Mpox 2022 to 2025 Update: A Comprehensive Review on Its Complications, Transmission, Diagnosis, and Treatment
Abstract
1. Introduction
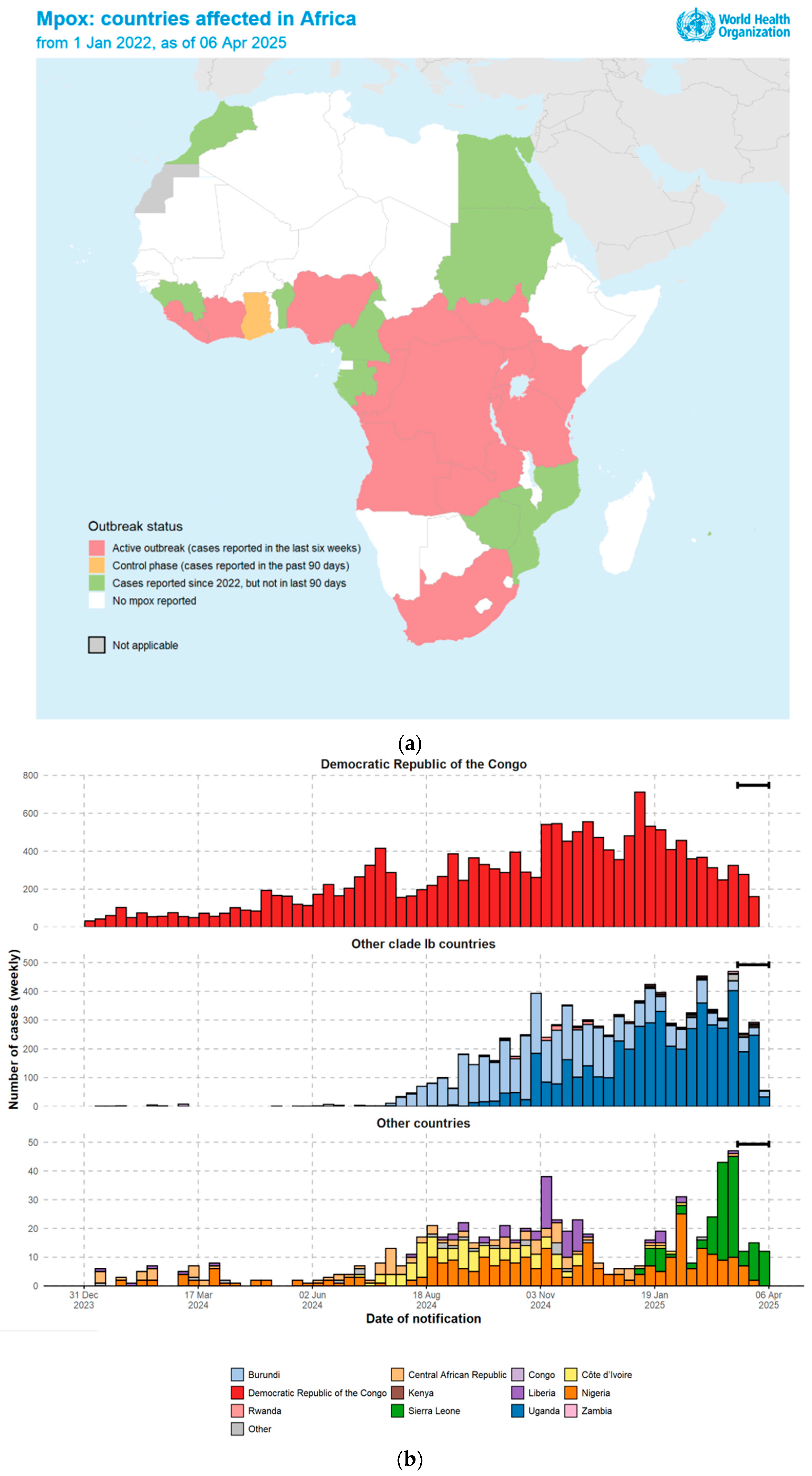
1.1. The 2024 Outbreak
1.2. The 2022 Outbreak
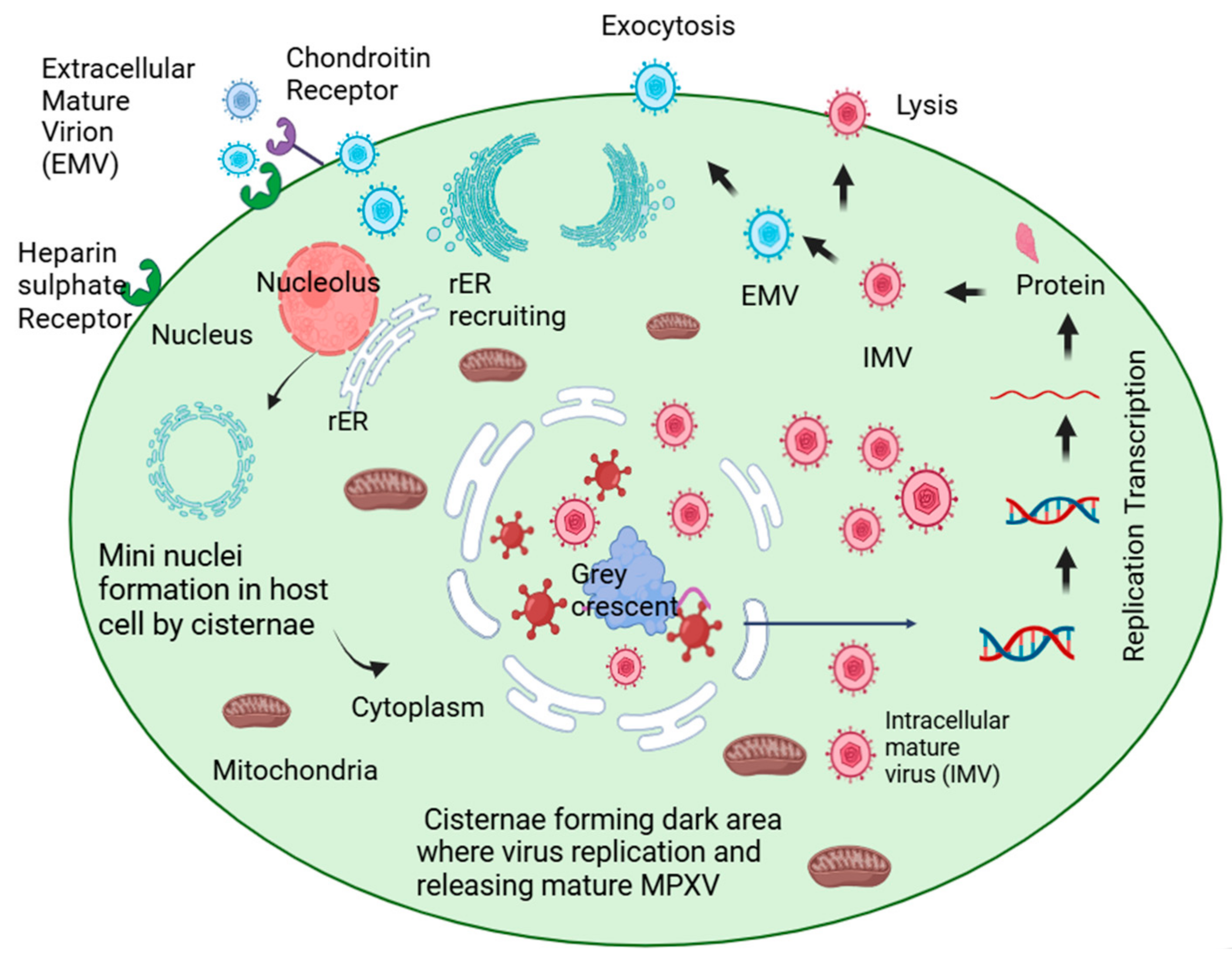
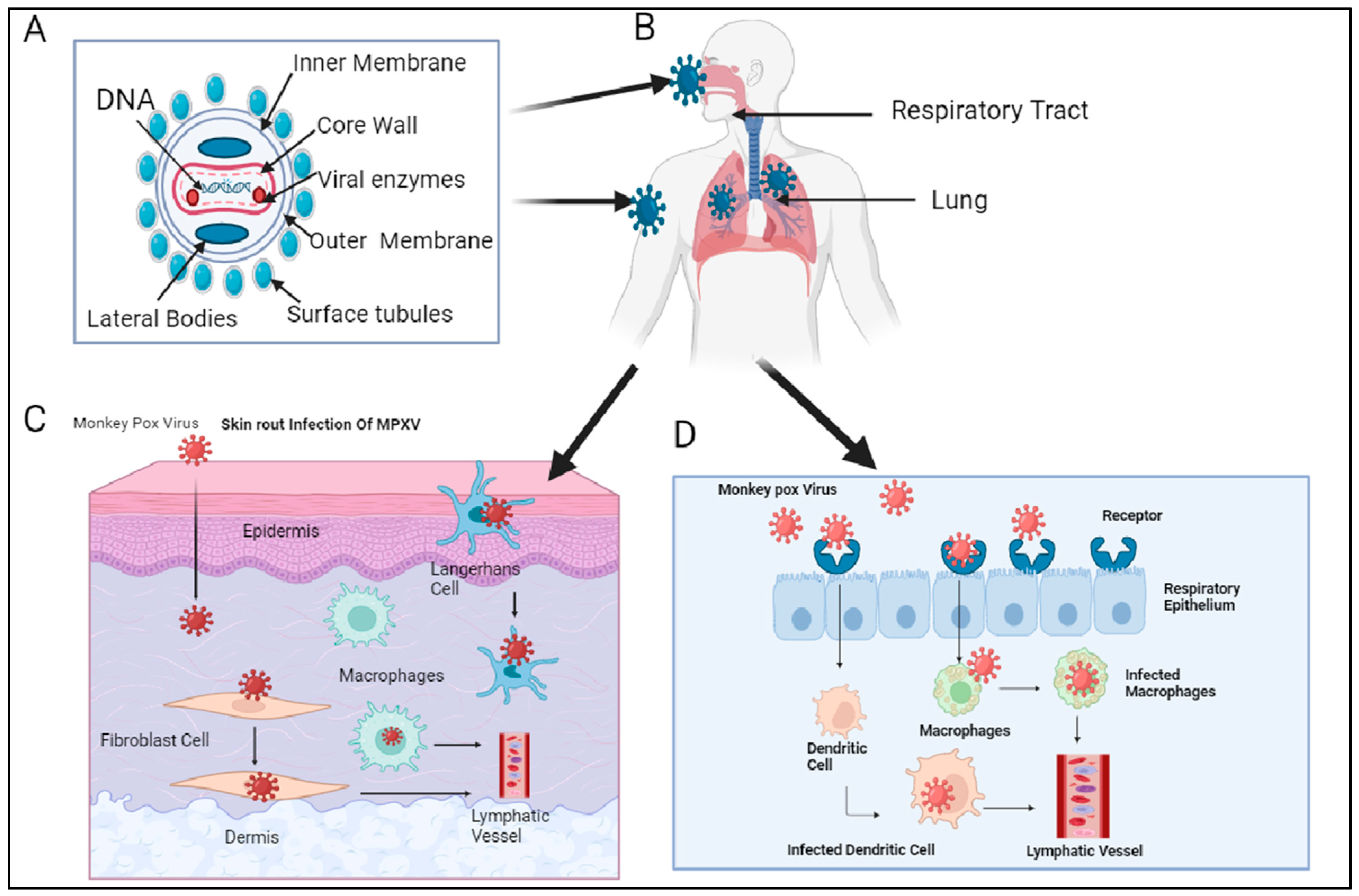
2. Host Reservoirs and Transmission
3. Complications Associated with Mpox
3.1. Neurological and Psychiatric Complications
3.2. Dermatological Complications
3.3. Complications Related to the Coinfection of Mpox Virus and HIV
3.4. Complications Associated with Heart (Myocarditis)
3.5. Hypotension Sepsis Complication
3.6. Ocular Complication
3.7. Fulminant Mpox
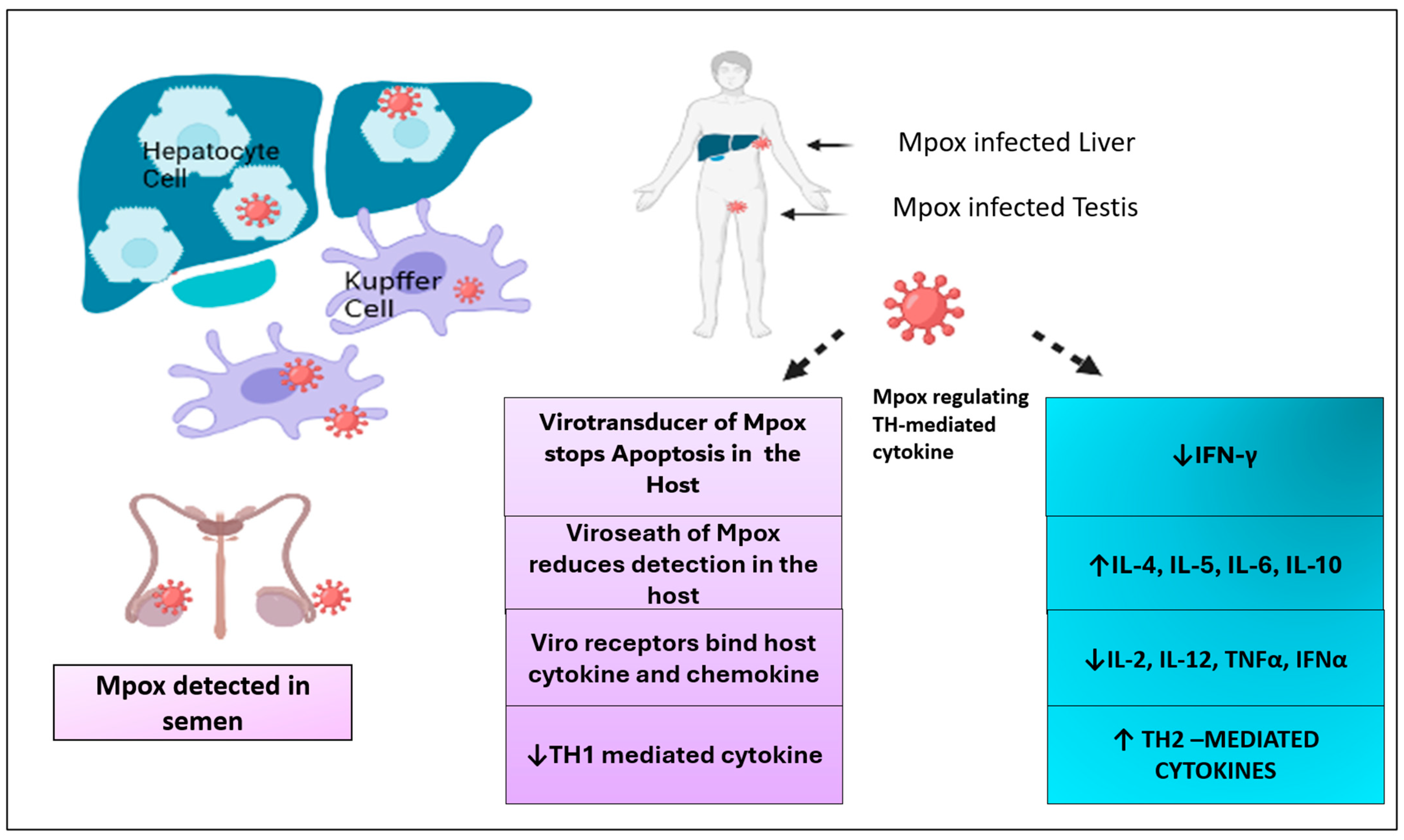
3.8. Complications Associated with Immune System
4. Diagnosis of Mpox
5. Treatment of Mpox
5.1. Vaccine Strategies Against Mpox
5.2. Various Therapies for the Management of Mpox
5.2.1. Tecovirimat
5.2.2. Cidofovir and Brincidofovir
5.2.3. Resveratrol
5.2.4. Plant Metabolite as a Therapeutic Drug
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Multi-Country Outbreak of Mpox, External Situation Report #51—29 April 2025. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report--51---29-april-2025 (accessed on 20 May 2025).
