Biomedical Interventions for HIV Prevention and Control: Beyond Vaccination
Abstract
1. Introduction
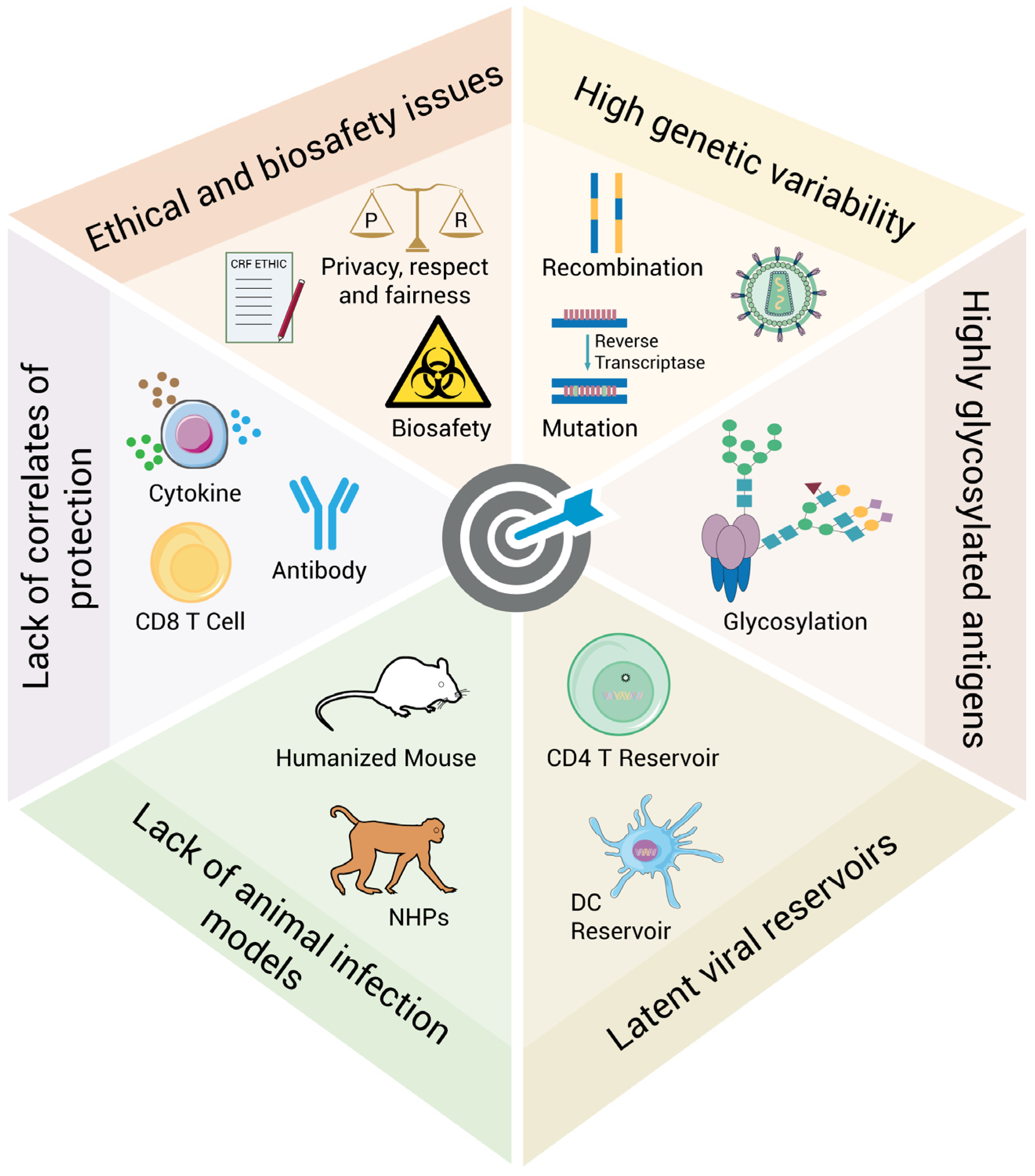
2. Challenges for the Development of Biomedical Interventions Against HIV Acquisition and Transmission
2.1. High Genetic Variability
2.2. Highly Glycosylated Antigens
2.3. Latent Viral Reservoirs
2.4. Lack of Animal Infection Models
2.5. Lack of Correlates of Protection
2.6. Ethical and Biosafety Issues
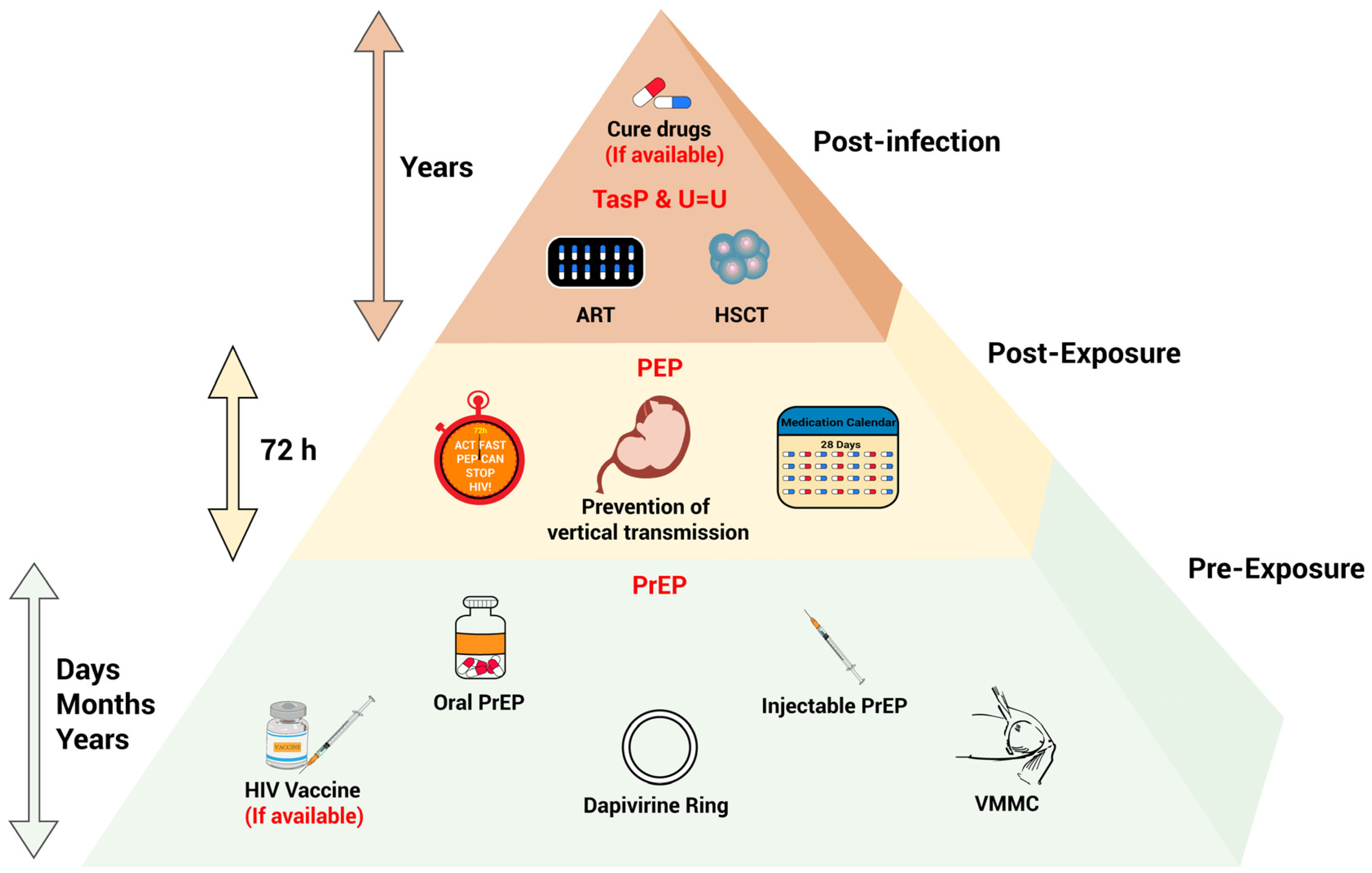
3. Advances of Biomedical Interventions for HIV Prevention and Control
3.1. PrEP
3.2. PEP
3.3. Treatment as Prevention (TasP) and U=U Principle
3.4. Testing as Prevention
3.5. Prevention of Vertical Transmission
3.6. Voluntary Medical Male Circumcision (VMMC)
3.7. Gene Editing Technology
3.8. Passive Infusion of Broadly Neutralizing Antibody
4. Conclusions and Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Govindan, R.; Stephenson, K.E. HIV Vaccine Development at a Crossroads: New B and T Cell Approaches. Vaccines 2024, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Siliciano, J.D.; Siliciano, R.F. HIV cure: The daunting scale of the problem. Science 2024, 383, 703–705. [Google Scholar] [CrossRef] [PubMed]
- UNAIDs. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 10 April 2025).
