Abstract
This descriptive, observational, cross-sectional study evaluated HTLV-1 and HTLV-2 infections in Ananindeua, northern Brazil. Individuals were screened for anti-HTLV-1/2 using ELISA (Murex HTLV-I + II, DiaSorin). Reactive or indeterminate samples underwent confirmation via Western blot (HTLV Blot 2.4 kit, MP Diagnostics) and/or RT-qPCR. A questionnaire examined behavioral and risk factors for HTLV-1/2 infection. HTLV-positive individuals received counseling, nurse follow-up, and specialized medical care. Among the 228 individuals investigated, 6 (2.7%) were infected with HTLV-1: 4 men (66.67%) and 2 women (33.33%), aged 51–73 years. The only significant risk factor observed was blood transfusion. Additionally, 80 other individuals residing in the municipality of Ananindeua independently visited the laboratory for an HTLV-1/2 diagnosis. Among them, 23 were diagnosed with HTLV-1 infection, and 1 with HTLV-2. Among the 30 positive individuals, 80% were asymptomatic, while 20% exhibited clinical manifestations associated with HTLV infection, including HAM and Sézary syndrome. These results indicate a notable prevalence of HTLV-1 infection in the municipality of Ananindeua emphasizing the significance of diagnosing the infection to assess its prevalence across the country accurately.
1. Introduction
Human T-lymphotropic viruses 1 and 2 (HTLV-1 and HTLV-2) were described as the first human oncogenic retroviruses, isolated in the 1980s from patients with cutaneous T-cell lymphoma and a T-cell variant of hairy cell leukemia, respectively [1,2]. The viruses present tropism for CD4+ and CD8+ T cells and are currently associated with adult T-cell leukemia/lymphoma (ATLL) and inflammatory diseases like HTLV-1-associated myelopathy (HAM), uveitis, infective dermatitis, arthritis, and other illnesses [3,4,5,6,7,8,9]. HTLV-1/2 are transmitted by direct contact with contaminated bodily fluids, and the main transmission routes are blood transfusion, sharing of contaminated syringes and needles, sexual intercourse without condoms, and mother-to-child transmission during pregnancy, childbirth, and, mainly, breastfeeding [5,10,11,12,13,14].
HTLV-1/2 are neglected and endemic infections present in different regions of the world, with the highest prevalences observed in Southern Japan, the Americas, Africa, and the Caribbean. The number of infected people worldwide is underestimated, but the global estimates range from 5 million to 10 million people living with HTLV-1/2 (PLHTLV) worldwide [5,14,15].
The origin of HTLV occurred through human interaction with simian T-lymphotropic viruses 1 and 2 (STLV-1/2), and the viruses spread throughout the world approximately 100,000 years ago due to human migration from Africa to other continents, reaching the Americas through the Bering Strait [16,17,18].
The introduction of HTLV-1 in Brazil was probably due to the human migration of African people during the slave trade, and HTLV-2 by the ancient human migration from Asia [18]. Brazil is considered one of the endemic countries for HTLV-1/2 infection, with an estimated 800,000 to 2.5 million people infected [19]. Additionally, epidemiological data demonstrated a high prevalence rate of infected people in some states of the northern and northeastern regions of Brazil, such as Bahia, Maranhão, and Pará [13,20,21].
The State of Pará is one of the federative units of Brazil with the highest prevalence of HTLV-1/2 infection, with the viruses disseminated among several population groups, such as quilombolas, pregnant women, blood donors, and indigenous people [18,20,21,22,23,24]. It is important to highlight that studies conducted in the urban region describe the circulation of both HTLV-1 and HTLV-2 in the state capital, Belém [25,26,27]. Nevertheless, there are several groups without any information on their epidemiological statuses regarding the presence of HTLV-1/2 in this region.
This study aimed to evaluate the serological and molecular evidence of HTLV-1 and HTLV-2 infections among residents of Ananindeua, Pará. This city is part of the metropolitan area of Belém and is the second most populous municipality in the state, playing a significant role due to its economy and geographic location.
2. Materials and Methods
2.1. Study Design and Population
This is a descriptive, observational, cross-sectional study that occurred from 2020 to 2024. The first approach occurred from 2020 to 2021, aiming to evaluate the prevalence of HTLV-1/2 infection in residents from Ananindeua. Serological screening and confirmatory tests were performed through an active search for people infected with HTLV-1/2, and the participants were selected through campaigns in community centers, churches, and public places. During this period, a total of 228 randomly selected individuals from various age groups and both genders were tested for HTLV-1/2 infection.
