Briese, T.; St George, K.; Ksiazek, T.G.; et al. High Diversity and Ancient Common Ancestry of Lymphocytic Choriomeningitis Virus. Emerg. Infect. Dis. 2010, 16, 1093–1100. [Google Scholar] [CrossRef]
- King, B.R.; Samacoits, A.; Eisenhauer, P.L.; Ziegler, C.M.; Bruce, E.A.; Zenklusen, D.; Zimmer, C.; Mueller, F.; Botten, J. Visualization of Arenavirus RNA Species in Individual Cells by Single-Molecule Fluorescence In Situ Hybridization Suggests a Model of Cyclical Infection and Clearance during Persistence. J. Virol. 2018, 92, e02241-17. [Google Scholar] [CrossRef]
- Fuller-Pace, F.V.; Southern, P.J. Detection of virus-specific RNA-dependent RNA polymerase activity in extracts from cells infected with lymphocytic choriomeningitis virus: In vitro synthesis of full-length viral RNA species. J. Virol. 1989, 63, 1938–1944. [Google Scholar] [CrossRef]
- Lee, K.J.; Perez, M.; Pinschewer, D.D.; de la Torre, J.C. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 2002, 76, 6393–6397. [Google Scholar] [CrossRef]
- Fehling, S.K.; Lennartz, F.; Strecker, T. Multifunctional Nature of the Arenavirus RING Finger Protein Z. Viruses 2012, 4, 2973–3011. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Ly, H. Inhibition of Innate Immune Responses Is Key to Pathogenesis by Arenaviruses. J. Virol. 2016, 90, 3810–3818. [Google Scholar] [CrossRef] [PubMed]
- Agnihothram, S.S.; York, J.; Nunberg, J.H. Role of the Stable Signal Peptide and Cytoplasmic Domain of G2 in Regulating Intracellular Transport of the Junín Virus Envelope Glycoprotein Complex. J. Virol. 2006, 80, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- Bederka, L.H.; Bonhomme, C.J.; Ling, E.L.; Buchmeier, M.J. Arenavirus Stable Signal Peptide Is the Keystone Subunit for Glycoprotein Complex Organization. mBio 2014, 5, e02063-14. [Google Scholar] [CrossRef]
- Cao, W.; Henry, M.D.; Borrow, P.; Yamada, H.; Elder, J.H.; Ravkov, E.V.; Nichol, S.T.; Compans, R.W.; Campbell, K.P.; Oldstone, M.B.A. Identification of α-Dystroglycan as a Receptor for Lymphocytic Choriomeningitis Virus and Lassa Fever Virus. Science 1998, 282, 2079–2081. [Google Scholar] [CrossRef]
- Burns, J.W.; Buchmeier, M.J. Protein-protein interactions in lymphocytic choriomeningitis virus. Virology 1991, 183, 620–629. [Google Scholar] [CrossRef]
- Radoshitzky, S.R.; Buchmeier, M.J.; Charrel, R.N.; Gonzalez, J.-P.J.; Günther, S.; Hepojoki, J.; Kuhn, J.H.; Lukashevich, I.S.; Romanowski, V.; Salvato, M.S.; et al. ICTV Virus Taxonomy Profile: Arenaviridae 2023. J. Gen. Virol. 2023, 104, 001891. [Google Scholar] [CrossRef]
- Childs, J.E.; Peters, C.J. Ecology and epidemiology of arenaviruses and their hosts. In The Arenaviridae; Salvato, M., Ed.; Plenum Press: New York, NY, USA, 1993. [Google Scholar]
- Gryseels, S.; Baird, S.J.E.; Borremans, B.; Makundi, R.; Leirs, H.; Goüy De Bellocq, J. When Viruses Don’t Go Viral: The Importance of Host Phylogeographic Structure in the Spatial Spread of Arenaviruses. PLoS Pathog. 2017, 13, e1006073. [Google Scholar] [CrossRef]
- Childs, J.E.; Klein, S.L.; Glass, G.E. A Case Study of Two Rodent-Borne Viruses: Not Always the Same Old Suspects. Front. Ecol. Evol. 2019, 7, 35. [Google Scholar] [CrossRef]