- Grinspoon, S.K.; Fitch, K.V.; Zanni, M.V.; Fichtenbaum, C.J.; Umbleja, T.; Aberg, J.A.; Overton, E.T.; Malvestutto, C.D.; Bloomfield, G.S.; Currier, J.S.; et al. Pitavastatin to Prevent Cardiovascular Disease in HIV Infection. N. Engl. J. Med. 2023, 389, 687–699. [Google Scholar] [CrossRef]
- McMyn, N.F.; Varriale, J.; Fray, E.J.; Zitzmann, C.; MacLeod, H.; Lai, J.; Singhal, A.; Moskovljevic, M.; Garcia, M.A.; Lopez, B.M.; et al. The latent reservoir of inducible, infectious HIV-1 does not decrease despite decades of antiretroviral therapy. J. Clin. Investig. 2023, 133, e171554. [Google Scholar] [CrossRef]
- Colby, D.J.; Trautmann, L.; Pinyakorn, S.; Leyre, L.; Pagliuzza, A.; Kroon, E.; Rolland, M.; Takata, H.; Buranapraditkun, S.; Intasan, J.; et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med. 2018, 24, 923–926. [Google Scholar] [CrossRef]
- Chanie, G.S.; Belachew, E.A.; Seid, A.M.; Limenh, L.W.; Mitku, M.L.; Beyna, A.T.; Mengesha, A.K.; Melese, M.; Esubalew, D.; Gela, Y.Y.; et al. Patients reported neuropsychiatric adverse events and associated factors among PLHIV patients receiving DTG-based regimen antiretroviral therapy real-life clinical practice in Ethiopia: Multi center crossetional study. BMC Psychiatry 2025, 25, 383. [Google Scholar] [CrossRef]
- Corti, N.; Menzaghi, B.; Orofino, G.; Guastavigna, M.; Lagi, F.; Di Biagio, A.; Taramasso, L.; De Socio, G.V.; Molteni, C.; Madeddu, G.; et al. Risk of Cardiovascular Events in People with HIV (PWH) Treated with Integrase Strand-Transfer Inhibitors: The Debate Is Not Over; Results of the SCOLTA Study. Viruses 2024, 16, 613. [Google Scholar] [CrossRef]
- Mwebaza, J.; Meya, D.; Musiime, V.; Birungi, C. Prevalence of neuropsychiatric adverse events and associated factors among adult patients on dolutegravir attending Mulago ISS clinic. HIV Med. 2023, 24, 491–501. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Fauci, A.S. Ending the HIV/AIDS Pandemic(1). Emerg. Infect. Dis. 2018, 24, 413–416. [Google Scholar] [CrossRef]
- Hu, W.S.; Hughes, S.H. HIV-1 reverse transcription. Cold Spring Harb. Perspect. Med. 2012, 2, a006882. [Google Scholar] [CrossRef] [PubMed]
- Nikolaitchik, O.A.; Islam, S.; Kitzrow, J.P.; Duchon, A.; Cheng, Z.; Liu, Y.; Rawson, J.M.O.; Shao, W.; Nikolaitchik, M.; Kearney, M.F.; et al. HIV-1 usurps transcription start site heterogeneity of host RNA polymerase II to maximize replication fitness. Proc. Natl. Acad. Sci. USA 2023, 120, e2305103120. [Google Scholar] [CrossRef] [PubMed]
- Rudometov, A.P.; Chikaev, A.N.; Rudometova, N.B.; Antonets, D.V.; Lomzov, A.A.; Kaplina, O.N.; Ilyichev, A.A.; Karpenko, L.I. Artificial Anti-HIV-1 Immunogen Comprising Epitopes of Broadly Neutralizing Antibodies 2F5, 10E8, and a Peptide Mimic of VRC01 Discontinuous Epitope. Vaccines 2019, 7, 83. [Google Scholar] [CrossRef]
- King, S.R.; Duggal, N.K.; Ndongmo, C.B.; Pacut, C.; Telesnitsky, A. Pseudodiploid genome organization AIDS full-length human immunodeficiency virus type 1 DNA synthesis. J. Virol. 2008, 82, 2376–2384. [Google Scholar] [CrossRef]
- Iglesias-Sanchez, M.J.; Lopez-Galindez, C. Each genomic RNA in HIV-1 heterozygous virus generate new virions. Virology 2005, 333, 316–323. [Google Scholar] [CrossRef]
- Rawson, J.M.O.; Nikolaitchik, O.A.; Keele, B.F.; Pathak, V.K.; Hu, W.S. Recombination is required for efficient HIV-1 replication and the maintenance of viral genome integrity. Nucleic Acids Res. 2018, 46, 10535–10545. [Google Scholar] [CrossRef]
- Onafuwa-Nuga, A.; Telesnitsky, A. The remarkable frequency of human immunodeficiency virus type 1 genetic recombination. Microbiol. Mol. Biol. Rev. MMBR 2009, 73, 451–480. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, M.; Hong, Z.; Huang, S.; Zhou, S.; Lyu, H.; Li, J.; Lin, Y.; Huang, H.; Tang, W.; et al. High Genetic Diversity of HIV-1 and Active Transmission Clusters among Male-to-Male Sexual Contacts (MMSCs) in Zhuhai, China. Viruses 2023, 15, 1947. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Zhang, K.; Yuan, Y.; Zhao, J.; Cui, M.; Yin, D.; Wen, Z.; Chen, Z.; Li, L.; et al. Characteristics of genotype, drug resistance, and molecular transmission network among newly diagnosed HIV-1 infections in Shenzhen, China. J. Med. Virol. 2023, 95, e28973. [Google Scholar] [CrossRef]
- Etienne, L.; Hahn, B.H.; Sharp, P.M.; Matsen, F.A.; Emerman, M. Gene loss and adaptation to hominids underlie the ancient origin of HIV-1. Cell Host Microbe 2013, 14, 85–92. [Google Scholar] [CrossRef]
- Schmitt, K.; Mohan Kumar, D.; Curlin, J.; Remling-Mulder, L.; Stenglein, M.; O’Connor, S.; Marx, P.; Akkina, R. Modeling the evolution of SIV sooty mangabey progenitor virus towards HIV-2 using humanized mice. Virology 2017, 510, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, J. The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 2012, 18, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Worobey, M. Dating the age of the SIV lineages that gave rise to HIV-1 and HIV-2. PLoS Comput. Biol. 2009, 5, e1000377. [Google Scholar] [CrossRef]
- Korber, B.; Gaschen, B.; Yusim, K.; Thakallapally, R.; Kesmir, C.; Detours, V. Evolutionary and immunological implications of contemporary HIV-1 variation. Br. Med. Bull. 2001, 58, 19–42. [Google Scholar] [CrossRef]
- Schlub, T.E.; Grimm, A.J.; Smyth, R.P.; Cromer, D.; Chopra, A.; Mallal, S.; Venturi, V.; Waugh, C.; Mak, J.; Davenport, M.P. Fifteen to twenty percent of HIV substitution mutations are associated with recombination. J. Virol. 2014, 88, 3837–3849. [Google Scholar] [CrossRef]
- Okada, A.; Iwatani, Y. APOBEC3G-Mediated G-to-A Hypermutation of the HIV-1 Genome: The Missing Link in Antiviral Molecular Mechanisms. Front. Microbiol. 2016, 7, 2027. [Google Scholar] [CrossRef]
- Sadler, H.A.; Stenglein, M.D.; Harris, R.S.; Mansky, L.M. APOBEC3G contributes to HIV-1 variation through sublethal mutagenesis. J. Virol. 2010, 84, 7396–7404. [Google Scholar] [CrossRef]
- Zhu, T.; Niu, G.; Zhang, Y.; Chen, M.; Li, C.-Y.; Hao, L.; Zhang, Z. Host-mediated RNA editing in viruses. Biol. Direct 2023, 18, 12. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, J.; Chen, Z.; Pan, W.; Chen, Z.; Yan, Y.; Dai, J. Glycosylation of viral proteins: Implication in virus-host interaction and virulence. Virulence 2022, 13, 670–683. [Google Scholar] [CrossRef]
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Sulli, C.; Nilo, A.; Yasmeen, A.; Ozorowski, G.; Sanders, R.W.; Ward, A.B.; Klasse, P.J.; Moore, J.P.; Doranz, B.J. High-Throughput Protein Engineering Improves the Antigenicity and Stability of Soluble HIV-1 Envelope Glycoprotein SOSIP Trimers. J. Virol. 2017, 91, 18. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, M.; Jiang, X.; Pang, L.; Navarra, G.; Rabbani, S.; Binder, F.; Schwardt, O.; Ernst, B. Kinetic properties of carbohydrate-lectin interactions: FimH antagonists. ChemMedChem 2014, 9, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, C.N.; Offer, J.; Zitzmann, N.; Dwek, R.A. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature 2007, 446, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Seabright, G.E.; Doores, K.J.; Burton, D.R.; Crispin, M. Protein and Glycan Mimicry in HIV Vaccine Design. J. Mol. Biol. 2019, 431, 2223–2247. [Google Scholar] [CrossRef]
- Moore, P.L.; Gray, E.S.; Wibmer, C.K.; Bhiman, J.N.; Nonyane, M.; Sheward, D.J.; Hermanus, T.; Bajimaya, S.; Tumba, N.L.; Abrahams, M.R.; et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat. Med. 2012, 18, 1688–1692. [Google Scholar] [CrossRef]
- Koch, M.; Pancera, M.; Kwong, P.D.; Kolchinsky, P.; Grundner, C.; Wang, L.; Hendrickson, W.A.; Sodroski, J.; Wyatt, R. Structure-based, targeted deglycosylation of HIV-1 gp120 and effects on neutralization sensitivity and antibody recognition. Virology 2003, 313, 387–400. [Google Scholar] [CrossRef]
- Shan, L.; Deng, K.; Gao, H.; Xing, S.; Capoferri, A.A.; Durand, C.M.; Rabi, S.A.; Laird, G.M.; Kim, M.; Hosmane, N.N.; et al. Transcriptional Reprogramming during Effector-to-Memory Transition Renders CD4(+) T Cells Permissive for Latent HIV-1 Infection. Immunity 2017, 47, 766–775.e3. [Google Scholar] [CrossRef]
- Eriksson, S.; Graf, E.H.; Dahl, V.; Strain, M.C.; Yukl, S.A.; Lysenko, E.S.; Bosch, R.J.; Lai, J.; Chioma, S.; Emad, F.; et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013, 9, e1003174. [Google Scholar] [CrossRef]