From 2022 onwards, with the consolidation of the Service for Assistance to People Living with HTLV (SAPEVH), and considering the number of seropositive individuals in the first sampling, a second effort was launched to increase the number of people investigated among the population of Ananindeua, selecting residents of that municipality who spontaneously sought the SAPEVH in the period between 2022 and 2024, seeking a diagnosis for HTLV-1/2 infection.
All participants were informed about the objective, risks, and benefits of this study and gave their consent by signing Informed Consent forms. Individuals of both sexes were randomly selected to join the present study by spontaneous demand and were submitted to a questionnaire investigating behavioral and risk factors for HTLV-1/2 infection. The SAPEVH aimed to provide diagnosis for the general public, and a follow-up for those who were seropositive for HTLV-1/2, including serological screening, confirmatory tests, and, for seropositive patients, counseling and follow-up with a nurse and specialized medical assistance. From 2022 to 2024, a total of 80 people were assisted by this service.
This study was approved by the Human Research Ethics Committee of the Health Sciences Institute of the Federal University of Pará and the National Research Ethics Committee (CONEP), in compliance with fundamental ethical and scientific requirements, respecting Resolution N° 466/12 of the Ministry of Health, which regulates research involving human beings under number CAAE: 27290619.2.0000.0018 and opinion 4.351.470.
2.2. Serological Tests
A peripheral venous blood sample (4.5 mL) was obtained and screened for anti-HTLV-1/2 by ELISA (Murex HTLV-I + II, DiaSorin, Dartford, UK) according to the manufacturer’s instructions. Samples considered reactive or indeterminate were submitted to confirmatory tests using Western blot (HTLV Blot 2.4 kit, MP Diagnostics, Singapore, Republic of Singapore) and/or Real-Time Polymerase Chain Reaction (RT-qPCR).
2.3. DNA Extraction
Seropositive samples were submitted to DNA extraction for the detection of HTLV proviral DNA. DNA was extracted from 200 µL of leukocytes using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol.
2.4. Real-Time PCR
The molecular analysis of HTLV infection was conducted via qPCR using the TaqMan system (Applied Biosystems, Foster City, CA, USA) on the Applied Biosystems StepOne Plus Real-Time PCR platform. The albumin gene (141 bp) was used as endogenous control, and the non-homologous viral pol gene regions (186 bp) of HTLV-1 and HTLV-2 were used as molecular markers to identify viral particles [28]. Each reaction contained 12.5 µL of TaqMan Universal PCR Master Mix (2X) (Applied Biosystems, Foster City, CA, USA), 6.0 µL of ultrapure water, 0.5 µL of each primer, 0.5 µL of each probe, and 5.0 µL of DNA, resulting in a total volume of 25 µL. The following temperature cycles were carried out: 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s, and then 60 °C for binding primers and probes for 1 min. The following primers were used in those reactions: 5′-ccctacaatccaaccagctcag-3′ (HTLV-1F), 5′-gtggtgaagctgccatcgggtttt-3′ (HTLV-1R), 5′-cgattgtgtacaggccgattg-3′ (HTLV-2F), 5′-caggagggcatgtcgatgtag-3′ (HTLV-2R), 5′-gctgtcatctcttgtgggctgt-3′ (AlbuminF), and 5′-aaactcatgggagctgctggtt-3′ (Albumin R). The probe sequences were as follows: FAM-5′-ctttactgacaaacccgacctacccatgga-3′-MGB (HTLV-1), FAM-5′-tgtcccgtctcaggtggtctatgttcca-3′-MGB (HTLV-2), and FAM-5’-cctgtcatgcccacacaaatctc-3′-MGB (Albumin).
2.5. Statistical Analysis
Data obtained from questionnaire responses, including population characteristics, risk factors, and demographic data, were tabulated in Microsoft Office Excel and then analyzed and described using descriptive statistical analysis. The risk factors associated with HTLV-1/2 infection were analyzed by Pearson’s chi-square test and G test, considering a level of 5% (p-value < 0.05). Data analysis was performed by the software BioEstat version 5.0 [29].
3. Results
3.1. Serological Screening, Molecular Analyses, and Prevalence
In the first approach of the present study, 228 individuals were enrolled in the investigation, of whom 39.47% were males and 60.53% were females. The screening serology test showed six individuals seropositive for HTLV-1 (2.7%), who were then submitted for molecular confirmation by qPCR. All samples were confirmed for HTLV-1 infection, of which four were males (66%) and two were females (33%), with ages ranging from 51 to 73 years old (Table 1).

Table 1.
General characteristics of the study population.