- Brown, K.; Leggat, P.A. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop. Med. Infect. Dis. 2016, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Neglected Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.A.; Mikheev, M.V.; Sisler, J.R.; et al. Human Monkeypox and Smallpox Viruses: Genomic Comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Massung, R.F.; Esposito, J.J. Comparison of the Genome DNA Sequences of Bangladesh-1975 and India-1967 Variola Viruses. Virus Res. 1995, 36, 107–118. [Google Scholar] [CrossRef]
- Hendrickson, R.C.; Wang, C.; Hatcher, E.L.; Lefkowitz, E.J. Orthopoxvirus Genome Evolution: The Role of Gene Loss. Viruses 2010, 2, 1933–1967. [Google Scholar] [CrossRef]
- Witt, A.S.A.; de Trindade, G.S.; de Souza, F.G.; Serafim, M.S.M.; da Costa, A.V.B.; Silva, M.V.F.; de Melo Iani, F.C.; Rodrigues, R.A.L.; Kroon, E.G.; Abrahão, J.S. Ultrastructural Analysis of Monkeypox Virus Replication in Vero Cells. J. Med. Virol. 2023, 95, e28536. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox Virus Isolation from a Semen Sample Collected in the Early Phase of Infection in a Patient with Prolonged Seminal Viral Shedding. Lancet Infect. Dis. 2022, 22, 1267–1269. [Google Scholar] [CrossRef]