- Buckley, S.M.; Casals, J. Lassa fever, a new virus disease of man from West Africa. 3. Isolation and characterization of the virus. Am. J. Trop. Med. Hyg. 1970, 19, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Rajini, B.; Zeng, J.; Suvas, P.K.; Dech, H.M.; Onami, T.M. Both systemic and mucosal LCMV immunization generate robust viral-specific IgG in mucosal secretions, but elicit poor LCMV-specific IgA. Viral Immunol. 2010, 23, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.H.; Knight, E.H. Natural routes for post-natal transmission of murine lymphocytic choriomeningitis. Lab. Anim. 1973, 7, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, R.; Stille, W.; Blumenthal, W.; Helm, E.B.; Keller, K.; Baldus, O. Syrian hamsters as vectors of lymphocytic choriomeningitis. Dtsch. Med. Wochenschr. 1972, 97, 1725–1731. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Huang, S.-J.; Wang, Z.-D.; Wei, F.; Feng, X.-M.; Jiang, D.-X.; Liu, Q. Isolation and genomic characterization of lymphocytic choriomeningitis virus in ticks from northeastern China. Transbound. Emerg. Dis. 2018, 65, 1733–1739. [Google Scholar] [CrossRef]
- Leong, K.M.; Terrell, S.P.; Savage, A. Causes of mortality in captive cotton-top tamarins (Saguinus oedipus). Zoo Biol. 2004, 23, 127–137. [Google Scholar] [CrossRef]
- Scanga, C.A.; Holmes, K.V.; Montali, R.J. Serologic Evidence of Infection with Lymphocytic Choriomeningitis Virus, the Agent of Callitrichid Hepatitis, in Primates in Zoos, Primate Research Centers, and a Natural Reserve. J. Zoo Wildl. Med. 1993, 24, 469–474. [Google Scholar]
- Arruda, L.B.; Haider, N.; Olayemi, A.; Simons, D.; Ehichioya, D.; Yinka-Ogunleye, A.; Ansumana, R.; Thomason, M.J.; Asogun, D.; Ihekweazu, C.; et al. The niche of One Health approaches in Lassa fever surveillance and control. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 29. [Google Scholar] [CrossRef]
- Olayemi, A.; Fichet-Calvet, E. Systematics, Ecology, and Host Switching: Attributes Affecting Emergence of the Lassa Virus in Rodents across Western Africa. Viruses 2020, 12, 312. [Google Scholar] [CrossRef]
- Ter Meulen, J.; Lukashevich, I.; Sidibe, K.; Inapogui, A.; Marx, M.; Dorlemann, A.; Yansane, M.L.; Koulemou, K.; Chang-Claude, J.; Schmitz, H. Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea. Am. J. Trop. Med. Hyg. 1996, 55, 661–666. [Google Scholar] [CrossRef]
- Salazar-Bravo, J.; Ruedas, L.A.; Yates, T.L. Mammalian reservoirs of arenaviruses. Curr. Top. Microbiol. Immunol. 2002, 262, 25–63. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.C.; Saron, M.F.; Brouqui, P.; Bourgeade, A. Lymphocytic choriomeningitis virus in southern France: Four case reports and a review of the literature. Eur. J. Epidemiol. 1997, 13, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Castellar, A.; Guevara, M.; Rodas, J.D.; Londoño, A.F.; Arroyave, A.; Díaz, F.J.; Levis, S.; Blanco, P.J. Primera evidencia de infección por el virus de la coriomeningitis linfocítica (arenavirus) en roedores Mus musculus capturados en la zona urbana del municipio de Sincelejo, Sucre, Colombia. Biomédica 2017, 37, 75–85. [Google Scholar]
- Alburkat, H.A.T.; Pulkkinen, E.; Virtanen, J.; Vapalahti, O.; Sironen, T.; Jääskeläinen, A.J. Serological and molecular screening of arenaviruses in suspected tick-borne encephalitis cases in Finland. Epidemiol. Infect. 2024, 152, e20. [Google Scholar] [CrossRef] [PubMed]
- Koroknai, A.; Nagy, A.; Nagy, O.; Csonka, N.; Mezei, E.; Szomor, K.; Takács, M. Lymphocytic Choriomeningitis Virus Infections in Hungary between 2017–2023—Investigation of the First Congenital Infections. Diagnostics 2024, 14, 1436. [Google Scholar] [CrossRef]
- Gregg, M.B. Recent outbreaks of lymphocytic choriomeningitis in the United States of America. Bull. World Health Organ. 1975, 52, 549–553. [Google Scholar]
- Knust, B.; Ströher, U.; Edison, L.; Albariño, C.G.; Lovejoy, J.; Armeanu, E.; House, J.; Cory, D.; Horton, C.; Fowler, K.L.; et al. Lymphocytic Choriomeningitis Virus in Employees and Mice at Multipremises Feeder-Rodent Operation, United States, 2012. Emerg. Infect. Dis. 2014, 20, 240–247. [Google Scholar] [CrossRef]