The epidemiological characteristics of the 228 individuals indicated that 55.70% self-identified as mixed race, 20.18% reported having a secondary education level, and 29.82% declared having an income of one to two minimum wages. There was no significant difference between the infected and non-infected groups.
Most HTLV-1 patients received a family income higher than the minimum wage, 16.67% reported a family income less than or equal to the minimum wage, 50% reported living with three to four minimum wages, and 16.67% declared receiving more than five minimum wages. Regarding the educational situation, there was a predominance of a high school educational level: 33.33% declared to have completed elementary school, 50% finished high school, and 16.67% reported an unfinished university degree (Table 1).
Between 2022 and 2024, a total of 80 individuals living in the municipality of Ananindeua voluntarily sought the SAPEVH for a diagnosis of HTLV-1/2 infection, comprising 25 males and 55 females. Their ages ranged from 13 to 84 years old. Twenty-four subjects tested positive for HTLV-1/2 in the screening, and 20 were confirmed by RT-qPCR. Of the four remaining samples, one showed amplification of proviral DNA for the HTLV-2 pol-II region and three were undetectable by RT-qPCR. However, when subjected to Western blot as an alternative for diagnosis, the three samples showed reactivity for HTLV-1. Of the 24 HTLV-1/2-positive patients who attended the SAPEVH in the period from 2022 to 2024, 14 were female (58.33%) and 10 were male (41.67%), and their ages ranged from 19 to 72 years old.
3.2. Clinical Findings
After the confirmatory laboratory diagnosis, patients were evaluated by a multidisciplinary health team in search of clinical manifestations of diseases associated with HTLV-1/2. Of the 30 positive individuals, 80% were asymptomatic, and 20% presented clinical manifestations associated with HTLV infection. Patients #7, #8, and #22 presented systemic lupus erythematosus, fibromyalgia, and motor deficit, respectively. Patients #13 and #20 were clinically diagnosed with HAM, while patient #23 presented Sézary syndrome (Table 2).

Table 2.
Characteristics of individuals residing in Ananindeua who spontaneously sought diagnostic services through the SAPEVH.
3.3. Epidemiological Characteristics and Risk Factors Associated with HTLV-1/2 Infection
The most significant risk factor associated with HTLV-1/2 infection was blood transfusion (p = 0.0028; odds ratio = 4.18; IC95%: 1.73–10.11; p < 0.05), but it was not related to having received a transfusion before 1993 (p = 0.3431), when mandatory testing for HTLV-1/2 was implemented in Brazilian Blood Centers. However, other important risk factors were also reported by infected individuals (without statistical significance), such as unprotected sexual intercourse, breastfeeding during childhood, the presence of tattoos or piercings, the use of illicit drugs, having sex for money, and a previous diagnosis of another STI (Table 3).

Table 3.
Risk factors associated with HTLV infection.
During the course of the investigation, it was possible to pinpoint other family members who were included in the present study for screening and confirmation. Family #1 was identified with eight members that tested positive for HTLV-1; the index case was a man (37 y). Family clusters were detected among four other families (#2, #3, #4, and #5) with the occurrence of infection among couples, suggesting that sexual intercourse was the main route of transmission. Unfortunately, it was not possible to detect infection among the children of these couples. (Figure 1). Furthermore, intrafamilial transmission could not be investigated for the only individual infected with HTLV-2.
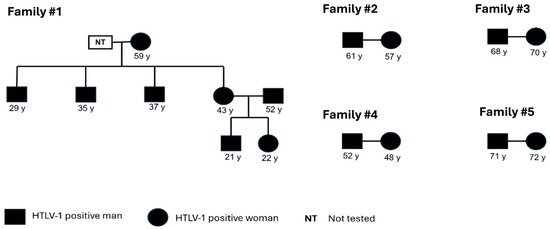
Figure 1.
Pedigree showing possible intrafamilial transmission routes. All families present horizontal transmission, and Family #1 presents both vertical and horizontal transmission.
4. Discussion
The present study reported a prevalence of 2.7% HTLV-1 infection in the urban population of Ananindeua, Pará, Brazil. As far as we know, despite the small sample size studied, this is the highest prevalence of HTLV-1 infection in an urban population in Brazil, surpassing the figure (1.18%) observed in Salvador [30].
This study represents the first epidemiological investigation of HTLV infection in the population residing in the municipality of Ananindeua. Silva et al. [31] reported the prevalence of HTLV infection, as high as 2%, in the metropolitan region of the capital city, Belém, but it is important to mention that the sample included individuals residing in Ananindeua. In contrast, in our previous study, conducted only with the population of Belém [25], the prevalence found was only 0.38%. Differences in the study population, despite geographical proximity and a shared economic context, may account for the disparity in prevalence rates.