- Moss, B. Understanding the Biology of Monkeypox Virus to Prevent Future Outbreaks. Nat. Microbiol. 2024, 9, 1408–1416. [Google Scholar] [CrossRef]
- Mathieu, E.; Spooner, F.; Dattani, S.; Ritchie, H.; Roser, M. Mpox. Our World in Data, 2022. Available online: https://ourworldindata.org/mpox (accessed on 1 May 2022).
- Masirika, L.M.; Udahemuka, J.C.; Schuele, L.; Ndishimye, P.; Otani, S.; Mbiribindi, J.B.; Marekani, J.M.; Mambo, L.M.; Bubala, N.M.; Boter, M.; et al. Ongoing Mpox Outbreak in Kamituga, South Kivu Province, Associated with Monkeypox Virus of a Novel Clade I Sub-Lineage, Democratic Republic of the Congo, 2024. Euro Surveill. 2024, 29, 2400106. [Google Scholar] [CrossRef]
- Preliminary Analysis of Full Genome Sequences of 58 MPXV Clade Ib Cases from Kamituga and Kamanyola, South-Kivu, DRC —MPXV/Evolution. Available online: https://virological.org/t/preliminary-analysis-of-full-genome-sequences-of-58-mpxv-clade-ib-cases-from-kamituga-and-kamanyola-south-kivu-drc/975 (accessed on 10 April 2025).