- Biggar, R.J.; Woodall, J.P.; Walter, P.D.; Haughie, G.E. Lymphocytic choriomeningitis outbreak associated with pet hamsters. Fifty-seven cases from New York State. JAMA 1975, 232, 494–500. [Google Scholar] [CrossRef]
- Fischer, S.A.; Graham, M.B.; Kuehnert, M.J.; Kotton, C.N.; Srinivasan, A.; Marty, F.M.; Comer, J.A.; Guarner, J.; Paddock, C.D.; DeMeo, D.L.; et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006, 354, 2235–2249. [Google Scholar] [CrossRef]
- Kilduff, S.; Steinman, B.; Xie, Y.; Kiss-Farengo, T.; Foca, M.; Hayde, N. Pet safety guidelines for pediatric transplant recipients. Pediatr. Transplant. 2024, 28, e14527. [Google Scholar] [CrossRef]
- Singleton, G.R.; Brown, P.R.; Pech, R.P.; Jacob, J.; Mutze, G.J.; Krebs, C.J. One hundred years of eruptions of house mice in Australia—A natural biological curio: Ecology of mice in Australia. Biol. J. Linn. Soc. 2005, 84, 617–627. [Google Scholar] [CrossRef]
- Palacios, G.; Druce, J.; Du, L.; Tran, T.; Birch, C.; Briese, T.; Conlan, S.; Quan, P.-L.; Hui, J.; Marshall, J.; et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008, 358, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Goldwater, P.N. A mouse zoonotic virus (LCMV): A possible candidate in the causation of SIDS. Med. Hypotheses 2022, 158, 110735. [Google Scholar] [CrossRef] [PubMed]
- Hogeboom, C. Does multiple sclerosis have a zoonotic origin? Correlations with lymphocytic choriomeningitis virus infection. Front. Immunol. 2023, 14, 1217176. [Google Scholar] [CrossRef]
- Asper, M.; Hofmann, P.; Osmann, C.; Funk, J.; Metzger, C.; Bruns, M.; Kaup, F.-J.; Schmitz, H.; Günther, S. First Outbreak of Callitrichid Hepatitis in Germany: Genetic Characterization of the Causative Lymphocytic Choriomeningitis Virus Strains. Virology 2001, 284, 203–213. [Google Scholar] [CrossRef][Green Version]
- Ramsay, E.C.; Montali, R.J.; Worley, M.; Stephensen, C.B.; Holmes, K.V. Callitrichid hepatitis: Epizootiology of a fatal hepatitis in zoo tamarins and marmosets. J. Zoo. Wildl. Med. 1989, 20, 178–183. [Google Scholar]
- Reuter, G.; Boros, Á.; Tóth, Z.; Gia Phan, T.; Delwart, E.; Pankovics, P. A highly divergent picornavirus in an amphibian, the smooth newt (Lissotriton vulgaris). J. Gen. Virol. 2015, 96, 2607–2613. [Google Scholar] [CrossRef]
- Mayo, M.A.; Pringle, C.R. Virus taxonomy—1997. J. Gen. Virol. 1998, 79(Pt. 4), 649–657. [Google Scholar] [CrossRef]
- Barbknecht, M.; Sepsenwol, S.; Leis, E.; Tuttle-Lau, M.; Gaikowski, M.; Knowles, N.J.; Lasee, B.; Hoffman, M.A. Characterization of a new picornavirus isolated from the freshwater fish Lepomis macrochirus. J. Gen. Virol. 2014, 95, 601–613. [Google Scholar] [CrossRef]
- Jääskeläinen, A.J.; Voutilainen, L.; Lehmusto, R.; Henttonen, H.; Lappalainen, M.; Kallio-Kokko, H.; Vaheri, A.; Vapalahti, O. Serological survey in the Finnish human population implies human-to-human transmission of Ljungan virus or antigenically related viruses. Epidemiol. Infect. 2016, 144, 1278–1285. [Google Scholar] [CrossRef]
- Lundstig, A.; McDonald, S.L.; Maziarz, M.; Weldon, W.C.; Vaziri-Sani, F.; Lernmark, Å.; Nilsson, A.-L. Neutralizing Ljungan virus antibodies in children with newly diagnosed type 1 diabetes. J. Gen. Virol. 2021, 102, 001602. [Google Scholar] [CrossRef]
- Niklasson, B.; Kinnunen, L.; Hörnfeldt, B.; Hörling, J.; Benemar, C.; Hedlund, K.O.; Matskova, L.; Hyypiä, T.; Winberg, G. A new picornavirus isolated from bank voles (Clethrionomys glareolus). Virology 1999, 255, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Fevola, C.; Rossi, C.; Rosso, F.; Girardi, M.; Rosà, R.; Manica, M.; Delucchi, L.; Rocchini, D.; Garzon-Lopez, C.X.; Arnoldi, D.; et al. Geographical Distribution of Ljungan Virus in Small Mammals in Europe. Vector Borne Zoonotic Dis. 2020, 20, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Tolf, C.; Gullberg, M.; Johansson, E.S.; Tesh, R.B.; Andersson, B.; Lindberg, A.M. Molecular characterization of a novel Ljungan virus (Parechovirus; Picornaviridae) reveals a fourth genotype and indicates ancestral recombination. J. Gen. Virol. 2009, 90, 843–853. [Google Scholar] [CrossRef]