The Ananindeua municipality originated from the quilombo community of Abacatal, which was initially formed by escaped African slaves. This community dates back to the 18th century and played a significant role in the formation of Ananindeua [32,33]. During the colonial period, the forced human migration was clearly increased from several parts of the African continent, and people were brought to Brazil as slaves, particularly to important ports, including Rio de Janeiro, Salvador, Recife, São Luiz, and Belem [18,34]. The high prevalence of HTLV-1 in Ananindeua may likely be related to the African origin of the virus and its introduction to Brazil during the slave trade in the colonial period.
Of the 30 individuals infected with HTLV-1/2 analyzed in this study, the majority were females, a finding that agrees with previous reports, in which women were found to be more affected by HTLV-1/2 infection than men [24,35,36,37,38,39]. The questionnaires revealed that females over 50 years old were the most affected group in this study. These findings are consistent with previous studies, which identified a correlation between age and the increase in prevalence rates for HTLV infection [14,40]. The association between age and sex may be due to the increased likelihood of acquiring the virus over time, as well as the fact that the virus is more efficiently transmitted from men to women. Kaplan et al. [41] hypothesized that the virus is more efficiently transmitted in a male-to-female manner due to the higher viral load present in men’s semen. Other studies have suggested that this difference in transmission could be related to the higher number of lymphocytes found in semen compared to vaginal secretions [42].
The main risk factors associated with seropositivity to HTLV-1/2, included the practice of unprotected sex, breastfeeding, and low income, although those were not considered statistically significant. Nevertheless, the presence of substantial evidence indicating intrafamilial transmission suggests that unprotected sexual activity may play a significant role in this mode of transmission. The sexual transmission of HTLV represents an important risk factor for the spread of the virus. There is substantial evidence showing the importance of this epidemiological transmission within close communities such as Indigenous people in Brazil [18,24,43,44], as well as among urban communities in the Amazon region of Brazil [45]. A study conducted in Salvador, Brazil, has shown that the sexual transmission route remains the most important mechanism of transmission, showing the increase in the prevalence in older age groups of infected people, which supports the idea of the acquisition of the virus over time [46].
In the case of family #1, we hypothesized that breastfeeding was the main transmission route, as the mother reported breastfeeding for 6 months or more. Long-term breastfeeding and the high proviral load present in breast milk are known risk factors for HTLV transmission [47]. It is recommended that HTLV-1/2-positive mothers refrain from breastfeeding, as there is evidence showing that the duration of breastfeeding (up to 6 months) is associated with an increased risk of transmission [13,48]. However, this recommendation does not fully reflect the situation in developing countries, where breast milk often serves as the primary source of nutrition for infants during their first 2 years. In this context, poverty and the lack of public health policies underscore the need for further studies and the development of effective strategies to prevent HTLV transmission from mother to child through breastfeeding [49].
Our results showed, for the first time, a high prevalence of HTLV-1 infection in Ananindeua municipality, which, to our knowledge, is the highest-ever reported prevalence for an urban population in Brazil. These findings highlight the urgent need for ongoing epidemiological surveillance in Ananindeua and other regions of Brazil with a strong mixture of African descendants where HTLV-1/2 prevalence has not been investigated. They also emphasize the importance of promoting services that offer diagnostic and confirmatory tests for individuals living with HTLV-1/2 to gain a clearer understanding of the true prevalence across the country.
Author Contributions
A.C.R.V., I.M.V.C.V. and R.I. conceived and designed the present study. F.T.L., G.d.S.P.N., L.C.C.P.S., A.C.R.L., B.S.B., I.N.A. and F.B.F. carried out the laboratory experiments. D.d.C.S. and G.d.S.P.N. performed the statistical analyses. D.d.C.S. and A.C.R.V. wrote the draft of the manuscript. R.I. and A.C.R.V. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from the National Council for Scientific and Technological Development (CNPq) (Process No. 442522/2019-3; 302935/2021-5, and 402412/2021-4), Instituto Nacional de Ciência e Tecnologia em Viroses Emergentes e Reemergentes—INCT-VER (#406360/2022-7).
Institutional Review Board Statement
This study was approved by the Human Research Ethics Committee of the Health Sciences Institute of the Federal University of Pará and the National Research Ethics Committee (CONEP) and was conducted in compliance with fundamental ethical and scientific requirements, respecting Resolution N° 466/12 of the Ministry of Health, which regulates research involving human beings under number CAAE: 27290619.2.0000.0018 and opinion 4.351.470.