- Ndembi, N.; Folayan, M.O.; Ngongo, N.; Ntoumi, F.; Ogoina, D.; El Rabbat, M.; Okwo-Bele, J.-M.; Kaseya, J. Mpox Outbreaks in Africa Constitute a Public Health Emergency of Continental Security. Lancet Glob. Health 2024, 12, e1577–e1579. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Nazli Khatib, M.; Datt Sharma, G.; Pratap Singh, M.; Bushi, G.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Ndabashinze, R. Mpox 2024: New Variant, New Challenges, and the Looming Pandemic. Clin. Infect. Pract. 2024, 24, 100394. [Google Scholar] [CrossRef]
- Multi-Country Outbreak of Mpox, External Situation Report#35—12 August 2024. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-35--12-august-2024 (accessed on 10 April 2025).
- One Case of Mpox Clade 1 Reported in Sweden—The Public Health Agency of Sweden. Available online: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/disease-information-about-mpox/one-case-of-mpox-clade-i-reported-in-sweden/ (accessed on 10 April 2025).
- Thailand Confirms Asia’s First Known Case of New Deadlier Mpox Variant. The Guardian. 2024. Available online: https://www.theguardian.com/world/article/2024/aug/23/thailand-mpox-clade-1b-strain-new-variant-case (accessed on 23 August 2023).
- CDC Mpox in the United States and Around the World: Current Situation. Available online: https://www.cdc.gov/mpox/situation-summary/index.html (accessed on 25 February 2025).
- Situation Report: Mpox Multi-Country Outbreak—Region of the Americas—31 January 2025—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/situation-report-mpox-multi-country-outbreak-region-americas-31-january-2025 (accessed on 25 February 2025).
- Mpox. Available online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 25 February 2025).
- Moore, M.J.; Rathish, B.; Zahra, F. Mpox (Monkeypox); StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Monkeypox. World Health Organization (WHO), 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 25 May 2022).
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 Outbreak and the Pathobiology of the Monkeypox Virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- Laurenson-Schafer, H.; Sklenovská, N.; Hoxha, A.; Kerr, S.M.; Ndumbi, P.; Fitzner, J.; Almiron, M.; de Sousa, L.A.; Briand, S.; Cenciarelli, O.; et al. Description of the First Global Outbreak of Mpox: An Analysis of Global Surveillance Data. Lancet Glob. Health 2023, 11, e1012–e1023. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Perez Duque, M.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R.; et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022, 27, 2200424. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Lopardo, G.; Verbanaz, S.; Orduna, T.; Lloveras, S.; Azeñas-Burgoa, J.M.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Barbosa, A.N.; Diaz-Quijano, F.; et al. Latin America: Situation and Preparedness Facing the Multi-Country Human Monkeypox Outbreak. Lancet Reg. Health Am. 2022, 13, 100318. [Google Scholar] [CrossRef] [PubMed]
- The WHO Declaration of Monkeypox as a Global Public Health Emergency|Infectious Diseases|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/2794922 (accessed on 10 February 2024).
- Harris, E. What to Know About Monkeypox. JAMA 2022, 327, 2278–2279. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S.; et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782–785. [Google Scholar] [CrossRef]
- Multi-Country Monkeypox Outbreak in Non-Endemic Countries. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (accessed on 10 February 2025).
- Okyay, R.A. Another Epidemic in the Shadow of COVID 19 Pandemic: A Review of Monkeypox. EJMO 2022, 6, 95–99. [Google Scholar] [CrossRef]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.-J.T. Maternal and Fetal Outcomes Among Pregnant Women With Human Monkeypox Infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020, 71, e210–e214. [Google Scholar] [CrossRef] [PubMed]
- Badenoch, J.B.; Conti, I.; Rengasamy, E.R.; Watson, C.J.; Butler, M.; Hussain, Z.; Carter, B.; Rooney, A.G.; Zandi, M.S.; Lewis, G.; et al. Neurological and Psychiatric Presentations Associated with Human Monkeypox Virus Infection: A Systematic Review and Meta-Analysis. EClinicalMedicine 2022, 52, 101644. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Abdulqadir, S.O.; Hussein, S.H.; Omar, R.M.; Ahmed, N.A.; Essa, R.A.; Dhama, K.; Lorenzo, J.M.; Abdulla, A.Q. The Impact of Monkeypox Outbreak on Mental Health and Counteracting Strategies: A Call to Action. Int. J. Surg. 2022, 106, 106943. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Abdulqadir, S.O.; Omar, R.M.; Abdullah, A.J.; Rahman, H.A.; Hussein, S.H.; Mohammed Amin, H.I.; Chandran, D.; Sharma, A.K.; Dhama, K.; et al. Knowledge, Attitude and Worry in the Kurdistan Region of Iraq during the Mpox (Monkeypox) Outbreak in 2022: An Online Cross-Sectional Study. Vaccines 2023, 11, 610. [Google Scholar] [CrossRef]
- Abaza, H.; Agadi, K.; Anand, A.; Elsaid, M. Clinical Manifestations of Monkeypox. Adv. Exp. Med. Biol. 2023, 1410, 7–11. [Google Scholar] [CrossRef]
- Wang, X.; Lun, W. Skin Manifestation of Human Monkeypox. J. Clin. Med. 2023, 12, 914. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Jiang, R.-M.; Zheng, Y.-J.; Zhou, L.; Feng, L.-Z.; Ma, L.; Xu, B.-P.; Xu, H.-M.; Liu, W.; Xie, Z.-D.; Deng, J.-K.; et al. Diagnosis, Treatment, and Prevention of Monkeypox in Children: An Experts’ Consensus Statement. World J. Pediatr. 2023, 19, 231–242. [Google Scholar] [CrossRef]