- Gunst, J.D.; Gohil, J.; Li, J.Z.; Bosch, R.J.; White Catherine Seamon, A.; Chun, T.W.; Mothe, B.; Gittens, K.; Praiss, L.; De Scheerder, M.A.; et al. Time to HIV viral rebound and frequency of post-treatment control after analytical interruption of antiretroviral therapy: An individual data-based meta-analysis of 24 prospective studies. Nat. Commun. 2025, 16, 906. [Google Scholar] [CrossRef]
- López-Huertas, M.R.; Gutiérrez, C.; Madrid-Elena, N.; Hernández-Novoa, B.; Olalla-Sierra, J.; Plana, M.; Delgado, R.; Rubio, R.; Muñoz-Fernández, M.; Moreno, S. Prolonged administration of maraviroc reactivates latent HIV in vivo but it does not prevent antiretroviral-free viral rebound. Sci. Rep. 2020, 10, 22286. [Google Scholar] [CrossRef]
- Castro-Gonzalez, S.; Colomer-Lluch, M.; Serra-Moreno, R. Barriers for HIV Cure: The Latent Reservoir. AIDS Res. Hum. Retroviruses 2018, 34, 739–759. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.M.; Garcia, J.V.; Hazuda, D.J.; Haynes, B.F. Latency reversal and viral clearance to cure HIV-1. Science 2016, 353, aaf6517. [Google Scholar] [CrossRef] [PubMed]
- Pankrac, J.; Klein, K.; Mann, J.F.S. Eradication of HIV-1 latent reservoirs through therapeutic vaccination. AIDS Res. Ther. 2017, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Dubé, K.; Chomont, N. How does the timing of antiretroviral therapy initiation in acute infection affect HIV reservoirs? Curr. Opin. HIV AIDS 2015, 10, 18–28. [Google Scholar] [CrossRef]
- Nühn, M.M.; Gumbs, S.B.H.; Buchholtz, N.; Jannink, L.M.; Gharu, L.; de Witte, L.D.; Wensing, A.M.J.; Lewin, S.R.; Nijhuis, M.; Symons, J. Shock and kill within the CNS: A promising HIV eradication approach? J. Leukoc. Biol. 2022, 112, 1297–1315. [Google Scholar] [CrossRef]
- Fidler, S.; Stöhr, W.; Pace, M.; Dorrell, L.; Lever, A.; Pett, S.; Kinloch-de Loes, S.; Fox, J.; Clarke, A.; Nelson, M.; et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): A phase 2, randomised trial. Lancet 2020, 395, 888–898. [Google Scholar] [CrossRef]
- Wen, Z.; Li, P.; Yuan, Y.; Wang, C.; Li, M.; Wang, H.; Shi, M.; He, Y.; Cui, M.; Chen, L.; et al. Purging viral latency by a bifunctional HSV-vectored therapeutic vaccine in chronically SIV-infected macaques. eLife 2024, 13, RP95964. [Google Scholar] [CrossRef]
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef]
- Johnson, K.A.; Chen, M.J.; Kohn, R.; Sachdev, D.; Bacon, O.; Lee, S.; Cohen, S.E. Acute HIV at the Time of Initiation of Pre-exposure or Post-exposure Prophylaxis: Impact on Drug Resistance and Clinical Outcomes. J. Acquir. Immune Defic. Syndr. (1999) 2021, 87, 818–825. [Google Scholar] [CrossRef]
- Hessell, A.J.; Haigwood, N.L. Animal models in HIV-1 protection and therapy. Curr. Opin. HIV AIDS 2015, 10, 170–176. [Google Scholar] [CrossRef]
- Ambrose, Z.; KewalRamani, V.N.; Bieniasz, P.D.; Hatziioannou, T. HIV/AIDS: In search of an animal model. Trends Biotechnol. 2007, 25, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Canary, L.A.; Sun, X.; Vinton, C.L.; Funderburg, N.T.; Morcock, D.R.; Quinones, M.; Deming, C.B.; Perkins, M.; Hazuda, D.J.; et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J. Clin. Investig. 2013, 123, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Sliva, K. Latest animal models for anti-HIV drug discovery. Expert Opin. Drug Discov. 2015, 10, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Badamchi-Zadeh, A.; Tartaglia, L.J.; Abbink, P.; Bricault, C.A.; Liu, P.T.; Boyd, M.; Kirilova, M.; Mercado, N.B.; Nanayakkara, O.S.; Vrbanac, V.D.; et al. Therapeutic Efficacy of Vectored PGT121 Gene Delivery in HIV-1-Infected Humanized Mice. J. Virol. 2018, 92, 12. [Google Scholar] [CrossRef]
- Dash, P.K.; Kaminski, R.; Bella, R.; Su, H.; Mathews, S.; Ahooyi, T.M.; Chen, C.; Mancuso, P.; Sariyer, R.; Ferrante, P.; et al. Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat. Commun. 2019, 10, 2753. [Google Scholar] [CrossRef]
- Satija, N.; Patel, F.; Schmidt, G.; Doanman, D.V.; Kapoor, M.; La Porte, A.; Wang, Y.C.; Law, K.M.; Esposito, A.M.; Allette, K.; et al. Tracking HIV persistence across T cell lineages during early ART-treated HIV-1-infection using a reservoir-marking humanized mouse model. Nat. Commun. 2025, 16, 2233. [Google Scholar] [CrossRef]
- Barnett, E.; Kaginkar, S.; Schmitt, K.; Remling-Mulder, L.; Akkina, R. A dual-purpose humanized mouse model for testing antiviral strategies against both SIV and HIV. Front. Immunol. 2024, 15, 1491481. [Google Scholar] [CrossRef]
- Min, A.K.; Javidfar, B.; Missall, R.; Doanman, D.; Durens, M.; Graziani, M.; Mordelt, A.; Marro, S.G.; de Witte, L.; Chen, B.K.; et al. HIV-1 infection of genetically engineered iPSC-derived central nervous system-engrafted microglia in a humanized mouse model. J. Virol. 2023, 97, e0159523. [Google Scholar] [CrossRef]
- Karpel, M.E.; Boutwell, C.L.; Allen, T.M. BLT humanized mice as a small animal model of HIV infection. Curr. Opin. Virol. 2015, 13, 75–80. [Google Scholar] [CrossRef]
- Heeney, J.L.; Plotkin, S.A. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 2006, 7, 1281–1284. [Google Scholar] [CrossRef]
- Moog, C. Immune responses that correlate with HIV-1 protection? AIDS 2008, 22, 1461–1462. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Littman, D. HIV immunology needs a new direction. Nature 2008, 455, 591. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Lopalco, L.; Mazzotta, F.; Lo Caputo, S.; Veas, F.; Clerici, M. The ‘immunologic advantage’ of HIV-exposed seronegative individuals. AIDS 2009, 23, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Wang, M.; Wrin, T.; Petropoulos, C.; Gurwith, M.; Sinangil, F.; D’Souza, P.; Rodriguez-Chavez, I.R.; DeCamp, A.; Giganti, M.; et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J. Infect. Dis. 2010, 202, 595–605. [Google Scholar] [CrossRef]
- Tay, M.Z.; Liu, P.; Williams, L.D.; McRaven, M.D.; Sawant, S.; Gurley, T.C.; Xu, T.T.; Dennison, S.M.; Liao, H.X.; Chenine, A.L.; et al. Antibody-Mediated Internalization of Infectious HIV-1 Virions Differs among Antibody Isotypes and Subclasses. PLoS Pathog. 2016, 12, e1005817. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Charles, T.P.; Joag, V.; Bollimpelli, V.S.; Scott, M.K.D.; Wimmers, F.; Burton, S.L.; Labranche, C.C.; Petitdemange, C.; Gangadhara, S.; et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 2020, 26, 932–940. [Google Scholar] [CrossRef]
- Zaunders, J.; van Bockel, D. Innate and Adaptive Immunity in Long-Term Non-Progression in HIV Disease. Front. Immunol. 2013, 4, 95. [Google Scholar] [CrossRef]
- Li, Y.; Ni, Y.; He, Q.; Hu, X.; Zhang, Y.; He, X.; Ni, M. Survival Analysis and Immune Differences of HIV Long-Term Non-progressors in Xinjiang China: A 12-Year Prospective Cohort Observation. AIDS Behav. 2024, 28, 3151–3160. [Google Scholar] [CrossRef]
- Mylvaganam, G.H.; Rios, D.; Abdelaal, H.M.; Iyer, S.; Tharp, G.; Mavigner, M.; Hicks, S.; Chahroudi, A.; Ahmed, R.; Bosinger, S.E.; et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc. Natl. Acad. Sci. USA 2017, 114, 1976–1981. [Google Scholar] [CrossRef]
- Gao, D.; Wu, J.; Wu, Y.T.; Du, F.; Aroh, C.; Yan, N.; Sun, L.; Chen, Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 2013, 341, 903–906. [Google Scholar] [CrossRef]
- Berkhout, B. Evolution of Live-Attenuated HIV Vaccines. BioPharm Int. 2011, 24. Available online: https://www.biopharminternational.com/view/evolution-live-attenuated-hiv-vaccines (accessed on 10 April 2025).
- Verity Erin, E.; Zotos, D.; Wilson, K.; Chatfield, C.; Lawson Victoria, A.; Dwyer Dominic, E.; Cunningham, A.; Learmont, J.; Dyer, W.; Sullivan, J.; et al. Viral Phenotypes and Antibody Responses in Long-Term Survivors Infected with Attenuated Human Immunodeficiency Virus Type 1 Containing Deletions in the nef and Long Terminal Repeat Regions. J. Virol. 2007, 81, 9268–9278. [Google Scholar] [CrossRef] [PubMed]
- Slack, C.; Ndebele, P.; Allen, M.; Salzwedel, J. Shifts in UNAIDS ethics guidance and implications for ethics review of preventive HIV vaccine trials. J. Int. AIDS Soc. 2021, 24 (Suppl. S7), e25796. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ethical Considerations in HIV Prevention Trials. Available online: https://www.unaids.org/sites/default/files/media_asset/ethical-considerations-hiv-prevention-trials_en.pdf (accessed on 10 April 2025).