- Pounder, K.C.; Watts, P.C.; Niklasson, B.; Kallio, E.R.K.; Marston, D.A.; Fooks, A.R.; Begon, M.; McElhinney, L.M. Genome characterisation of two Ljungan virus isolates from wild bank voles (Myodes glareolus) in Sweden. Infect. Genet. Evol. 2015, 36, 156–164. [Google Scholar] [CrossRef]
- Johansson, E.S.; Niklasson, B.; Tesh, R.B.; Shafren, D.R.; Travassos da Rosa, A.P.A.; Lindberg, A.M. Molecular characterization of M1146, an American isolate of Ljungan virus (LV) reveals the presence of a new LV genotype. J. Gen. Virol. 2003, 84, 837–844. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, L.; Du, J.; Yang, L.; Ren, X.; Liu, B.; Jiang, J.; Yang, J.; Dong, J.; Sun, L.; et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 2018, 6, 178. [Google Scholar] [CrossRef]
- Niklasson, B.; Heller, K.E.; Schønecker, B.; Bildsøe, M.; Daniels, T.; Hampe, C.S.; Widlund, P.; Simonson, W.T.; Schaefer, J.B.; Rutledge, E.; et al. Development of type 1 diabetes in wild bank voles associated with islet autoantibodies and the novel ljungan virus. Int. J. Exp. Diabesity Res. 2003, 4, 35–44. [Google Scholar] [CrossRef]
- Niklasson, B.; Nyholm, E.; Feinstein, R.E.; Samsioe, A.; Hörnfeldt, B. Diabetes and myocarditis in voles and lemmings at cyclic peak densities—induced by Ljungan virus? Oecologia 2006, 150, 1–7. [Google Scholar] [CrossRef]
- Hauffe, H.C.; Fevola, C.; Rossi, C.; Rizzoli, A.; Niemimaa, J.; Henttonen, H. Is rodent-borne Ljungan virus responsible for mortality in migrating Norwegian lemmings (Lemmus lemmus)? In INTERACT: International Network for Terrestrial Research and Monitoring in the Arctic; Tories of Arctic Science; Callaghan, T.V., Savela, H., Eds.; DCE Danish Centre for Environment and Energy, Aarhus University: Aarhus, Denmark, 2015; pp. 120–121. [Google Scholar]
- Diven, D.G. An overview of poxviruses. J. Am. Acad. Dermatol. 2001, 44, 1–16. [Google Scholar] [CrossRef]
- Werden, S.J.; Rahman, M.M.; McFadden, G. Poxvirus host range genes. Adv. Virus Res. 2008, 71, 135–171. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Jones, S. Zoonotic poxvirus infections in humans. Curr. Opin. Infect. Dis. 2004, 17, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tack, D.M.; Reynolds, M.G. Zoonotic Poxviruses Associated with Companion Animals. Animals 2011, 1, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G.; Henderson, D.A. Diagnosis and management of smallpox. N. Engl. J. Med. 2002, 346, 1300–1308. [Google Scholar] [CrossRef]
- von Magnus, P.; Andersen, E.A.; Petersen, K.B.; Birch-Andersen, A. A pox-like disease in cynomolgus monkeys. Acta Path. Microbiol. Scand. 1959, 46–159. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Parvin, R.; Hossain, I.; Hasan, A.; Afrin, S.Z.; Shehata, A.A. Influenza and coronavirus zoonoses: An overview on pandemic events, viral genome, replication and emergency preparedness. Ger. J. Microbiol. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- WHO Monkeypox. 2022. Available online: https://www.who.int/news-room/questions-and-answers/item/monkeypox (accessed on 14 May 2025).
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- WHO Monkeypox: Public Health Advice for gay, Bisexual and Other Men Who Have Sex with Men. 2022. Available online: https://www.who.int/news/item/25-05-2022-monkeypox--public-health-advice-for-gay--bisexual-and-other-men-who-have-sex-with-men (accessed on 14 May 2025).