- Issa, A.W.; Alkhofash, N.F.; Gopinath, D.; Varma, S.R. Oral Manifestations in Monkeypox: A Scoping Review on Implications for Oral Health. Dent. J. 2023, 11, 132. [Google Scholar] [CrossRef]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and Clinical Characteristics of Confirmed Human Monkeypox Virus Cases in Individuals Attending a Sexual Health Centre in London, UK: An Observational Analysis. Lancet Infect. Dis. 2022, 22, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Acha, J.; Vela-Ganuza, M.; Sarró-Fuente, C.; López-Estebaranz, J.L. Monkeypox: A New Differential Diagnosis When Addressing Genital Ulcer Disease. Br. J. Dermatol. 2022, 187, 1050–1052. [Google Scholar] [CrossRef]
- Prasad, S.; Galvan Casas, C.; Strahan, A.G.; Fuller, L.C.; Peebles, K.; Carugno, A.; Leslie, K.S.; Harp, J.L.; Pumnea, T.; McMahon, D.E.; et al. A Dermatologic Assessment of 101 Mpox (Monkeypox) Cases from 13 Countries during the 2022 Outbreak: Skin Lesion Morphology, Clinical Course, and Scarring. J. Am. Acad. Dermatol. 2023, 88, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, R.M.; Baldo, M.E.; Santana, G.U.; da Costa, G.P.; Palma, L.F.; Campos, L. Phototherapies for the Management of a Large Painful Facial Cutaneous Lesion from Human Monkeypox Infection. Photodiagnosis Photodyn. Ther. 2023, 41, 103276. [Google Scholar] [CrossRef]
- Silva, M.S.T.; dos Santos, D.G.; Coutinho, C.; Ribeiro, M.P.D.; Cardoso, S.W.; Veloso, V.G.; Grinsztejn, B. The First Case of Acute HIV and Monkeypox Coinfection in Latin America. Braz. J. Infect. Dis. 2022, 27, 102736. [Google Scholar] [CrossRef]
- de Sousa, D.; Patrocínio, J.; Frade, J.; Correia, C.; Borges-Costa, J.; Filipe, P. Human Monkeypox Coinfection with Acute HIV: An Exuberant Presentation. Int. J. STD AIDS 2022, 33, 936–938. [Google Scholar] [CrossRef]
- Ortiz-Saavedra, B.; Montes-Madariaga, E.S.; Cabanillas-Ramirez, C.; Alva, N.; Ricardo-Martínez, A.; León-Figueroa, D.A.; Barboza, J.J.; Mohanty, A.; Padhi, B.K.; Sah, R. Epidemiologic Situation of HIV and Monkeypox Coinfection: A Systematic Review. Vaccines 2023, 11, 246. [Google Scholar] [CrossRef]
- Multi-Country Outbreak of Mpox, External Situation Report#12—14 December 2022. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-12--14-december-2022 (accessed on 15 June 2024).
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-Like Disease in Cynomolgus Monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Cohen, M.S.; Hoffman, I.F.; Royce, R.A.; Kazembe, P.; Dyer, J.R.; Daly, C.C.; Zimba, D.; Vernazza, P.L.; Maida, M.; Fiscus, S.A.; et al. Reduction of Concentration of HIV-1 in Semen after Treatment of Urethritis: Implications for Prevention of Sexual Transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997, 349, 1868–1873. [Google Scholar] [CrossRef]
- Gray, R.H.; Wawer, M.J.; Brookmeyer, R.; Sewankambo, N.K.; Serwadda, D.; Wabwire-Mangen, F.; Lutalo, T.; Li, X.; vanCott, T.; Quinn, T.C.; et al. Probability of HIV-1 Transmission per Coital Act in Monogamous, Heterosexual, HIV-1-Discordant Couples in Rakai, Uganda. Lancet 2001, 357, 1149–1153. [Google Scholar] [CrossRef]
- Mitjà, O.; Alemany, A.; Marks, M.; Mora, J.I.L.; Rodríguez-Aldama, J.C.; Silva, M.S.T.; Herrera, E.A.C.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in People with Advanced HIV Infection: A Global Case Series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef]
- Rodriguez-Nava, G.; Kadlecik, P.; Filardo, T.D.; Ain, D.L.; Cooper, J.D.; McCormick, D.W.; Webber, B.J.; O’Laughlin, K.; Petersen, B.W.; Narasimhan, S.; et al. Myocarditis Attributable to Monkeypox Virus Infection in 2 Patients, United States, 2022. Emerg. Infect. Dis. 2022, 28, 2508–2512. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Kelleman, M.; West, Z.; Peter, A.; Dove, M.; Butto, A.; Oster, M.E. Comparison of Multisystem Inflammatory Syndrome in Children-Related Myocarditis, Classic Viral Myocarditis, and COVID-19 Vaccine-Related Myocarditis in Children. J. Am. Heart Assoc. 2022, 11, e024393. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.F.; Khan, A.H.; Sperling, L.S. Cardiac Complications after Smallpox Vaccination. South. Med. J. 2009, 102, 615–619. [Google Scholar] [CrossRef]
- Maqbool, K.U.; Arsh, H.; Kumar, D.; Veena, F.; Punshi, A.K.; Payal, F.; Kumar, S.; Kumar, S.; Rani, D.; Malik, J. Cardiovascular Manifestations of Human Monkeypox Virus: An Updated Review. Curr. Probl. Cardiol. 2023, 48, 101869. [Google Scholar] [CrossRef]
- Di Bari, S.; Mondi, A.; Pinnetti, C.; Mazzotta, V.; Carletti, F.; Matusali, G.; Vincenti, D.; Gagliardini, R.; Santoro, R.; Fontana, C.; et al. A Case of Severe Mpox Complicated with Streptococcus Pyogenes Sepsis in a Patient with HIV Infection. Pathogens 2023, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Rayati Damavandi, A.; Semnani, F.; Hassanpour, K. A Review of Monkeypox Ocular Manifestations and Complications: Insights for the 2022 Outbreak. Ophthalmol. Ther. 2023, 12, 55–69. [Google Scholar] [CrossRef]
- Carvalho, E.M.; Medeiros, M.; Veloso, V.G.; Biancardi, A.L.; Curi, A.L.L. Monkeypox Infection Causing Conjunctival Vesicles and Anterior Uveitis. Ocul. Immunol. Inflamm. 2024, 32, 266–267. [Google Scholar] [CrossRef]
- Mukit, F.A.; Louie, E.M.; Cape, H.T.; Bohn, S.N. A Suspected Case of a Neonatal Monkeypox Infection with Ocular Involvement. Cureus 2023, 15, e38819. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Mentreddy, A.; Schallhorn, J.; Chan, M.; Aung, S.; Doernberg, S.B.; Babik, J.; Miles, K.; Yang, K.; Lydon, E.; et al. Isolated Ocular Mpox without Skin Lesions, United States. Emerg. Infect. Dis. 2023, 29, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- CDC Interim Clinical Considerations for Management of Ocular Mpox. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/ocular-infection.html (accessed on 25 February 2025).