- Haire, B.; Folayan, M.O.; Hankins, C.; Sugarman, J.; McCormack, S.; Ramjee, G.; Warren, M. Ethical considerations in determining standard of prevention packages for HIV prevention trials: Examining PrEP. Dev. World Bioeth. 2013, 13, 87–94. [Google Scholar] [CrossRef]
- Dawson, L.; Garner, S.; Anude, C.; Ndebele, P.; Karuna, S.; Holt, R.; Broder, G.; Handibode, J.; Hammer, S.M.; Sobieszczyk, M.E. Testing the waters: Ethical considerations for including PrEP in a phase IIb HIV vaccine efficacy trial. Clin. Trials 2015, 12, 394–402. [Google Scholar] [CrossRef]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapía, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef]
- Baeten, J.M.; Donnell, D.; Ndase, P.; Mugo, N.R.; Campbell, J.D.; Wangisi, J.; Tappero, J.W.; Bukusi, E.A.; Cohen, C.R.; Katabira, E.; et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012, 367, 399–410. [Google Scholar] [CrossRef]
- Choopanya, K.; Martin, M.; Suntharasamai, P.; Sangkum, U.; Mock, P.A.; Leethochawalit, M.; Chiamwongpaet, S.; Kitisin, P.; Natrujirote, P.; Kittimunkong, S.; et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013, 381, 2083–2090. [Google Scholar] [CrossRef]
- Stalter, R.M.; Dong, T.Q.; Hendrix, C.W.; Palanee-Phillips, T.; van der Straten, A.; Hillier, S.L.; Kiweewa, F.M.; Mgodi, N.M.; Marzinke, M.A.; Bekker, L.G.; et al. Assessing Per-Sex-Act HIV-1 Risk Reduction Among Women Using the Dapivirine Vaginal Ring. J. Infect. Dis. 2024, 229, 1158–1165. [Google Scholar] [CrossRef]
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F.; et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N. Engl. J. Med. 2016, 375, 2121–2132. [Google Scholar] [CrossRef]
- Delany-Moretlwe, S.; Hughes, J.P.; Bock, P.; Ouma, S.G.; Hunidzarira, P.; Kalonji, D.; Kayange, N.; Makhema, J.; Mandima, P.; Mathew, C.; et al. Cabotegravir for the prevention of HIV-1 in women: Results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022, 399, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Landovitz, R.J.; Donnell, D.; Clement, M.E.; Hanscom, B.; Cottle, L.; Coelho, L.; Cabello, R.; Chariyalertsak, S.; Dunne, E.F.; Frank, I.; et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. N. Engl. J. Med. 2021, 385, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Bekker, L.G.; Das, M.; Abdool Karim, Q.; Ahmed, K.; Batting, J.; Brumskine, W.; Gill, K.; Harkoo, I.; Jaggernath, M.; Kigozi, G.; et al. Twice-Yearly Lenacapavir or Daily F/TAF for HIV Prevention in Cisgender Women. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.F.; Acevedo-Quiñones, M.; Agwu, A.L.; Avihingsanon, A.; Benson, P.; Blumenthal, J.; Brinson, C.; Brites, C.; Cahn, P.; Cantos, V.D.; et al. Twice-Yearly Lenacapavir for HIV Prevention in Men and Gender-Diverse Persons. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Young, T.N.; Arens, F.J.; Kennedy, G.E.; Laurie, J.W.; Rutherford, G. Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure. Cochrane Database Syst. Rev. 2007, 2007, Cd002835. [Google Scholar] [CrossRef]
- Broyles, L.N.; Luo, R.; Boeras, D.; Vojnov, L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: A systematic review. Lancet 2023, 402, 464–471. [Google Scholar] [CrossRef]
- Tudor Car, L.; van-Velthoven, M.H.; Brusamento, S.; Elmoniry, H.; Car, J.; Majeed, A.; Atun, R. Integrating prevention of mother-to-child HIV transmission (PMTCT) programmes with other health services for preventing HIV infection and improving HIV outcomes in developing countries. Cochrane Database Syst. Rev. 2011, Cd008741. [Google Scholar] [CrossRef]
- Auvert, B.; Taljaard, D.; Lagarde, E.; Sobngwi-Tambekou, J.; Sitta, R.; Puren, A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 Trial. PLoS Med. 2005, 2, e298. [Google Scholar] [CrossRef]
- Bailey, R.C.; Moses, S.; Parker, C.B.; Agot, K.; Maclean, I.; Krieger, J.N.; Williams, C.F.; Campbell, R.T.; Ndinya-Achola, J.O. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet 2007, 369, 643–656. [Google Scholar] [CrossRef]
- Gray, R.H.; Kigozi, G.; Serwadda, D.; Makumbi, F.; Watya, S.; Nalugoda, F.; Kiwanuka, N.; Moulton, L.H.; Chaudhary, M.A.; Chen, M.Z.; et al. Male circumcision for HIV prevention in men in Rakai, Uganda: A randomised trial. Lancet 2007, 369, 657–666. [Google Scholar] [CrossRef]
- Molina, J.M.; Capitant, C.; Spire, B.; Pialoux, G.; Cotte, L.; Charreau, I.; Tremblay, C.; Le Gall, J.M.; Cua, E.; Pasquet, A.; et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N. Engl. J. Med. 2015, 373, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Glidden, D.V.; Mayer, K.; Schechter, M.; Buchbinder, S.; Grinsztejn, B.; Hosek, S.; Casapia, M.; Guanira, J.; Bekker, L.G.; et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: An observational cohort study. Lancet HIV 2016, 3, e521–e528. [Google Scholar] [CrossRef]
- Solomon, M.M.; Lama, J.R.; Glidden, D.V.; Mulligan, K.; McMahan, V.; Liu, A.Y.; Guanira, J.V.; Veloso, V.G.; Mayer, K.H.; Chariyalertsak, S.; et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS 2014, 28, 851–859. [Google Scholar] [CrossRef]
- Parikh, U.M.; Mellors, J.W. How could HIV-1 drug resistance impact preexposure prophylaxis for HIV prevention? Curr. Opin. HIV AIDS 2022, 17, 213–221. [Google Scholar] [CrossRef]
- Landovitz, R.J.; Delany-Moretlwe, S.; Fogel, J.M.; Marzinke, M.A.; Piwowar-Manning, E.; Richardson, P.; Halvas, E.K.; Mellors, J.W.; Persaud, D.; Kofron, R.; et al. Features of HIV Infection in the Context of Long-Acting Cabotegravir Preexposure Prophylaxis. N. Engl. J. Med. 2024, 391, 1253–1256. [Google Scholar] [CrossRef]
- Segal-Maurer, S.; DeJesus, E.; Stellbrink, H.J.; Castagna, A.; Richmond, G.J.; Sinclair, G.I.; Siripassorn, K.; Ruane, P.J.; Berhe, M.; Wang, H.; et al. Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection. N. Engl. J. Med. 2022, 386, 1793–1803. [Google Scholar] [CrossRef]
- Wen, Z.; Shi, M.; Sun, C. Treatment as the Best Prevention: Twice-Yearly Lenacapavir, a Game Changer in Ending the AIDS Epidemic. Viruses 2024, 16, 1368. [Google Scholar] [CrossRef]
- Thigpen, M.C.; Kebaabetswe, P.M.; Paxton, L.A.; Smith, D.K.; Rose, C.E.; Segolodi, T.M.; Henderson, F.L.; Pathak, S.R.; Soud, F.A.; Chillag, K.L.; et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 2012, 367, 423–434. [Google Scholar] [CrossRef]
- McCormack, S.; Dunn, D.T.; Desai, M.; Dolling, D.I.; Gafos, M.; Gilson, R.; Sullivan, A.K.; Clarke, A.; Reeves, I.; Schembri, G.; et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016, 387, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.H.; Molina, J.M.; Thompson, M.A.; Anderson, P.L.; Mounzer, K.C.; De Wet, J.J.; DeJesus, E.; Jessen, H.; Grant, R.M.; Ruane, P.J.; et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020, 396, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.L.; Yang, K.H.; Prince, H.M.; Sykes, C.; White, N.; Malone, S.; Dellon, E.S.; Madanick, R.D.; Shaheen, N.J.; Hudgens, M.G.; et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J. Infect. Dis. 2016, 214, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; van Niekerk, N.; Kapiga, S.; Bekker, L.G.; Gama, C.; Gill, K.; Kamali, A.; Kotze, P.; Louw, C.; Mabude, Z.; et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N. Engl. J. Med. 2016, 375, 2133–2143. [Google Scholar] [CrossRef]
- WHO. WHO Recommends the Dapivirine Vaginal Ring as a New HIV Prevention Option for Women at Substantial Risk of HIV Infection. Available online: https://www.who.int/news/item/26-01-2021-who-recommends-the-dapivirine-vaginal-ring-as-a-new-choice-for-hiv-prevention-for-women-at-substantial-risk-of-hiv-infection (accessed on 10 April 2025).