Informed Consent Statement
Informed Consent was obtained from all subjects involved in this study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank the Federal University of Pará (UFPA) and the National Council for Scientific and Technological Development (CNPq) for funding this research. We thank the patients who agreed to participate in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Poiesz, B.; Ruscetti, F.; Gazdar, A.; Bunn, P.; Minna, J.; Gallo, R. Detection and isolation of type C retrovirus particles from Fresh and Cultured Lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, V.S.; Sarngadharan, M.G.; Robert-Guroff, M.; Miyoshi, I.; Blayney, D.; Golde, D.; Gallo, R.C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 1982, 218, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Barin, F.; Vernant, J.; Gout, O.; Maurs, L.; Calender, A.; De Thé, G. Antibodies to human t-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985, 326, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Schierhout, G.; McGregor, S.; Gessain, A.; Einsiedel, L.; Martinello, M.; Kaldor, J. Association between HTLV-1 infection and adverse health outcomes: A systematic review and meta-analysis of epidemiological studies. Lancet Infect. Dis. 2020, 20, 133–143. [Google Scholar] [CrossRef]
- Legrand, N.; McGregor, S.; Bull, R.; Bajis, S.; Valencia, B.M.; Ronnachit, A.; Einsiedel, L.; Gessain, A.; Kaldor, J.; Martinello, M. Clinical and public health implications of human T-lymphotropic virus type 1 infection. Clin. Microbiol. Rev. 2022, 35, e00078-21. [Google Scholar] [CrossRef]
- Kamoi, K. HTLV-1 in Ophthalmology. Front. Microbiol. 2020, 11, 388. [Google Scholar] [CrossRef]
- Eguchi, K.; Iwanaga, M.; Terada, K.; Aramaki, T.; Tuji, Y.; Kurushima, S.; Kojima, K.; Arima, K.; Iwamoto, N.; Ichinose, K.; et al. Clinical features and human T-cell leukemia virus type-1 (HTLV-1) proviral load in HTLV-1-positive patients with rheumatoid arthritis: Baseline data in a single center cohort study. Mod. Rheumatol. 2020, 30, 471–480. [Google Scholar] [CrossRef]
- Martin, F.; Taylor, G.P.; Jacobson, S. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev. Clin. Immunol. 2014, 10, 1531–1546. [Google Scholar] [CrossRef]
- Brites, C.; Grassi, M.F.; Quaresma, J.A.S.; Ishak, R.; Vallinoto, A.C.R. Pathogenesis of HTLV-1 infection and progression biomarkers: An Overview. Braz. J. Infect. Dis. 2021, 25, 101594. [Google Scholar] [CrossRef]
- Gotuzzo, E.; Arango, C.; de Queiroz-Campos, A.; Istúriz, R.E. Human T-cell lymphotropic virus-I in Latin America. Infect. Dis. Clin. N. Am. 2000, 14, 211–239. [Google Scholar] [CrossRef]
- Gonçalves, D.U.; Proietti, F.A.; Ribas, J.G.R.; Araújo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B.F. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Eusebio-Ponce, E.; Anguita, E.; Paulino-Ramirez, R.; Candel, F.J. HTLV-1 Infection: An emerging risk. pathogenesis, epidemiology, diagnosis and associated diseases. Rev. Esp. Quimioter. 2019, 32, 485–496. [Google Scholar] [PubMed]
- Rosadas, C.; Menezes, M.L.B.; Galvão-Castro, B.; Assone, T.; Miranda, A.E.; Aragón, M.G.; Caterino-De-Araujo, A.; Taylor, G.P.; Ishak, R. Blocking HTLV-1/2 Silent Transmission in Brazil: Current public health policies and proposal for additional strategies. PloS Negl. Trop. Dis. 2021, 15, e0009717. [Google Scholar] [CrossRef]
- Gessain, A.; Mahieux, R. Tropical spastic paraparesis and HTLV-1 associated myelopathy: Clinical, epidemiological, virological and therapeutic aspects. Rev. Neurol. 2012, 168, 257–269. [Google Scholar] [CrossRef]
- ECDC—European Centre for Disease Prevention and Control. Geographical Distribution of Areas with a High Prevalence of HTLV-1 Infection; European Centre for Disease Prevention and Control: Solna, Sweden, 2015; ISBN 978-92-9193-625-0. [CrossRef]
- Vallinoto, A.C.R.; Ishak, M.O.G.; Azevedo, V.N.; Vicente, A.C.P.; Otsuki, K.; Hall, W.W.; Ishak, R. Molecular epidemiology of human T-lymphotropic virus type II infection in Amerindian and urban populations of the amazon region of Brazil. Hum. Biol. 2002, 74, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Castro, B.; Alcântara, L.C., Jr.; Grassi, M.F.; Mota-Miranda, A.C.A.; de Queiroz, A.T.L.; Rego, F.F.A.; Mota, A.C.A.; Pereira, S.A.; Magalhães, T.; Tavares-Neto, J.; et al. HTLV-I Epidemiology And Origin in Salvador, State of Bahia: The city with the highest prevalence of this infection in Brazil. Gaz. Médic. Bahia 2009, 79, 3–10. Available online: http://gmbahia.ufba.br/index.php/gmbahia/article/viewFile/1053/1022 (accessed on 25 November 2024).