- Clinical Management and Infection Prevention and Control for Monkeypox: Interim Rapid Response Guidance, 10 June 2022. Available online: https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1 (accessed on 25 February 2025).
- Maredia, H.; Sartori-Valinotti, J.C.; Ranganath, N.; Tosh, P.K.; O’Horo, J.C.; Shah, A.S. Supportive Care Management Recommendations for Mucocutaneous Manifestations of Monkeypox Infection. Mayo Clin. Proc. 2023, 98, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarim, B.; Ticknor, I.L.; Torres, A.R.; Mohammed, T.O.; Rees, J.S.; Baghchechi, M.; Streams, B.N. Cutaneous Findings of Fulminant Monkeypox in a Patient with HIV/AIDS. JAAD Case Rep. 2023, 38, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Calin, R.; Périllaud-Dubois, C.; Marot, S.; Kerrou, K.; Peytavin, G.; Bachir, M.; Kirch, A.L.; Lassel, L.; Fallet, V.; Gozlan, J.; et al. Mpox Hepatic and Pulmonary Lesions in HIV/Hepatitis B Virus Co-Infected Patient, France. Emerg. Infect. Dis. 2024, 30, 2445–2447. [Google Scholar] [CrossRef] [PubMed]
- Warner, N.C.; Shishido, A.; Fulco, P.P.; Sastry, S. Immune Reconstitution Inflammatory Syndrome Due to Monkeypox in Two Patients with AIDS. AIDS 2023, 37, 1187–1188. [Google Scholar] [CrossRef]
- Rao, A.K. Interim Clinical Treatment Considerations for Severe Manifestations of Mpox—United States, February 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 232–243. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The Virology of Human Monkeypox Virus (hMPXV): A Brief Overview. Virus Res. 2022, 322, 198932. [Google Scholar] [CrossRef]
- Alkhalil, A.; Hammamieh, R.; Hardick, J.; Ichou, M.A.; Jett, M.; Ibrahim, S. Gene Expression Profiling of Monkeypox Virus-Infected Cells Reveals Novel Interfaces for Host-Virus Interactions. Virol. J. 2010, 7, 173. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef]
- Li, H.; Huang, Q.-Z.; Zhang, H.; Liu, Z.-X.; Chen, X.-H.; Ye, L.-L.; Luo, Y. The Land-Scape of Immune Response to Monkeypox Virus. eBioMedicine 2022, 87, 104424. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and Consequences of Jak-STAT Signaling in the Immune System. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Marco, M. del M.; Alejo, A.; Hudson, P.; Damon, I.K.; Alcami, A. The Highly Virulent Variola and Monkeypox Viruses Express Secreted Inhibitors of Type I Interferon. FASEB J. 2010, 24, 1479–1488. [Google Scholar] [CrossRef]
- Esteban, D.J.; Nuara, A.A.; Buller, R.M.L. Interleukin-18 and Glycosaminoglycan Binding by a Protein Encoded by Variola Virus. J. Gen. Virol. 2004, 85, 1291–1299. [Google Scholar] [CrossRef]
- Hudson, P.N.; Self, J.; Weiss, S.; Braden, Z.; Xiao, Y.; Girgis, N.M.; Emerson, G.; Hughes, C.; Sammons, S.A.; Isaacs, S.N.; et al. Elucidating the Role of the Complement Control Protein in Monkeypox Pathogenicity. PLoS ONE 2012, 7, e35086. [Google Scholar] [CrossRef] [PubMed]
- De Pace, V.; Bruzzone, B.; Ricucci, V.; Domnich, A.; Guarona, G.; Garzillo, G.; Qosja, R.; Ciccarese, G.; Di Biagio, A.; Orsi, A.; et al. Molecular Diagnosis of Human Monkeypox Virus during 2022–23 Outbreak: Preliminary Evaluation of Novel Real-Time Qualitative PCR Assays. Microorganisms 2024, 12, 664. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Ahn, J.H.; Nam, J.-H. Diagnosis of Viral Infection Using Real-Time Polymerase Chain Reaction. J. Bacteriol. Virol. 2018, 48, 1–13. [Google Scholar] [CrossRef]
- Dronina, J.; Samukaite-Bubniene, U.; Ramanavicius, A. Advances and Insights in the Diagnosis of Viral Infections. J. Nanobiotechnology 2021, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Altindis, M.; Puca, E.; Shapo, L. Diagnosis of Monkeypox Virus—An Overview. Travel. Med. Infect. Dis. 2022, 50, 102459. [Google Scholar] [CrossRef]
- Sui, Y.; Xu, Q.; Liu, M.; Zuo, K.; Liu, X.; Liu, J. CRISPR-Cas12a-Based Detection of Monkeypox Virus. J. Infect. 2022, 85, 702–769. [Google Scholar] [CrossRef]
- Grant, R.J.; Baldwin, C.D.; Nalca, A.; Zoll, S.; Blyn, L.B.; Eshoo, M.W.; Matthews, H.; Sampath, R.; Whitehouse, C.A. Application of the Ibis-T5000 Pan-Orthopoxvirus Assay to Quantitatively Detect Monkeypox Viral Loads in Clinical Specimens from Macaques Experimentally Infected with Aerosolized Monkeypox Virus. Am. J. Trop. Med. Hyg. 2010, 82, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, I.; Saijo, M.; Shiota, T.; Ami, Y.; Suzaki, Y.; Nagata, N.; Hasegawa, H.; Sakai, K.; Fukushi, S.; Mizutani, T.; et al. Loop-Mediated Isothermal Amplification-Based Diagnostic Assay for Monkeypox Virus Infections. J. Med. Virol. 2009, 81, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Batéjat, C.; Grassin, Q.; Feher, M.; Hoinard, D.; Vanhomwegen, J.; Manuguerra, J.-C.; Leclercq, I. Heat Inactivation of Monkeypox Virus. J. Biosaf. Biosecurity 2022, 4, 121–123. [Google Scholar] [CrossRef]
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef]
- Commissioner, O. of the Mpox. FDA. 2025. Available online: https://www.fda.gov/vaccines-blood-biologics/jynneos (accessed on 20 May 2025).