- Hutson, C.L.; Gallardo-Romero, N.; Carroll, D.S.; Clemmons, C.; Salzer, J.S.; Nagy, T.; Hughes, C.M.; Olson, V.A.; Karem, K.L.; Damon, I.K. Transmissibility of the Monkeypox Virus Clades via Respiratory Transmission: Investigation Using the Prairie Dog-Monkeypox Virus Challenge System. PLoS ONE 2013, 8, e55488. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J.; et al. Exportation of Monkeypox Virus From the African Continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Mauldin, M.; Emerson, G.; Reynolds, M.; Lash, R.; Gao, J.; Zhao, H.; Li, Y.; Muyembe, J.-J.; Kingebeni, P.; et al. A Phylogeographic Investigation of African Monkeypox. Viruses 2015, 7, 2168–2184. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Sklenovská, N.; Van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef]
- Petersen, E.; Abubakar, I.; Ihekweazu, C.; Heymann, D.; Ntoumi, F.; Blumberg, L.; Asogun, D.; Mukonka, V.; Lule, S.A.; Bates, M.; et al. Monkeypox—Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int. J. Infect. Dis. 2019, 78, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sadeuh-Mba, S.A.; Yonga, M.G.; Els, M.; Batejat, C.; Eyangoh, S.; Caro, V.; Etoundi, A.; Carniel, E.; Njouom, R. Monkeypox virus phylogenetic similarities between a human case detected in Cameroon in 2018 and the 2017-2018 outbreak in Nigeria. Infect. Genet. Evol. 2019, 69, 8–11. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Parker, S.; Nuara, A.; Buller, R.M.L.; Schultz, D.A. Human monkeypox: An emerging zoonotic disease. Future Microbiol. 2007, 2, 17–34. [Google Scholar] [CrossRef]
- Eder, I.; Vollmar, P.; Pfeffer, M.; Naether, P.; Rodloff, A.C.; Meyer, H. Two Distinct Clinical Courses of Human Cowpox, Germany, 2015. Viruses 2017, 9, 375. [Google Scholar] [CrossRef]
- Heer, R.S.; Porter, B.; Jones, N. Necrotic facial ulceration caused by cowpox virus. J. Med. Virol. 2023, 95, e28372. [Google Scholar] [CrossRef]
- Eis-Hübinger, A.M.; Gerritzen, A.; Schneweis, K.E.; Pfeiff, B.; Pullmann, H.; Mayr, A.; Czerny, C.P. Fatal cowpox-like virus infection transmitted by cat. Lancet 1990, 336, 880. [Google Scholar] [CrossRef]
- Becker, C.; Kurth, A.; Hessler, F.; Kramp, H.; Gokel, M.; Hoffmann, R.; Kuczka, A.; Nitsche, A. Cowpox Virus Infection in Pet Rat Owners. Dtsch. Ärztebl. Int. 2009, 106, 329. [Google Scholar] [CrossRef] [PubMed]
- Jeske, K.; Weber, S.; Pfaff, F.; Imholt, C.; Jacob, J.; Beer, M.; Ulrich, R.G.; Hoffmann, D. Molecular Detection and Characterization of the First Cowpox Virus Isolate Derived from a Bank Vole. Viruses 2019, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Campe, H.; Zimmermann, P.; Glos, K.; Bayer, M.; Bergemann, H.; Dreweck, C.; Graf, P.; Weber, B.K.; Meyer, H.; Büttner, M.; et al. Cowpox virus transmission from pet rats to humans, Germany. Emerg. Infect. Dis. 2009, 15, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Sárdy, M.; Glos, K.; Korting, H.C.; Ruzicka, T.; Wollenberg, A. The Munich outbreak of cutaneous cowpox infection: Transmission by infected pet rats. Acta Derm. Venereol. 2012, 92, 126–131. [Google Scholar] [CrossRef]
- Baxby, D.; Bennett, M.; Getty, B. Human cowpox 1969–93: A review based on 54 cases. Br. J. Dermatol. 1994, 131, 598–607. [Google Scholar] [CrossRef]
- Wolfs, T.F.W.; Wagenaar, J.A.; Niesters, H.G.M.; Osterhaus, A.D.M.E. Rat-to-human transmission of Cowpox infection. Emerg. Infect. Dis. 2002, 8, 1495–1496. [Google Scholar] [CrossRef]
- Kurth, A.; Wibbelt, G.; Gerber, H.-P.; Petschaelis, A.; Pauli, G.; Nitsche, A. Rat-to-elephant-to-human transmission of cowpox virus. Emerg. Infect. Dis. 2008, 14, 670–671. [Google Scholar] [CrossRef]
- Obermeier, P.E.; Buder, S.C.; Hillen, U. Poxvirus infections in dermatology—the neglected, the notable, and the notorious. J. Dtsch. Dermatol. Ges. 2024, 22, 56–93. [Google Scholar] [CrossRef]