- Ishak, R.; Ishak, M.d.O.G.; Azevedo, V.N.; Machado, L.F.A.; Vallinoto, I.M.C.; Queiroz, M.A.F.; Costa, G.d.L.C.; Guerreiro, J.F.; Vallinoto, A.C.R. HTLV in South America: Origins of a silent ancient human infection. Virus Evol. 2020, 6, veaa053. [Google Scholar] [CrossRef]
- Carneiro-Proietti, A.B.F.; Ribas, J.G.R.; Catalan-Soares, B.C.; Martins, M.L.; Brito-Melo, G.E.A.; Martins-Filho, O.A.; Pinheiro, S.R.; Araújo, A.d.Q.-C.; Galvão-Castro, B.; de Oliveira, M.S.P.; et al. Infecção e doença pelos vírus linfotrópicos humanos de células T (HTLV-I/II) no Brasil. Rev. Soc. Bras. Med. Trop. 2002, 35, 499–508. [Google Scholar] [CrossRef]
- Catalan-Soares, B.; Carneiro-Proietti, A.B.d.F.; Proietti, F.A. Heterogeneous geographic distribution of human T-cell lymphotropic viruses I and II (HTLV-I/II): Serological screening prevalence rates in blood donors from large urban areas in Brazil. Cad. Saúde Pública 2005, 21, 926–931. [Google Scholar] [CrossRef]
- Coordenação-Geral de Vigilância das Infecções Sexualmente Transmissíveis (CGIST/DCCI/SVS); Rosadas, C.; Miranda, A.E.; Gonçalves, D.U.; Caterino-de-Araujo, A.; Assone, T.; Ishak, R. Prevalência da infecção por HTLV-1/2 no Brasil. Bol. Epidemiol. Secr. Vigilânc. Saúde Minist. Saúde 2020, 51. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2020/boletim_epidemiologico_svs_48.pdf (accessed on 25 November 2024).
- Vallinoto, A.; Pontes, G.; Muto, N.; Lopes, I.; Machado, L.; Azevedo, V.; Carvalhaes, F.; Santos, S.; Guerreiro, J.; Ishak, M.; et al. Identification of human T-cell lymphotropic virus infection in a semi-isolated afro-brazilian quilombo located in the Marajó Island (Pará, Brazil). Mem. Inst. Oswaldo Cruz 2006, 101, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, C.G.; Tamegão-Lopes, B.P.; Santos, E.J.M.D.; Ventura, A.M.R.; Moraes-Pinto, M.I.; de Menezes Succi, R.C. Descriptive Study of HTLV Infection in a population of pregnant women from the state of Pará, Northern Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 453–456. [Google Scholar] [CrossRef]
- Abreu, I.N.; Lima, C.N.C.; Sacuena, E.R.P.; Lopes, F.T.; da Silva Torres, M.K.; Santos, B.C.d.; de Oliveira Freitas, V.; de Figueiredo, L.G.C.P.; Pereira, K.A.S.; de Lima, A.C.R.; et al. HTLV-1/2 in Indigenous peoples of the Brazilian amazon: Seroprevalence, molecular characterization and sociobehavioral factors related to risk of infection. Viruses 2022, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.T.; de Sousa, R.S.; Gomes, J.L.C.; Vallinoto, M.C.; de Lima, A.C.R.; Lima, S.S.; Freitas, F.B.; Feitosa, R.N.M.; da Silva, A.N.M.R.; Machado, L.F.A.; et al. The relevance of a diagnostic and counseling service for people living with HTLV-1/2 in a metropolis of the Brazilian Amazon. Front. Public Health 2022, 10, 864861. [Google Scholar] [CrossRef]
- Ferreira, L.d.S.C.; Costa, J.H.G.; da Costa, C.A.; Melo, M.d.F.C.d.; Andrade, M.L.; Martins, L.C.; Ishikawa, E.A.Y.; de Sousa, M.S. Soroprevalência do vírus linfotrópico de células T humanas em comunidades ribeirinhas da região nordeste do estado do Pará, Brasil. Rev. Pan-Amaz. Saúde 2010, 1, 103–108. [Google Scholar] [CrossRef]
- Ishak, R.; Vallinoto, A.C.R.; Azevedo, V.N.; Ishak, M.d.O.G. Epidemiological aspects of retrovirus (HTLV) infection among Indian populations in the amazon region of Brazil. Cad. Saúde Pública 2003, 19, 901–914. [Google Scholar] [CrossRef]