- Deputy, N.P.; Deckert, J.; Chard, A.N.; Sandberg, N.; Moulia, D.L.; Barkley, E.; Dalton, A.F.; Sweet, C.; Cohn, A.C.; Little, D.R.; et al. Vaccine Effectiveness of JYNNEOS against Mpox Disease in the United States. N. Engl. J. Med. 2023, 388, 2434–2443. [Google Scholar] [CrossRef]
- Huber, M.N.; Lim, S. Estimated Effectiveness of JYNNEOS Vaccine in Preventing Mpox: A Multijurisdictional Case-Control Study—United States, August 19, 2022–March 31, 2023. Ann. Emerg. Med. 2023, 82, 520–522. [Google Scholar] [CrossRef]
- Pischel, L.; Martini, B.A.; Yu, N.; Cacesse, D.; Tracy, M.; Kharbanda, K.; Ahmed, N.; Patel, K.M.; Grimshaw, A.A.; Malik, A.A.; et al. Vaccine Effectiveness of 3rd Generation Mpox Vaccines against Mpox and Disease Severity: A Systematic Review and Meta-Analysis. Vaccine 2024, 42, 126053. [Google Scholar] [CrossRef]
- Chan-Tack, K.M.; Harrington, P.R.; Choi, S.-Y.; Myers, L.; O’Rear, J.; Seo, S.; McMillan, D.; Ghantous, H.; Birnkrant, D.; Sherwat, A.I. Assessing a Drug for an Eradicated Human Disease: US Food and Drug Administration Review of Tecovirimat for the Treatment of Smallpox. Lancet Infect. Dis. 2019, 19, e221–e224. [Google Scholar] [CrossRef] [PubMed]
- Sherwat, A.; Brooks, J.T.; Birnkrant, D.; Kim, P. Tecovirimat and the Treatment of Monkeypox—Past, Present, and Future Considerations. N. Engl. J. Med. 2022, 387, 579–581. [Google Scholar] [CrossRef]
- Vernuccio, R.; León, A.M.; Poojari, C.S.; Buchrieser, J.; Selverian, C.; Jaleta, Y.; Meola, A.; Guivel-Benhassine, F.; Porrot, F.; Haouz, A.; et al. Mechanisms of tecovirimat antiviral activity and poxvirus resistance. Res. Sq. 2024. preprint. [Google Scholar] [CrossRef]
- Braddick, M.; Singh, K.P. Therapeutic Agents for the Treatment of Human Mpox. Curr. Opin. Infect. Dis. 2024, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- The Antiviral Tecovirimat Is Safe but Did Not Improve Clade I Mpox Resolution in Democratic Republic of the Congo. Available online: https://www.nih.gov/news-events/news-releases/antiviral-tecovirimat-safe-did-not-improve-clade-i-mpox-resolution-democratic-republic-congo (accessed on 12 April 2025).
- SIGA Technologies, Inc.—Interim Results from STOMP Study of SIGA’s Tecovirimat in Treatment of Mpox Announced. Available online: https://investor.siga.com/investors/news/news-details/2024/Interim-Results-from-STOMP-Study-of-SIGAs-Tecovirimat-in-Treatment-of-Mpox-Announced/default.aspx (accessed on 12 April 2025).
- Desai, A.N.; Thompson, G.R., III; Neumeister, S.M.; Arutyunova, A.M.; Trigg, K.; Cohen, S.H. Compassionate Use of Tecovirimat for the Treatment of Monkeypox Infection. JAMA 2022, 328, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.; Leeds, J.M.; Tyavanagimatt, S.; Hruby, D.E. Development of ST-246® for Treatment of Poxvirus Infections. Viruses 2010, 2, 2409–2435. [Google Scholar] [CrossRef] [PubMed]
- Frenois-Veyrat, G.; Gallardo, F.; Gorgé, O.; Marcheteau, E.; Ferraris, O.; Baidaliuk, A.; Favier, A.-L.; Enfroy, C.; Holy, X.; Lourenco, J.; et al. Tecovirimat Is Effective against Human Monkeypox Virus in Vitro at Nanomolar Concentrations. Nat. Microbiol. 2022, 7, 1951–1955. [Google Scholar] [CrossRef]
- Warner, B.M.; Klassen, L.; Sloan, A.; Deschambault, Y.; Soule, G.; Banadyga, L.; Cao, J.; Strong, J.E.; Kobasa, D.; Safronetz, D. In Vitro and in Vivo Efficacy of Tecovirimat against a Recently Emerged 2022 Monkeypox Virus Isolate. Sci. Transl. Med. 2022, 14, eade7646. [Google Scholar] [CrossRef]
- Postal, J.; Guivel-Benhassine, F.; Porrot, F.; Grassin, Q.; Crook, J.M.; Vernuccio, R.; Caro, V.; Vanhomwegen, J.; Guardado-Calvo, P.; Simon-Lorière, E.; et al. Antiviral Activity of Tecovirimat against Monkeypox Virus Clades 1a, 1b, 2a, and 2b. Lancet Infect. Dis. 2025, 25, e126–e127. [Google Scholar] [CrossRef]
- Delaune, D.; Iseni, F. Drug Development against Smallpox: Present and Future. Antimicrob. Agents Chemother. 2020, 64, e01683-19. [Google Scholar] [CrossRef]
- Magee, W.C.; Aldern, K.A.; Hostetler, K.Y.; Evans, D.H. Cidofovir and (S)-9-[3-Hydroxy-(2-Phosphonomethoxy)Propyl]Adenine Are Highly Effective Inhibitors of Vaccinia Virus DNA Polymerase When Incorporated into the Template Strand. Antimicrob. Agents Chemother. 2008, 52, 586–597. [Google Scholar] [CrossRef]