- Bonnekoh, B.; Falk, K.; Reckling, K.; Kenklies, S.; Nitsche, A.; Ghebremedhin, B.; Pokrywka, A.; Franke, I.; Thriene, B.; König, W.; et al. Cowpox infection transmitted from a domestic cat. J. Dtsch. Dermatol. Ges. 2008, 6, 210–213. [Google Scholar] [CrossRef]
- Brown, P.A.; Touzain, F.; Briand, F.X.; Gouilh, A.M.; Courtillon, C.; Allée, C.; Lemaitre, E.; De Boisséson, C.; Blanchard, Y.; Eterradossi, N. First complete genome sequence of European turkey coronavirus suggests complex recombination history related with US turkey and guinea fowl coronaviruses. J. Gen. Virol. 2016, 97, 110–120. [Google Scholar] [CrossRef]
- ICTV International Committee on Taxonomy of Viruses. Master Species List 2019 V1; ICTV International Committee on Taxonomy of Viruses: Moscow, Russia, 2020. [Google Scholar]
- Chen, G.-Q.; Zhuang, Q.-Y.; Wang, K.-C.; Liu, S.; Shao, J.-Z.; Jiang, W.-M.; Hou, G.-Y.; Li, J.-P.; Yu, J.-M.; Li, Y.-P.; et al. Identification and Survey of a Novel Avian Coronavirus in Ducks. PLoS ONE 2013, 8, e72918. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.J.; Cook, J.K.A. Spotlight on avian coronaviruses. Avian Pathol. 2020, 49, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-Y.; Yang, W.-H.; Zhou, J.-H.; Li, B.; Zhang, W.; Shi, Z.-L.; Zhang, Y.-Z. Detection of alpha-and betacoronaviruses in rodents from Yunnan, China. Virol. J. 2017, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Santos-Medellín, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought Stress Results in a Compartment-Specific Restructuring of the Rice Root-Associated Microbiomes. mBio 2017, 8, e00764-17. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wang, Y.; Lenoch, J.; Kohler, D.; DeLiberto, T.J.; Tang, C.Y.; Li, T.; Tao, Y.J.; Guan, M.; Compton, S.; Zeiss, C.; et al. SARS-CoV-2 Exposure in Norway Rats (Rattus norvegicus) from New York City. mBio 2023, 14, e0362122. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Root, J.J.; Porter, S.M.; Walker, A.E.; Guilbert, L.; Hawvermale, D.; Pepper, A.; Maison, R.M.; Hartwig, A.E.; Gordy, P.; et al. Peridomestic Mammal Susceptibility to Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Emerg. Infect. Dis. 2021, 27, 2073–2080. [Google Scholar] [CrossRef]
- Montagutelli, X.; Prot, M.; Levillayer, L.; Salazar, E.B.; Jouvion, G.; Conquet, L.; Beretta, M.; Donati, F.; Albert, M.; Gambaro, F.; et al. Variants with the N501Y mutation extend SARS-CoV-2 host range to mice, with contact transmission. arXiv 2021. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Vladimirova, D.; Dietert, K.; Abdelgawad, A.; Gruber, A.D.; Osterrieder, N.; Trimpert, J. SARS-CoV-2 infection of Chinese hamsters (Cricetulus griseus) reproduces COVID-19 pneumonia in a well-established small animal model. Transbound. Emerg. Dis. 2021, 68, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.W.; Siu, G.K.H.; Yuan, S.; Ip, J.D.; Cai, J.P.; Chu, A.W.H.; Chan, W.M.; Abdullah, S.M.U.; Luo, C.; Chan, B.P.C.; et al. Probable Animal-to-Human Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Delta Variant AY.127 Causing a Pet Shop-Related Coronavirus Disease 2019 (COVID-19) Outbreak in Hong Kong. Clin. Infect. Dis. 2022, 75, e76–e81. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Zhan, S.; Vilander, A.C.; Fagre, A.C.; Kiaris, H.; Schountz, T. SARS-CoV-2 infects multiple species of North American deer mice and causes clinical disease in the California mouse. bioRxiv 2022, 23:2022.08.22.504888. [Google Scholar] [CrossRef]
- Fagre, A.; Lewis, J.; Eckley, M.; Zhan, S.; Rocha, S.M.; Sexton, N.R.; Burke, B.; Geiss, B.; Peersen, O.; Bass, T.; et al. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLoS Pathog. 2021, 17, e1009585. [Google Scholar] [CrossRef]