- Krogstad, E.A.; Montgomery, E.T.; Atujuna, M.; Minnis, A.M.; O’Rourke, S.; Ahmed, K.; Bekker, L.G.; van der Straten, A. Design of an Implant for Long-Acting HIV Pre-Exposure Prophylaxis: Input from South African Health Care Providers. AIDS Patient Care STDs 2019, 33, 157–166. [Google Scholar] [CrossRef]
- Chua, C.Y.X.; Jain, P.; Ballerini, A.; Bruno, G.; Hood, R.L.; Gupte, M.; Gao, S.; Di Trani, N.; Susnjar, A.; Shelton, K.; et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J. Control. Release 2018, 286, 315–325. [Google Scholar] [CrossRef]
- Johnson, L.M.; Krovi, S.A.; Li, L.; Girouard, N.; Demkovich, Z.R.; Myers, D.; Creelman, B.; van der Straten, A. Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics 2019, 11, 315. [Google Scholar] [CrossRef]
- Gunawardana, M.; Remedios-Chan, M.; Sanchez, D.; Fanter, R.; Webster, S.; Webster, P.; Moss, J.A.; Trinh, M.; Beliveau, M.; Ramirez, C.M.; et al. Preclinical Considerations for Long-acting Delivery of Tenofovir Alafenamide from Subdermal Implants for HIV Pre-exposure Prophylaxis. Pharm. Res. 2023, 40, 1657–1672. [Google Scholar] [CrossRef]
- Li, L.; Areson, C.; van der Straten, A.; Johnson, L.M. Effects of Polymer Blending on the Performance of a Subcutaneous Biodegradable Implant for HIV Pre-Exposure Prophylaxis (PrEP). Int. J. Mol. Sci. 2021, 22, 6529. [Google Scholar] [CrossRef]
- Barrett, S.E.; Teller, R.S.; Forster, S.P.; Li, L.; Mackey, M.A.; Skomski, D.; Yang, Z.; Fillgrove, K.L.; Doto, G.J.; Wood, S.L.; et al. Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrob. Agents Chemother. 2018, 62, e01058-18. [Google Scholar] [CrossRef]
- Kovarova, M.; Benhabbour, S.R.; Massud, I.; Spagnuolo, R.A.; Skinner, B.; Baker, C.E.; Sykes, C.; Mollan, K.R.; Kashuba, A.D.M.; García-Lerma, J.G.; et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat. Commun. 2018, 9, 4156. [Google Scholar] [CrossRef]
- Su, J.T.; Simpson, S.M.; Sung, S.; Tfaily, E.B.; Veazey, R.; Marzinke, M.; Qiu, J.; Watrous, D.; Widanapathirana, L.; Pearson, E.; et al. A Subcutaneous Implant of Tenofovir Alafenamide Fumarate Causes Local Inflammation and Tissue Necrosis in Rabbits and Macaques. Antimicrob. Agents Chemother. 2020, 64, e01893-19. [Google Scholar] [CrossRef] [PubMed]
- Gatto, G.J.; Krovi, A.; Li, L.; Massud, I.; Holder, A.; Gary, J.; Mills, P.; Mitchell, J.; Luecke, E.; Demkovich, Z.R.; et al. Comparative Pharmacokinetics and Local Tolerance of Tenofovir Alafenamide (TAF) From Subcutaneous Implant in Rabbits, Dogs, and Macaques. Front. Pharmacol. 2022, 13, 923954. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.J.; Benn, P.D. Postexposure prophylaxis for HIV following sexual exposure. Curr. Opin. HIV AIDS 2010, 5, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Follis, K.E.; Sabo, A.; Beck, T.W.; Grant, R.F.; Bischofberger, N.; Benveniste, R.E.; Black, R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 1995, 270, 1197–1199. [Google Scholar] [CrossRef]
- Otten, R.A.; Smith, D.K.; Adams, D.R.; Pullium, J.K.; Jackson, E.; Kim, C.N.; Jaffe, H.; Janssen, R.; Butera, S.; Folks, T.M. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J. Virol. 2000, 74, 9771–9775. [Google Scholar] [CrossRef]
- Ayieko, J.; Petersen, M.L.; Kabami, J.; Mwangwa, F.; Opel, F.; Nyabuti, M.; Charlebois, E.D.; Peng, J.; Koss, C.A.; Balzer, L.B.; et al. Uptake and outcomes of a novel community-based HIV post-exposure prophylaxis (PEP) programme in rural Kenya and Uganda. J. Int. AIDS Soc. 2021, 24, e25670. [Google Scholar] [CrossRef]
- Isah, A.; Igboeli, N.U.; Dim, O.F.; Ekwuofu, A.A. HIV infections averted at PEPFAR-APIN clinics in Nigeria: A ten-year retrospective evaluation of the clinical outcomes of post-exposure prophylaxis services. Afr. J. AIDS Res. AJAR 2023, 22, 46–53. [Google Scholar] [CrossRef]
- Smith, D.K.; Grohskopf, L.A.; Black, R.J.; Auerbach, J.D.; Veronese, F.; Struble, K.A.; Cheever, L.; Johnson, M.; Paxton, L.A.; Onorato, I.M.; et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: Recommendations from the U.S. Department of Health and Human Services. MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2005, 54, 1–20. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Update: Provisional Public Health Service recommendations for chemoprophylaxis after occupational exposure to HIV. MMWR. Morb. Mortal. Wkly. Rep. 1996, 45, 468–480. [Google Scholar]
- Announcement: Updated Guidelines for Antiretroviral Postexposure Prophylaxis after Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 458. [CrossRef] [PubMed]
- Cresswell, F.; Asanati, K.; Bhagani, S.; Boffito, M.; Delpech, V.; Ellis, J.; Fox, J.; Furness, L.; Kingston, M.; Mansouri, M.; et al. UK guideline for the use of HIV post-exposure prophylaxis 2021. HIV Med. 2022, 23, 494–545. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for HIV Post-Exposure Prophylaxis. Available online: https://iris.who.int/bitstream/handle/10665/378221/9789240095137-eng.pdf?sequence=1 (accessed on 10 April 2025).
- Eaton, L.A.; Kalichman, S. Risk compensation in HIV prevention: Implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr. HIV/AIDS Rep. 2007, 4, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.E.; Neilands, T.B.; Krone, M.R.; Katz, M.H.; Franses, K.; Grant, R.M.; Busch, M.P.; Hecht, F.M.; Shacklett, B.L.; Kahn, J.O.; et al. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin. Infect. Dis. 2005, 41, 1507–1513. [Google Scholar] [CrossRef]
- Kahn, J.O.; Martin, J.N.; Roland, M.E.; Bamberger, J.D.; Chesney, M.; Chambers, D.; Franses, K.; Coates, T.J.; Katz, M.H. Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: The San Francisco PEP Study. J. Infect. Dis. 2001, 183, 707–714. [Google Scholar] [CrossRef]
- Winston, A.; McAllister, J.; Amin, J.; Cooper, D.A.; Carr, A. The use of a triple nucleoside-nucleotide regimen for nonoccupational HIV post-exposure prophylaxis. HIV Med. 2005, 6, 191–197. [Google Scholar] [CrossRef]
- Mayer, K.H.; Mimiaga, M.J.; Cohen, D.; Grasso, C.; Bill, R.; Van Derwarker, R.; Fisher, A. Tenofovir DF plus lamivudine or emtricitabine for nonoccupational postexposure prophylaxis (NPEP) in a Boston Community Health Center. J. Acquir. Immune Defic. Syndr. (1999) 2008, 47, 494–499. [Google Scholar] [CrossRef]
- Tosini, W.; Muller, P.; Prazuck, T.; Benabdelmoumen, G.; Peyrouse, E.; Christian, B.; Quertainmont, Y.; Bouvet, E.; Rabaud, C. Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation. AIDS 2010, 24, 2375–2380. [Google Scholar] [CrossRef]
- Diaz-Brito, V.; León, A.; Knobel, H.; Peraire, J.; Domingo, P.; Clotet, B.; Dalmau, D.; Cruceta, A.; Arnaiz, J.A.; Gatell, J.M.; et al. Post-exposure prophylaxis for HIV infection: A clinical trial comparing lopinavir/ritonavir versus atazanavir each with zidovudine/lamivudine. Antivir. Ther. 2012, 17, 337–346. [Google Scholar] [CrossRef]
- McAllister, J.; Read, P.; McNulty, A.; Tong, W.W.; Ingersoll, A.; Carr, A. Raltegravir-emtricitabine-tenofovir as HIV nonoccupational post-exposure prophylaxis in men who have sex with men: Safety, tolerability and adherence. HIV Med. 2014, 15, 13–22. [Google Scholar] [CrossRef]
- Leal, L.; León, A.; Torres, B.; Inciarte, A.; Lucero, C.; Mallolas, J.; Laguno, M.; Martínez-Rebollar, M.; González-Cordón, A.; Manzardo, C.; et al. A randomized clinical trial comparing ritonavir-boosted lopinavir versus raltegravir each with tenofovir plus emtricitabine for post-exposure prophylaxis for HIV infection. J. Antimicrob. Chemother. 2016, 71, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.; León, A.; Torres, B.; Inciarte, A.; Lucero, C.; Mallolas, J.; Laguno, M.; Martínez-Rebollar, M.; González-Cordón, A.; Manzardo, C.; et al. A randomized clinical trial comparing ritonavir-boosted lopinavir versus maraviroc each with tenofovir plus emtricitabine for post-exposure prophylaxis for HIV infection. J. Antimicrob. Chemother. 2016, 71, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Fätkenheuer, G.; Jessen, H.; Stoehr, A.; Jung, N.; Jessen, A.B.; Kümmerle, T.; Berger, M.; Bogner, J.R.; Spinner, C.D.; Stephan, C.; et al. PEPDar: A randomized prospective noninferiority study of ritonavir-boosted darunavir for HIV post-exposure prophylaxis. HIV Med. 2016, 17, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Valin, N.; Fonquernie, L.; Daguenel, A.; Campa, P.; Anthony, T.; Guiguet, M.; Girard, P.M.; Meyohas, M.C. Evaluation of tolerability with the co-formulation elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate for post-HIV exposure prophylaxis. BMC Infect. Dis. 2016, 16, 718. [Google Scholar] [CrossRef]
- Milinkovic, A.; Benn, P.; Arenas-Pinto, A.; Brima, N.; Copas, A.; Clarke, A.; Fisher, M.; Schembri, G.; Hawkins, D.; Williams, A.; et al. Randomized controlled trial of the tolerability and completion of maraviroc compared with Kaletra® in combination with Truvada® for HIV post-exposure prophylaxis (MiPEP Trial). J. Antimicrob. Chemother. 2017, 72, 1760–1768. [Google Scholar] [CrossRef]
- Chauveau, M.; Billaud, E.; Bonnet, B.; Merrien, D.; Hitoto, H.; Bouchez, S.; Michau, C.; Hall, N.; Perez, L.; Sécher, S.; et al. Tenofovir DF/emtricitabine/rilpivirine as HIV post-exposure prophylaxis: Results from a multicentre prospective study. J. Antimicrob. Chemother. 2019, 74, 1021–1027. [Google Scholar] [CrossRef]
- Nie, J.; Sun, F.; He, X.; Liu, J.; Wang, M.; Li, C.; Gu, S.; Chen, Z.; Li, Y.; Chen, Y. Tolerability and Adherence of Antiretroviral Regimens Containing Long-Acting Fusion Inhibitor Albuvirtide for HIV Post-Exposure Prophylaxis: A Cohort Study in China. Infect. Dis. Ther. 2021, 10, 2611–2623. [Google Scholar] [CrossRef]
- Liu, A.; Xin, R.; Zhang, H.; Dai, L.; Wu, R.E.; Wang, X.; Li, A.; Hua, W.; Li, J.; Shao, Y.; et al. An open-label evaluation of safety and tolerability of coformulated bictegravir/emtricitabine/tenofovir alafenamide for post-exposure prophylaxis following potential exposure to human immunodeficiency virus-1. Chin. Med. J. 2022, 135, 2725–2729. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; van Lunzen, J.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA 2016, 316, 171–181. [Google Scholar] [CrossRef] [PubMed]
- LeMessurier, J.; Traversy, G.; Varsaneux, O.; Weekes, M.; Avey, M.T.; Niragira, O.; Gervais, R.; Guyatt, G.; Rodin, R. Risk of sexual transmission of human immunodeficiency virus with antiretroviral therapy, suppressed viral load and condom use: A systematic review. CMAJ Can. Med. Assoc. J. = J. De L’association Medicale Can. 2018, 190, E1350–E1360. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Mhandire, D.; Dandara, C.; Kyei, G.B. Promoting Undetectable Equals Untransmittable in Sub-Saharan Africa: Implication for Clinical Practice and ART Adherence. Int. J. Environ. Res. Public Health 2020, 17, 6163. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Available online: https://iris.who.int/bitstream/handle/10665/186275/9789241509565_eng.pdf?sequence=1 (accessed on 10 April 2025).