- Tamegão-Lopes, B.P.; Rezende, P.R.; Maradei-Pereira, L.M.C.; de Lemos, J.A.R. Carga proviral do HTLV-1 e HTLV-2: Um método simples através da pcr quantitativa em tempo real [HTLV-1 and HTLV-2 proviral load: A simple method using quantitative Real-Time Pcr]. Rev. Soc. Bras. Med. Trop. 2006, 39, 548–552. [Google Scholar] [CrossRef]
- Ayres, M.; Ayres, J.; Ayres, D.; Santos, A. Bioestat 5.3: Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas; Sociedade Civil Mamirauá: Belém, Brazil; Available online: https://bioestat.software.informer.com/5.0/ (accessed on 25 November 2024).
- Pereira, F.M.; Almeida, M.d.C.C.d.; Santos, F.L.N.; Carreiro, R.P.; Regis-Silva, C.G.; Galvão-Castro, B.; Grassi, M.F.R. Evidence of new endemic clusters of human T-cell leukemia virus (HTLV) infection in Bahia, Brazil. Front. Microbiol. 2019, 10, 1002. [Google Scholar] [CrossRef]
- Silva, I.C.; Pinheiro, B.T.; Nobre, A.F.S.; Coelho, J.L.; Pereira, C.C.C.; Ferreira, L.d.S.C.; de Almeida, C.P.S.; Viana, M.d.N.D.S.d.A.; de Almeida, D.S.; Falcão, J.R.; et al. Moderate endemicity of the human T-lymphotropic virus infection in the metropolitan region of Belém, Pará, Brazil. Rev. Bras. Epidemiol. 2018, 21, e180018. [Google Scholar] [CrossRef]
- Marin, R.; Castro, E. No Caminho das Pedras de Abacatal: Experiência Social de Grupos Negros no PARÁ, 2nd ed.; Universidade Federal do Pará: Belém, Brazil, 1999; ISBN 857143011x. [Google Scholar]
- Araújo, A.d.S.; Anjos DRd Silva RdSe Santos MASd Martins, C.M.; Almeida, R.H.C. Análise socioeconômica de agricultores da comunidade quilombola do Abacatal, Ananindeua, estado do Pará, Brasil. Biota Amaz. 2017, 7, 30–37. Available online: http://www.repositorio.ufra.edu.br:8080/jspui/handle/123456789/982 (accessed on 25 November 2024).
- Amoussa, A.E.R.; Wilkinson, E.; Giovanetti, M.; Rego, F.F.d.A.; Araujo, T.H.A.; Gonçalves, M.d.S.; de Oliveira, T.; Alcantara, L.C.J. HTLV-1aa introduction into Brazil and its association with the trans-Atlantic slave trade. Infect. Genet. Evol. 2017, 48, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.A.; Bidinotto, A.B.; Dartora, W.J.; Pedrotti, L.G.; de Oliveira, V.M.; Wendland, E.M. Prevalence of Human T-Lymphotropic Virus Type 1 And 2 (HTLV-1/-2) Infection in pregnant women in Brazil: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alencar, S.P.; Souza, M.d.C.; Fonseca, R.R.d.S.; Menezes, C.R.; Azevedo, V.N.; Ribeiro, A.L.R.; Lima, S.S.; Laurentino, R.V.; Barbosa, M.d.A.d.A.P.; Freitas, F.B.; et al. Prevalence and molecular epidemiology of human T-lymphotropic virus (HTLV) infection in people living with HIV/Aids in the Pará state, Amazon region of Brazil. Front. Microbiol. 2020, 11, 572381. [Google Scholar] [CrossRef] [PubMed]
- Dourado, I.; Alcantara, L.C.; Barreto, M.L.; Teixeira, M.d.G.; Galvão-Castro, B. HTLV-I in the general population of Salvador, Brazil: A city with African ethnic and sociodemographic characteristics. J. Acquir. Immune Defic. Syndr. 2003, 34, 527–531. [Google Scholar] [CrossRef]