- Magee, W.C.; Hostetler, K.Y.; Evans, D.H. Mechanism of Inhibition of Vaccinia Virus DNA Polymerase by Cidofovir Diphosphate. Antimicrob. Agents Chemother. 2005, 49, 3153–3162. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A Multitargeted Agent for Age-Associated Chronic Diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.; Rezaei-Tavirani, M. Resveratrol: A Miraculous Natural Compound for Diseases Treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Palamara, A.T.; Nencioni, L.; Aquilano, K.; De Chiara, G.; Hernandez, L.; Cozzolino, F.; Ciriolo, M.R.; Garaci, E. Inhibition of Influenza A Virus Replication by Resveratrol. J. Infect. Dis. 2005, 191, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, J.; Zhang, Z.; Li, W.; Sun, Y.; Zhang, Q.; Feng, X.; Zhu, W. Effects of Resveratrol on the Amelioration of Insulin Resistance in KKAy Mice. Can. J. Physiol. Pharmacol. 2012, 90, 237–242. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, H.; Liu, T.; Yang, Z.; Xu, L.; Xu, Y.; Ge, H.M.; Tan, R.-X.; Li, E. Inhibition of Herpes Simplex Virus Infection by Oligomeric Stilbenoids through ROS Generation. Antivir. Res. 2012, 95, 30–36. [Google Scholar] [CrossRef]
- Clouser, C.L.; Chauhan, J.; Bess, M.A.; van Oploo, J.L.; Zhou, D.; Dimick-Gray, S.; Mansky, L.M.; Patterson, S.E. Anti-HIV-1 Activity of Resveratrol Derivatives and Synergistic Inhibition of HIV-1 by the Combination of Resveratrol and Decitabine. Bioorg Med. Chem. Lett. 2012, 22, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Saito, H.; Ikeda, M.; Hokari, R.; Kato, N.; Hibi, T.; Miura, S. An Antioxidant Resveratrol Significantly Enhanced Replication of Hepatitis C Virus. World J. Gastroenterol. 2010, 16, 184–192. [Google Scholar] [CrossRef]
- Galindo, I.; Hernáez, B.; Berná, J.; Fenoll, J.; Cenis, J.L.; Escribano, J.M.; Alonso, C. Comparative Inhibitory Activity of the Stilbenes Resveratrol and Oxyresveratrol on African Swine Fever Virus Replication. Antivir. Res. 2011, 91, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Takami, A.; Trung, L.Q.; Kato, S.; Nakao, S. Resveratrol Prevents EBV Transformation and Inhibits the Outgrowth of EBV-Immortalized Human B Cells. PLoS ONE 2012, 7, e51306. [Google Scholar] [CrossRef]
- Xie, X.-H.; Zang, N.; Li, S.; Wang, L.; Deng, Y.; He, Y.; Yang, X.; Liu, E. Resveratrol Inhibits Respiratory Syncytial Virus-Induced IL-6 Production, Decreases Viral Replication, and Downregulates TRIF Expression in Airway Epithelial Cells. Inflammation 2012, 35, 1392–1401. [Google Scholar] [CrossRef]
- Xu, J.; Yin, Z.; Li, L.; Cheng, A.; Jia, R.; Song, X.; Lu, H.; Dai, S.; Lv, C.; Liang, X.; et al. Inhibitory Effect of Resveratrol against Duck Enteritis Virus in Vitro. PLoS ONE 2013, 8, e65213. [Google Scholar] [CrossRef]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Realegeno, S.; Pant, A.; Satheshkumar, P.S.; Yang, Z. Suppression of Poxvirus Replication by Resveratrol. Front. Microbiol. 2017, 8, 2196. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Ahmed, S.R.; Shahid, S.B.; Ahmed, T.; Tamanna, H.K.; Marma, H. Therapeutic Promises of Plant Metabolites against Monkeypox Virus: An in Silico Study. Adv. Virol. 2023, 2023, 9919776. [Google Scholar] [CrossRef] [PubMed]
- Bajrai, L.H.; Alharbi, A.S.; El-Day, M.M.; Bafaraj, A.G.; Dwivedi, V.D.; Azhar, E.I. Identification of Antiviral Compounds against Monkeypox Virus Profilin-like Protein A42R from Plantago Lanceolata. Molecules 2022, 27, 7718. [Google Scholar] [CrossRef]
- Van Vliet, K.; Mohamed, M.R.; Zhang, L.; Villa, N.Y.; Werden, S.J.; Liu, J.; McFadden, G. Poxvirus Proteomics and Virus-Host Protein Interactions. Microbiol. Mol. Biol. Rev. 2009, 73, 730–749. [Google Scholar] [CrossRef]
- Witke, W. The Role of Profilin Complexes in Cell Motility and Other Cellular Processes. Trends Cell Biol. 2004, 14, 461–469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.; Chaudhary, A.A.; Srivastava, U.; Gupta, S.; Rustagi, S.; Rudayni, H.A.; Kashyap, V.K.; Kumar, S. Mpox 2022 to 2025 Update: A Comprehensive Review on Its Complications, Transmission, Diagnosis, and Treatment. Viruses 2025, 17, 753. https://doi.org/10.3390/v17060753
Yadav R, Chaudhary AA, Srivastava U, Gupta S, Rustagi S, Rudayni HA, Kashyap VK, Kumar S. Mpox 2022 to 2025 Update: A Comprehensive Review on Its Complications, Transmission, Diagnosis, and Treatment. Viruses. 2025; 17(6):753. https://doi.org/10.3390/v17060753
Chicago/Turabian StyleYadav, Rajesh, Anis Ahmad Chaudhary, Ujjwal Srivastava, Saurabh Gupta, Sarvesh Rustagi, Hassan Ahmed Rudayni, Vivek Kumar Kashyap, and Sanjay Kumar. 2025. "Mpox 2022 to 2025 Update: A Comprehensive Review on Its Complications, Transmission, Diagnosis, and Treatment" Viruses 17, no. 6: 753. https://doi.org/10.3390/v17060753
APA StyleYadav, R., Chaudhary, A. A., Srivastava, U., Gupta, S., Rustagi, S., Rudayni, H. A., Kashyap, V. K., & Kumar, S. (2025). Mpox 2022 to 2025 Update: A Comprehensive Review on Its Complications, Transmission, Diagnosis, and Treatment. Viruses, 17(6), 753. https://doi.org/10.3390/v17060753