- Griffin, B.D.; Chan, M.; Tailor, N.; Mendoza, E.J.; Leung, A.; Warner, B.M.; Duggan, A.T.; Moffat, E.; He, S.; Garnett, L.; et al. SARS-CoV-2 infection and transmission in the North American deer mouse. Nat. Commun. 2021, 12, 3612. [Google Scholar] [CrossRef]
- Wernike, K.; Drewes, S.; Mehl, C.; Hesse, C.; Imholt, C.; Jacob, J.; Ulrich, R.G.; Beer, M. No Evidence for the Presence of SARS-CoV-2 in Bank Voles and Other Rodents in Germany, 2020–2022. Pathogens 2022, 11, 1112. [Google Scholar] [CrossRef]
- Nazari, N.; Shojaee, S.; Mohebali, M.; Teimouri, A.; Ghadiri, K.; Raeghi, S.; Shiee, M.R.; Azarakhsh, Y.; Bozorgomid, A. Toxoplasma gondii And Neospora caninum In Brain Tissue Of Rodents In North-West Iran. Vet. Med. Res. Rep. 2019, 10, 223–227. [Google Scholar] [CrossRef]
- Esson, C.; Samelius, G.; Strand, T.M.; Lundkvist, Å.; Michaux, J.R.; Råsbäck, T.; Wahab, T.; Mijiddorj, T.N.; Berger, L.; Skerratt, L.F.; et al. The prevalence of rodent-borne zoonotic pathogens in the South Gobi desert region of Mongolia. Infect. Ecol. Epidemiol. 2023, 13, 2270258. [Google Scholar] [CrossRef]
- Sánchez-Soto, M.F.; Gaona, O.; Vigueras-Galván, A.L.; Suzán, G.; Falcón, L.I.; Vázquez-Domínguez, E. Prevalence and transmission of the most relevant zoonotic and vector-borne pathogens in the Yucatan peninsula: A review. PLoS Negl. Trop. Dis. 2024, 18, e0012286. [Google Scholar] [CrossRef]
- Islam, M.M.; Farag, E.; Hassan, M.M.; Jaffrey, S.S.; Atta, M.; Al-Marri, A.M.; Al-Zeyara, A.M.; Al Romaihi, H.; Bansal, D.; Mkhize-Kwitshana, Z.L. Rodent-borne zoonoses in Qatar: A possible One-Health framework for the intervention of future epidemic. One Health 2023, 16, 100517. [Google Scholar] [CrossRef]
- Mistrick, J.; Kipp, E.J.; Weinberg, S.I.; Adams, C.C.; Larsen, P.A.; Craft, M.E. Microbiome diversity and zoonotic bacterial pathogen prevalence in Peromyscus mice from agricultural landscapes and synanthropic habitat. Mol. Ecol. 2024, 33, e17309. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Robb, G.N.; Alagaili, A.N.; Hastriter, M.W.; Apanaskevich, D.A.; Ueckermann, E.A.; Bennett, N.C. Ectoparasite fauna of rodents collected from two wildlife research centres in Saudi Arabia with discussion on the implications for disease transmission. Acta Trop. 2015, 147, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bron, G.M.; Malavé, C.M.; Boulerice, J.T.; Osorio, J.E.; Rocke, T.E. Plague-Positive Mouse Fleas on Mice Before Plague Induced Die-Offs in Black-Tailed and White-Tailed Prairie Dogs. Vector Borne Zoonotic Dis. 2019, 19, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Mojahed, N.; Mohammadkhani, M.A.; Mohamadkhani, A. Climate Crises and Developing Vector-Borne Diseases: A Narrative Review. Iran. J. Public Health 2022, 51, 2664–2673. [Google Scholar] [CrossRef]
- Patwary, M.M.; Rodriguez-Morales, A.J. Deadly Flood and Landslides amid COVID-19 Crisis: A Public Health Concern for the World’s Largest Refugee Camp in Bangladesh. Prehosp. Disaster Med. 2022, 37, 292–293. [Google Scholar] [CrossRef]
- Acosta-España, J.D.; Romero-Alvarez, D.; Luna, C.; Rodriguez-Morales, A.J. Infectious disease outbreaks in the wake of natural flood disasters: Global patterns and local implications. Infez. Med. 2024, 32, 451–462. [Google Scholar] [CrossRef]