- Metzger, V.T.; Lloyd-Smith, J.O.; Weinberger, L.S. Autonomous targeting of infectious superspreaders using engineered transmissible therapies. PLoS Comput. Biol. 2011, 7, e1002015. [Google Scholar] [CrossRef][Green Version]
- Pitchai, F.N.N.; Tanner, E.J.; Khetan, N.; Vasen, G.; Levrel, C.; Kumar, A.J.; Pandey, S.; Ordonez, T.; Barnette, P.; Spencer, D.; et al. Engineered deletions of HIV replicate conditionally to reduce disease in nonhuman primates. Science 2024, 385, eadn5866. [Google Scholar] [CrossRef]
- Viguerie, A.; Gopalappa, C.; Lyles, C.M.; Farnham, P.G. The effects of HIV self-testing on HIV incidence and awareness of status among men who have sex with men in the United States: Insights from a novel compartmental model. Epidemics 2024, 49, 100796. [Google Scholar] [CrossRef]
- Booton, R.D.; Ong, J.J.; Lee, A.; Liu, A.; Huang, W.; Wei, C.; Tang, W.; Ma, W.; Vickerman, P.; Tucker, J.D.; et al. Modelling the impact of an HIV testing intervention on HIV transmission among men who have sex with men in China. HIV Med. 2021, 22, 467–477. [Google Scholar] [CrossRef]
- WHO. Mother-to-Child Transmission of HIV. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/prevention/mother-to-child-transmission-of-hiv (accessed on 10 April 2025).
- Kesho Bora Study, G.; de Vincenzi, I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): A randomised controlled trial. Lancet. Infect. Dis. 2011, 11, 171–180. [Google Scholar] [CrossRef]
- European Mode of Delivery, C. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: A randomised clinical trial. Lancet 1999, 353, 1035–1039. [Google Scholar] [CrossRef]
- Hirbod, T.; Bailey, R.C.; Agot, K.; Moses, S.; Ndinya-Achola, J.; Murugu, R.; Andersson, J.; Nilsson, J.; Broliden, K. Abundant expression of HIV target cells and C-type lectin receptors in the foreskin tissue of young Kenyan men. Am. J. Pathol. 2010, 176, 2798–2805. [Google Scholar] [CrossRef]
- Galiwango, R.M.; Yegorov, S.; Joag, V.; Prodger, J.; Shahabi, K.; Huibner, S.; Muyanja, E.; Kabuubi, B.R.; Namuniina, A.; Nalutaaya, A.; et al. Characterization of CD4(+) T cell subsets and HIV susceptibility in the inner and outer foreskin of Ugandan men. Am. J. Reprod. Immunol. 2019, 82, e13143. [Google Scholar] [CrossRef] [PubMed]
- Galiwango, R.M.; Park, D.E.; Huibner, S.; Onos, A.; Aziz, M.; Roach, K.; Anok, A.; Nnamutete, J.; Isabirye, Y.; Wasswa, J.B.; et al. Immune milieu and microbiome of the distal urethra in Ugandan men: Impact of penile circumcision and implications for HIV susceptibility. Microbiome 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Hungate, B.A.; Tobian, A.A.; Serwadda, D.; Ravel, J.; Lester, R.; Kigozi, G.; Aziz, M.; Galiwango, R.M.; Nalugoda, F.; et al. Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. mBio 2013, 4, e00076. [Google Scholar] [CrossRef] [PubMed]
- Tobian, A.A.; Serwadda, D.; Quinn, T.C.; Kigozi, G.; Gravitt, P.E.; Laeyendecker, O.; Charvat, B.; Ssempijja, V.; Riedesel, M.; Oliver, A.E.; et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N. Engl. J. Med. 2009, 360, 1298–1309. [Google Scholar] [CrossRef]
- Tobian, A.A.; Gray, R.H.; Quinn, T.C. Male circumcision for the prevention of acquisition and transmission of sexually transmitted infections: The case for neonatal circumcision. Arch. Pediatr. Adolesc. Med. 2010, 164, 78–84. [Google Scholar] [CrossRef]
- UNAIDs. UNAIDS Data 2021. Available online: https://www.unaids.org/en/resources/documents/2021/2021_unaids_data (accessed on 3 May 2025).