- de Alcântara Maneschy, C.; Barile, K.A.D.S.; de Castro, J.A.A.; Palmeira, M.K.; de Castro, R.B.H.; de Melo Amaral, C.E. Seroprevalence of the human T lymphotropic virus (HTLV 1 AND HTLV 2) in blood donor candidates in the state of Pará, northern Brazil. Res. Soc. Dev. 2022, 11, e1111427082. Available online: https://rsdjournal.org/index.php/rsd/article/view/27082 (accessed on 25 November 2024). [CrossRef]
- Miranda, C.; Utsch-Gonçalves, D.; Piassi, F.C.C.; Loureiro, P.; Gomes, I.; Ribeiro, M.A.; de Almeida-Neto, C.; Blatyta, P.; Amorim, L.; Mateos, S.O.G.; et al. Prevalence and risk factors for human T-cell lymphotropic virus (HTLV) in blood donors in Brazil—A 10-year study (2007–2016). Front. Med. 2022, 9, 844265. [Google Scholar] [CrossRef]
- Eshima, N.; Iwata, O.; Iwata, S.; Tabata, M.; Higuchi, Y.; Matsuishi, T.; Karukaya, S. Age and gender specific prevalence of HTLV-1. J. Clin. Virol. 2009, 45, 135–138. [Google Scholar] [CrossRef]
- Kaplan, J.E.; Khabbaz, R.F.; Murphy, E.L.; Hermansen, S.; Roberts, C.; Lal, R.; Heneine, W.; Wright, D.; Matijas, L.; Thomson, R.; et al. Male-to-female transmission of human T-cell lymphotropic virus types I and II: Association with viral load. the retrovirus epidemiology donor study group. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 12, 193–201. [Google Scholar] [CrossRef]
- Paiva, A.; Casseb, J. Sexual transmission of human T-cell lymphotropic virus type 1. Rev. Soc. Bras. Med. Trop. 2014, 47, 265–274. [Google Scholar] [CrossRef]
- Ishak, R.; Harrington, W.J., Jr.; Azevedo, V.N.; Eiraku, N.; Ishak, M.O.; Guerreiro, J.F.; Santos, S.B.; Kubo, T.; Monken, C.; Alexander, S.; et al. Identification of human T cell lymphotropic virus type IIa infection in the Kayapo, an Indigenous population of Brazil. AIDS Res. Hum. Retroviruses 1995, 11, 813–821. [Google Scholar] [CrossRef]
- Martel, M.; Gotuzzo, E. HTLV-1 is also a sexually transmitted infection. Front. Public Health 2022, 10, 840295. [Google Scholar] [CrossRef] [PubMed]
- da Costa, C.A.; Furtado, K.C.Y.O.; Ferreira, L.d.S.C.; Almeida, D.d.S.; Linhares, A.d.C.; Ishak, R.; Vallinoto, A.C.R.; de Lemos, J.A.R.; Martins, L.C.; Ishikawa, E.A.Y.; et al. Familial transmission of human T-cell lymphotropic virus: Silent dissemination of an emerging but neglected infection. PLoS Negl. Trop. Dis. 2013, 7, e2272. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Boa-Sorte, N.; Grassi, M.F.R.; Taylor, G.P.; Teixeira, M.G.; Barreto, M.L.; Dourado, I.; Galvão-Castro, B. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS ONE 2017, 12, e0171303. [Google Scholar] [CrossRef]
- Carneiro-Proietti, A.B.F.; Amaranto-Damasio, M.S.; Leal-Horiguchi, C.F.; Bastos, R.H.C.; Seabra-Freitas, G.; Borowiak, D.R.; Ribeiro, M.A.; Proietti, F.A.; Ferreira, A.S.D.; Martins, M.L. Mother-to-child transmission of human T-cell lymphotropic viruses-1/2: What we know, and what are the gaps in understanding and preventing this route of infection. J. Pediatr. Infect. Dis. Soc. 2024, 3 (Suppl. 1), S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Rosadas, C.; Taylor, G.P. Current interventions to prevent HTLV-1 mother-to-child transmission and their effectiveness: A systematic review and meta-analysis. Microorganisms 2022, 10, 2227. [Google Scholar] [CrossRef]
- Millen, S.; Thoma-Kress, A.K. Milk transmission of HTLV-1 and the need for innovative prevention strategies. Front. Med. 2022, 9, 867147. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).