- Sutiningsih, D.; Sari, D.P.; Permatasari, C.D.; Azzahra, N.A.; Rodriguez-Morales, A.J.; Yuliawati, S.; Maharani, N.E. Geospatial Analysis of Abiotic and Biotic Conditions Associated with Leptospirosis in the Klaten Regency, Central Java, Indonesia. Trop. Med. Infect. Dis. 2024, 9, 225. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Suárez, J.A.; Franco-Paredes, C.; Vilcarromero, S.; Mattar, S.; Gómez-Marín, J.E.; Villamil-Gómez, W.E.; Ruíz-Sáenz, J.; Cardona-Ospina, J.A.; Idarraga-Bedoya, S.E.; et al. Brazil burning! What is the potential impact of the Amazon wildfires on vector-borne and zoonotic emerging diseases?—A statement from an international experts meeting. Travel Med. Infect. Dis. 2019, 31, 101474. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Motahari, H.; Taghizadeh Khamesi, M.; Sharifi, A.; Campos, M.; Schraufnagel, D.E. Climate Change and Respiratory Infections. Ann. Am. Thorac. Soc. 2016, 13, 1223–1230. [Google Scholar] [CrossRef]
- Dąbrowska, J.; Menéndez Orellana, A.E.; Kilian, W.; Moryl, A.; Cielecka, N.; Michałowska, K.; Policht-Latawiec, A.; Michalski, A.; Bednarek, A.; Włóka, A. Between flood and drought: How cities are facing water surplus and scarcity. J. Environ. Manag. 2023, 345, 118557. [Google Scholar] [CrossRef]
- Kiefer, E.M.; Felton, D. A Review of Climate-Driven Threats to Recreational Water Users in Hawaii. Wilderness Environ. Med. 2025, 36, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, M.P.; Marín Montesinos, J.C.; Corduneanu, A.; Obregón, D.; Moutailler, S.; Cabezas-Cruz, A. Tick-borne viruses and their risk to public health in the Caribbean: Spotlight on bats as reservoirs in Cuba. Heliyon 2024, 10, e26118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-R.; Liu, T.; Gao, X.; Wang, H.-B.; Xiao, J.-H. Impact of climate change on the global circulation of West Nile virus and adaptation responses: A scoping review. Infect. Dis. Poverty 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]


| Disease | Virus | Reservoir Species |
|---|---|---|
| Hantavirus Cardiopulmonary Syndrome | Amur/Soochong | Apodemus peninsulae |
| Dobrava | Apodemus flavicollis | |
| Hantaan | Apodemus agrarius | |
| Puumala | Myodes H | |
| Luxi | Eothenomys miletus | |
| Saaremaa | Apodemus agrarius | |
| Seoul | Rattus norvegicus and Rattus rattus, | |
| Tula | Microtus arvalis | |
| Hemorrhagic Fever with Renal Syndrome | Anajatuba | Oligoryzomys fornesi |
| Araucaria | Oligoryzomys nigripes, Oxymycterus judex, Akodon montensis | |
| Araraquara | Bolomys lasiurus | |
| Bayou | Oryzomys palustris | |
| Bermejo | Bolomys lasiurus | |
| Black Creek Canal | Sigmodon hispidus | |
| Castelo dos sonhos | Oligoryzomys eliurus | |
| Choclo | Oligoryzomys fulvescens | |
| Itapua | Oligoryzomys nigripes | |
| Juquitiba | Oligoryzomys nigripes | |
| Laguna Negra | Calomys laucha | |
| Lechiguanas | Oligoryzomys flavescens | |
| Maporal | Oligoryzomys delicatus | |
| Monongahela | Peromyscus leucopus | |
| Neembucu | Oligoryzomys chacoensis | |
| New York | Peromyscus leucopus | |
| Oran | Oligoryzomys longicaudatus | |
| Paranoa | Not known | |
| Rio Mamore | Oligoryzomys microtis | |
| Sin Nombre | Peromyscus maniculatus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, A.A.; Parvin, R.; Tasnim, S.; Duarte, P.M.; Rodriguez-Morales, A.J.; Basiouni, S. The Hidden Threat: Rodent-Borne Viruses and Their Impact on Public Health. Viruses 2025, 17, 809. https://doi.org/10.3390/v17060809
Shehata AA, Parvin R, Tasnim S, Duarte PM, Rodriguez-Morales AJ, Basiouni S. The Hidden Threat: Rodent-Borne Viruses and Their Impact on Public Health. Viruses. 2025; 17(6):809. https://doi.org/10.3390/v17060809
Chicago/Turabian StyleShehata, Awad A., Rokshana Parvin, Shadia Tasnim, Phelipe Magalhães Duarte, Alfonso J. Rodriguez-Morales, and Shereen Basiouni. 2025. "The Hidden Threat: Rodent-Borne Viruses and Their Impact on Public Health" Viruses 17, no. 6: 809. https://doi.org/10.3390/v17060809
APA StyleShehata, A. A., Parvin, R., Tasnim, S., Duarte, P. M., Rodriguez-Morales, A. J., & Basiouni, S. (2025). The Hidden Threat: Rodent-Borne Viruses and Their Impact on Public Health. Viruses, 17(6), 809. https://doi.org/10.3390/v17060809