- Gao, Y.; Zhan, Y.; Sun, Y.; Zheng, W.; Zhang, W.; Fu, L.; Guo, Z.; Lin, Y.F.; Li, Y.; Zheng, L.; et al. Efficacy of Voluntary Medical Male Circumcision to Prevent HIV Infection Among Men Who Have Sex With Men: A Randomized Controlled Trial. Ann. Intern. Med. 2024, 177, 719–728. [Google Scholar] [CrossRef]
- Yuan, T.; Fitzpatrick, T.; Ko, N.Y.; Cai, Y.; Chen, Y.; Zhao, J.; Li, L.; Xu, J.; Gu, J.; Li, J.; et al. Circumcision to prevent HIV and other sexually transmitted infections in men who have sex with men: A systematic review and meta-analysis of global data. Lancet. Glob. Health 2019, 7, e436–e447. [Google Scholar] [CrossRef]
- Hütter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müssig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abdul-Jawad, S.; McCoy, L.E.; Mok, H.P.; Peppa, D.; Salgado, M.; Martinez-Picado, J.; Nijhuis, M.; Wensing, A.M.J.; Lee, H.; et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019, 568, 244–248. [Google Scholar] [CrossRef]
- Hsu, J.; Van Besien, K.; Glesby, M.J.; Pahwa, S.; Coletti, A.; Warshaw, M.G.; Petz, L.; Moore, T.B.; Chen, Y.H.; Pallikkuth, S.; et al. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 2023, 186, 1115–1126.e8. [Google Scholar] [CrossRef]
- Jensen, B.O.; Knops, E.; Cords, L.; Lübke, N.; Salgado, M.; Busman-Sahay, K.; Estes, J.D.; Huyveneers, L.E.P.; Perdomo-Celis, F.; Wittner, M.; et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 2023, 29, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Dickter, J.K.; Aribi, A.; Cardoso, A.A.; Gianella, S.; Gendzekhadze, K.; Li, S.; Feng, Y.; Chaillon, A.; Laird, G.M.; Browning, D.L.; et al. HIV-1 Remission after Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2024, 390, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, D.; Wang, S. The CCR5-Delta32 Genetic Polymorphism and HIV-1 Infection Susceptibility: A Meta-analysis. Open Med. 2018, 13, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, H.; Gao, Y.; Chen, Z.; Xie, L.; Liu, Y.; Liu, Y.; Wang, X.; Li, H.; Lai, W.; et al. CRISPR/Cas9-Mediated CCR5 Ablation in Human Hematopoietic Stem/Progenitor Cells Confers HIV-1 Resistance In Vivo. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 1782–1789. [Google Scholar] [CrossRef]
- Tebas, P.; Stein, D.; Tang, W.W.; Frank, I.; Wang, S.Q.; Lee, G.; Spratt, S.K.; Surosky, R.T.; Giedlin, M.A.; Nichol, G.; et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 2014, 370, 901–910. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Liu, Y.; Xie, L.; Su, B.; Mou, D.; Wang, L.; Liu, T.; Wang, X.; Zhang, B.; et al. CRISPR-Edited Stem Cells in a Patient with HIV and Acute Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 1240–1247. [Google Scholar] [CrossRef]
- Kaminski, R.; Chen, Y.; Fischer, T.; Tedaldi, E.; Napoli, A.; Zhang, Y.; Karn, J.; Hu, W.; Khalili, K. Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing. Sci. Rep. 2016, 6, 22555. [Google Scholar] [CrossRef]
- Mancuso, P.; Chen, C.; Kaminski, R.; Gordon, J.; Liao, S.; Robinson, J.A.; Smith, M.D.; Liu, H.; Sariyer, I.K.; Sariyer, R.; et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat. Commun. 2020, 11, 6065. [Google Scholar] [CrossRef]
- Johnson, N.M.; Alvarado, A.F.; Moffatt, T.N.; Edavettal, J.M.; Swaminathan, T.A.; Braun, S.E. HIV-based lentiviral vectors: Origin and sequence differences. Mol. Ther. Methods Clin. Dev. 2021, 21, 451–465. [Google Scholar] [CrossRef]
- Munis, A.M. Gene Therapy Applications of Non-Human Lentiviral Vectors. Viruses 2020, 12, 1106. [Google Scholar] [CrossRef]
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum. Mol. Genet. 2014, 23, R40–R46. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, N.; Berkhout, B.; Das, A.T. CRISPR-Cas9 Can Inhibit HIV-1 Replication but NHEJ Repair Facilitates Virus Escape. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- Leggat, D.J.; Cohen, K.W.; Willis, J.R.; Fulp, W.J.; deCamp, A.C.; Kalyuzhniy, O.; Cottrell, C.A.; Menis, S.; Finak, G.; Ballweber-Fleming, L.; et al. Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science 2022, 378, eadd6502. [Google Scholar] [CrossRef]
- Haynes, B.F.; Wiehe, K.; Borrow, P.; Saunders, K.O.; Korber, B.; Wagh, K.; McMichael, A.J.; Kelsoe, G.; Hahn, B.H.; Alt, F.; et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol. 2023, 23, 142–158. [Google Scholar] [CrossRef]
- Sun, C.; Zuo, T.; Wen, Z. B cell engineering in vivo: Accelerating induction of broadly neutralizing antibodies against HIV-1 infection. Signal Transduct. Target. Ther. 2023, 8, 13. [Google Scholar] [CrossRef]
- McFarland, E.J.; Cunningham, C.K.; Muresan, P.; Capparelli, E.V.; Perlowski, C.; Morgan, P.; Smith, B.; Hazra, R.; Purdue, L.; Harding, P.A.; et al. Safety, Tolerability, and Pharmacokinetics of a Long-Acting Broadly Neutralizing Human Immunodeficiency Virus Type 1 (HIV-1) Monoclonal Antibody VRC01LS in HIV-1-Exposed Newborn Infants. J. Infect. Dis. 2021, 224, 1916–1924. [Google Scholar] [CrossRef]
- Cunningham, C.K.; McFarland, E.J.; Morrison, R.L.; Capparelli, E.V.; Safrit, J.T.; Mofenson, L.M.; Mathieson, B.; Valentine, M.E.; Perlowski, C.; Smith, B.; et al. Safety, Tolerability, and Pharmacokinetics of the Broadly Neutralizing Human Immunodeficiency Virus (HIV)-1 Monoclonal Antibody VRC01 in HIV-Exposed Newborn Infants. J. Infect. Dis. 2020, 222, 628–636. [Google Scholar] [CrossRef]
- Corey, L.; Gilbert, P.B.; Juraska, M.; Montefiori, D.C.; Morris, L.; Karuna, S.T.; Edupuganti, S.; Mgodi, N.M.; deCamp, A.C.; Rudnicki, E.; et al. Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N. Engl. J. Med. 2021, 384, 1003–1014. [Google Scholar] [CrossRef]
- Bar, K.J.; Sneller, M.C.; Harrison, L.J.; Justement, J.S.; Overton, E.T.; Petrone, M.E.; Salantes, D.B.; Seamon, C.A.; Scheinfeld, B.; Kwan, R.W.; et al. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N. Engl. J. Med. 2016, 375, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Davis-Gardner, M.E.; Alfant, B.; Weber, J.A.; Gardner, M.R.; Farzan, M. A Bispecific Antibody That Simultaneously Recognizes the V2- and V3-Glycan Epitopes of the HIV-1 Envelope Glycoprotein Is Broader and More Potent than Its Parental Antibodies. mBio 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pegu, A.; Rao, E.; Doria-Rose, N.; Beninga, J.; McKee, K.; Lord, D.M.; Wei, R.R.; Deng, G.; Louder, M.; et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 2017, 358, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Nishimura, Y.; Pegu, A.; Nason, M.C.; Klein, F.; Gazumyan, A.; Golijanin, J.; Buckler-White, A.; Sadjadpour, R.; Wang, K.; et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016, 533, 105–109. [Google Scholar] [CrossRef]
- Sok, D.; Burton, D.R. Recent progress in broadly neutralizing antibodies to HIV. Nat. Immunol. 2018, 19, 1179–1188. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Houser, K.V.; Doria-Rose, N.A.; Chen, G.L.; Rothwell, R.S.S.; Berkowitz, N.; Costner, P.; Holman, L.A.; Gordon, I.J.; Hendel, C.S.; et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: A phase 1 dose-escalation clinical trial. Lancet HIV 2019, 6, e667–e679. [Google Scholar] [CrossRef]
- Balazs, A.B.; Ouyang, Y.; Hong, C.M.; Chen, J.; Nguyen, S.M.; Rao, D.S.; An, D.S.; Baltimore, D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat. Med. 2014, 20, 296–300. [Google Scholar] [CrossRef]
- Gardner, M.R.; Fellinger, C.H.; Kattenhorn, L.M.; Davis-Gardner, M.E.; Weber, J.A.; Alfant, B.; Zhou, A.S.; Prasad, N.R.; Kondur, H.R.; Newton, W.A.; et al. AAV-delivered eCD4-Ig protects rhesus macaques from high-dose SIVmac239 challenges. Sci. Transl. Med. 2019, 11, aau5409. [Google Scholar] [CrossRef]
- Gardner, M.R.; Kattenhorn, L.M.; Kondur, H.R.; von Schaewen, M.; Dorfman, T.; Chiang, J.J.; Haworth, K.G.; Decker, J.M.; Alpert, M.D.; Bailey, C.C.; et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 2015, 519, 87–91. [Google Scholar] [CrossRef]
| Category | Applicable Population | Prophylactic Efficacy | Advantage | Disadvantage | ||
|---|---|---|---|---|---|---|
| Currently available biomedical interventions | PrEP | Oral PrEP | Uninfected people who are persistently at high risk of HIV infection | Sexual transmission: 90–99% [78,79] Drug injection transmission: 74% [80] | 1. Broad range of application 2. High efficacy for HIV prevention | 1. Highly affected by adherence, stigmatization issues 2. Long-term medication, drug side effects |
| Dapivirine ring | Uninfected women at high risk of HIV infection | Vaginal sexual transmission: 56–63% [81,82] | 1. Female-led interventions 2. Long duration of protection 3. No need for oral medication, those females unable or unwilling to use oral medication can use this mode of prevention 4. Localized medication, reducing systemic side effects of medications 5. Strong concealment | 1. Limited efficacy for HIV prevention 2. Limited to vaginal route of HIV transmission 3. Might cause local discomfort and inflammation | ||
| Injectable PrEP | Uninfected people who are persistently at high risk of HIV infection | CAB-LA: 69–88% [83,84] Lenacapavir: 96–100% [85,86] |
1. Long duration of protection 2. High efficacy for HIV prevention |
1. Cost-related treatment barriers 2. Limited treatment availability 3. Drug resistance 4. LEVI syndrome | ||
| PEP | Uninfected people who have been exposed to HIV or accidentally exposed | Occupational and nonoccupational exposure: 80–90% [87] (up to 99% for use within 2 h) | 1. Fast-acting, can be used for emergency prophylaxis 2. Short-term use and convenient | 1. Highly affected by initiation time and adherence 2. Drug side effects 3. Not applicable to people with persistent high risk | ||
| TasP and U=U | All PLWH | Nearly 100% [88] (near elimination of risk of HIV transmission) | 1. High prevention rate 2. Applicable to all people living with HIV 3. Reduce HIV stigma | 1. High compliance requirements 2. Long-term medication, drug side effects | ||
| Prevention of vertical transmission | HIV-positive women planning to become pregnant, pregnant, and breastfeeding | 99% [89] (risk of mother-to-child transmission reduced to <1%) | 1. High prevention rate 2. Improve the health of pregnant women and prevent newborns from being infected with HIV | 1. Highly affected by adherence and stigmatization issues 2. Poor accessibility in underdeveloped regions where the need is greatest | ||
| VMMC # | Adolescent and adult males | Reduce the risk of heterosexual transmission of HIV infection by 60% [90,91,92] | 1. No need to use drugs 2. Long-term protection without dependence on adherence 3. Reduce the risk of other sexually transmitted infections | 1. Limited preventive effect, need to be combined with other measures 2. Ineffective in MSM and female partners 3. Surgical risk | ||
| Potentially available biomedical interventions in the future | Gene editing technology | – | – |
1. Long-term protection without dependence on adherence 2. Removal of latent reservoirs for functional cure |
1. Off-target risk 2. CCR5 receptor deficiency leads to susceptibility to other viral infections | |
| Passive infusion of broadly neutralizing antibody | – | – |
1. Highly effective and broad-spectrum 2. Long-term protection without dependence on adherence 3. Both preventive and therapeutic |
1. High production and preservation costs 2. Unable to remove latent viral reservoirs 3. Repeated infusions can trigger anti-drug antibody (ADA) responses | ||
| Trial | Timeline | Location | Population | Sample Size | Regimen | Outcome |
|---|---|---|---|---|---|---|
| iPrEx [78] | 2007–2010 | United States, Brazil, Ecuador, Peru, South Africa, Thailand | MSM and transgender women | 2499 | Daily oral TDF/FTC | 36 infections in the TDF/FTC group, and 64 infections in the placebo group. Overall 44% reduction; up to 92% reduction with high adherence |
| TDF2 [102] | 2007–2010 | Botswana | Heterosexual men and women | 1219 | Daily oral TDF/FTC | 9 infections in the TDF-FTC group and 24 infections in the placebo group. 62% reduction |
| Bangkok Tenofovir [80] | 2005–2010 | Bangkok, Thailand | People who use injection drugs | 2413 | Daily oral TDF | 17 infections in the TDF group (0.35 per 100 person-years) and 33 infections in the placebo group (0.68 per 100 person-years). 44% reduction |
| Partners PrEP [79] | 2008–2011 | Kenya, Uganda | Heterosexual serodiscordant couples | 4758 | Daily oral TDF/FTC or TDF alone | 17 infections in the TDF group (0.65 per 100 person-years), 13 infections in the TDF/FTC group (0.50 per 100 person-years), and 52 in the placebo group (incidence, 1.99 per 100 person-years). 75% reduction with TDF/FTC; 67% reduction with TDF alone |
| PROUD [103] | 2012–2014 | United Kingdom | MSM | 544 | Daily oral TDF/FTC | 3 infections in the TDF/FTC group (1.2 per 100 person-years) and 20 infections in the placebo group (9.0 per 100 person-years). 86% reduction |
| IPERGAY [93] | 2012–2014 | France, Canada | MSM | 400 | On-demand TDF/FTC | 2 infections in the TDF-FTC group (0.91 per 100 person-years) and 14 infections in the placebo group (6.60 per 100 person-years). 86% reduction |
| DISCOVER [104] | 2016–2019 | United States, Canada, Europe | MSM and transgender women | 5387 | Daily oral TAF/FTC vs. TDF/FTC | 7 infections in the TAF/FTC group (0.16 infections per 100 person-years) and 15 infections in the TDF/FTC group (0.34 infections per 100 person-years). TAF/FTC was non-inferior to TDF/FTC |
| HPTN 083 [84] | 2016–2020 | United States, Latin America, Asia, Africa | MSM and transgender women | 4570 | Long-acting intramuscular cabotegravir (CAB-LA) every month vs. daily oral TDF/FTC | 13 infections in the CAB-LA group (0.41 per 100 person-years) and 39 in the TDF-FTC group (1.22 per 100 person-years). 66% more effective than daily TDF/FTC |
| HPTN 084 [83] | 2017–2021 | Africa | Cisgender women | 3224 | Intramuscular CAB-LA every month vs. daily oral TDF/FTC | 4 infections in the CAB-LA group (0.2 per 100 person-years) and 36 infections in the TDF-FTC group (1.85 per 100 person-years). 89% reduction with CAB-LA compared to TDF/FTC |
| PURPOSE 1 [85] | 2021–2024 | South Africa, Uganda | Cisgender adolescent girls and young women | 5338 | subcutaneous lenacapavir every 6 months vs. daily oral F/TAF vs. daily oral F/TDF | 0 infections in the lenacapavir group (0 per 100 person-years), 39 infections among in the F/TAF group (2.02 per 100 person-years), and 16 infections in the F/TDF group (1.69 per 100 person-years). Significantly lower than other two groups (100% efficacy in preventing HIV infections) |
| PURPOSE 2 [86] | 2021–2024 | United States, Argentina, Brazil, Mexico, Peru, Puerto Rico, South Africa, Thailand | Cisgender men, transgender women, transgender men, and gender-nonbinary persons | 3265 | subcutaneous lenacapavir every 6 months vs. daily oral F/TAF vs. daily oral F/TDF | 2 infections in the lenacapavir group (0.10 per 100 person-years) and in 9 infections in the F/TDF group (0.93 per 100 person-years). Significantly lower than other two groups (reduced overall risk of infection by 96%) |
| Trial | Location | Exposure | Sample Size | Regimen | Protection Rates | Adverse Reaction Rate |
|---|---|---|---|---|---|---|
| Kahn et al. [129] (2001) | United States | Nonoccupational | 401 | ZDV/3TC | 78% | Nausea (52%), fatigue (44%), headache (24%), diarrhea (15%), and anorexia (12%) |
| Winston et al. [130] (2005) | Australia | Nonoccupational | 385 | ZDV/3TC vs. ZDV/3TC/NFV vs. TDF/3TC/d4T | 75% vs. 68% vs. 85% | Transaminase elevation (11% vs. 9% vs. 19%), diarrhea (6% vs. 51% vs. 25%), fatigue (39% vs. 32% vs. 30%), headache (17% vs. 12% vs. 1%), and nausea (81% vs. 42% vs. 23%) |
| Mayer et al. [131] (2008) | United States | Nonoccupational | 371 | TDF/FTC vs. TDF/3TC vs. ZDV/3TC | 72% vs. 87% vs. 42% | Diarrhea (47% vs. 31% vs. 10%), fatigue (30% vs. 28% vs. 39%), nausea (22% vs. 19% vs. 56%), headache (22% vs. 19% vs. 25%), and dizziness (20% vs. 16% vs. 5%) |
| Tosini et al. [132] (2010) | France | Nonoccupational and occupational | 249 | TDF/FTC/LPV-r | 67% | Diarrhea (80%), asthenia (66%), and abdominal pain (44%) |
| Diaz-Brito et al. [133] (2012) | Spain | Nonoccupational | 200 | LPV-r vs. ATV | 64% vs. 64% | Gastrointestinal (70% vs. 41%), neuropsychiatric (11% vs. 16%), asthenia (17% vs. 23%) |
| McAllister et al. [134] (2014) | Australia | Nonoccupational | 120 | RAL/FTC/TDF vs. FTC/TDF | 92% vs. 91% | Fatigue (37% vs. 26%), nausea (24% vs. 18%), abdominal cramps (21% vs. 12%), myalgias (9% vs. 0%) |
| Leal et al. [135] (2016) | Spain | Nonoccupational | 243 | TDF/FTC/LPV-r vs. RAL | 66% vs. 80% | Gastrointestinal (57% vs. 58%), neuropsychiatric (14% vs. 23%), and asthenia (18% vs. 18%) |
| Leal et al. [136] (2016) | Spain | Nonoccupational | 237 | TDF/FTC/LPV-r vs. TDF/FTC/MVC | 56% vs. 68% | Gastrointestinal (56% vs. 58%), neuropsychiatric (15% vs. 20%), and asthenia (19% vs. 18%) |
| Fatkenheuer et al. [137] (2016) | Germany | Nonoccupational and occupational | 305 | DRV-r vs. LPV-r | 94% vs. 90% | Diarrhea (30% vs. 52%), nausea (16% vs. 28%), fatigue (13% vs. 18%), sleep disorder(0% vs. 4%) |
| Valin et al. [138] (2016) | France | Nonoccupational | 234 | FTC/TDF/ELV/COBI | 92% | Fatigue (26%), nausea (25%), diarrhoea (17%), abdominal cramps (16%) |
| Milinkovic et al. [139] (2017) | United Kingdom | Nonoccupational | 213 | TDF/FTC/LPV-r vs. TDF/FTC/MVC | 65% vs. 71% | Nausea or vomiting (39% vs. 30%, diarrhea (74% vs. 19%), fatigue (39% vs. 36%) |
| Chauveau et al. [140] (2019) | France | Nonoccupational and occupational | 158 | TDF/FTC/RPV | 86% | Fatigue (35%), nausea (22%), diarrhea (20%), abdominal cramps (16%), headache (11%) |
| Nie et al. [141] (2021) | China | Nonoccupational and occupational | 297 | ABT/DTG vs. ABT/TDF/3TC vs. DTG/TDF/3TC | 64% vs. 64% vs. 64% | Dizziness (7% vs. 7% vs. 7%), diarrhea (8% vs. 6% vs. 2%), asthenia (5% vs. 4% vs. 5%), and triglycerides increase (4% vs. 2% vs. 7%) |
| Liu et al. [142] (2022) | China | Nonoccupational | 108 | BIC/FTC/TAF | 96% | Creatinine elevation (4%), headache (2%), diarrhea (2%), and nausea (1%) |
| Lacombe et al. (2024) | France | Nonoccupational | 226 | DOR | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Wen, Z.; Shi, M.; Zou, H.; Sun, C. Biomedical Interventions for HIV Prevention and Control: Beyond Vaccination. Viruses 2025, 17, 756. https://doi.org/10.3390/v17060756
Liao Y, Wen Z, Shi M, Zou H, Sun C. Biomedical Interventions for HIV Prevention and Control: Beyond Vaccination. Viruses. 2025; 17(6):756. https://doi.org/10.3390/v17060756
Chicago/Turabian StyleLiao, Yu, Ziyu Wen, Minjuan Shi, Huachun Zou, and Caijun Sun. 2025. "Biomedical Interventions for HIV Prevention and Control: Beyond Vaccination" Viruses 17, no. 6: 756. https://doi.org/10.3390/v17060756
APA StyleLiao, Y., Wen, Z., Shi, M., Zou, H., & Sun, C. (2025). Biomedical Interventions for HIV Prevention and Control: Beyond Vaccination. Viruses, 17(6), 756. https://doi.org/10.3390/v17060756








