Viral Disruption of Blood–Testis Barrier Precedes Testicular Infection
Abstract
1. Introduction
| Virus | Family | Cytokines Associated | Tight Junction Proteins Affected |
|---|---|---|---|
| Mumps virus | Paramyxoviridae | TNF-α [29] IL-6 [30] CXCL10 [31] | ZO-1 [29] Occludin [29] |
| HIV | Retroviridae | none * | Occludin [32] Claudin-1 [32] N-cadherin [32] β-catenin [32] |
| Zika virus | Flaviviridae | TNF-α [33] IFN-γ [28] | Claudin-1 [28] ZO-1 [34] |
| Ebola/Marburg virus | Filoviridae | unclear | ZO-1 [35] ZO-2 [35] |
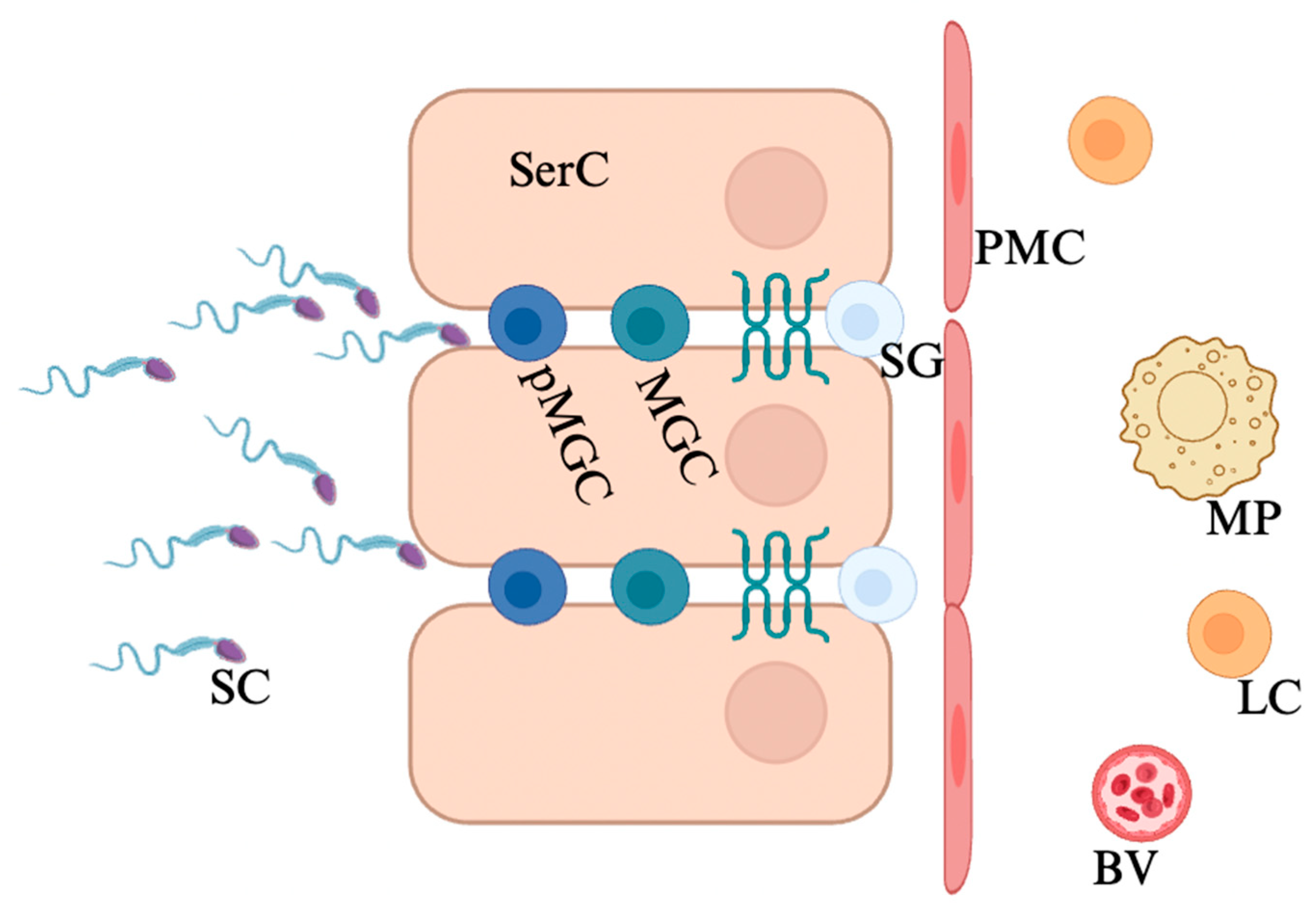
2. Blood–Testis Barrier
2.1. Structure and Function
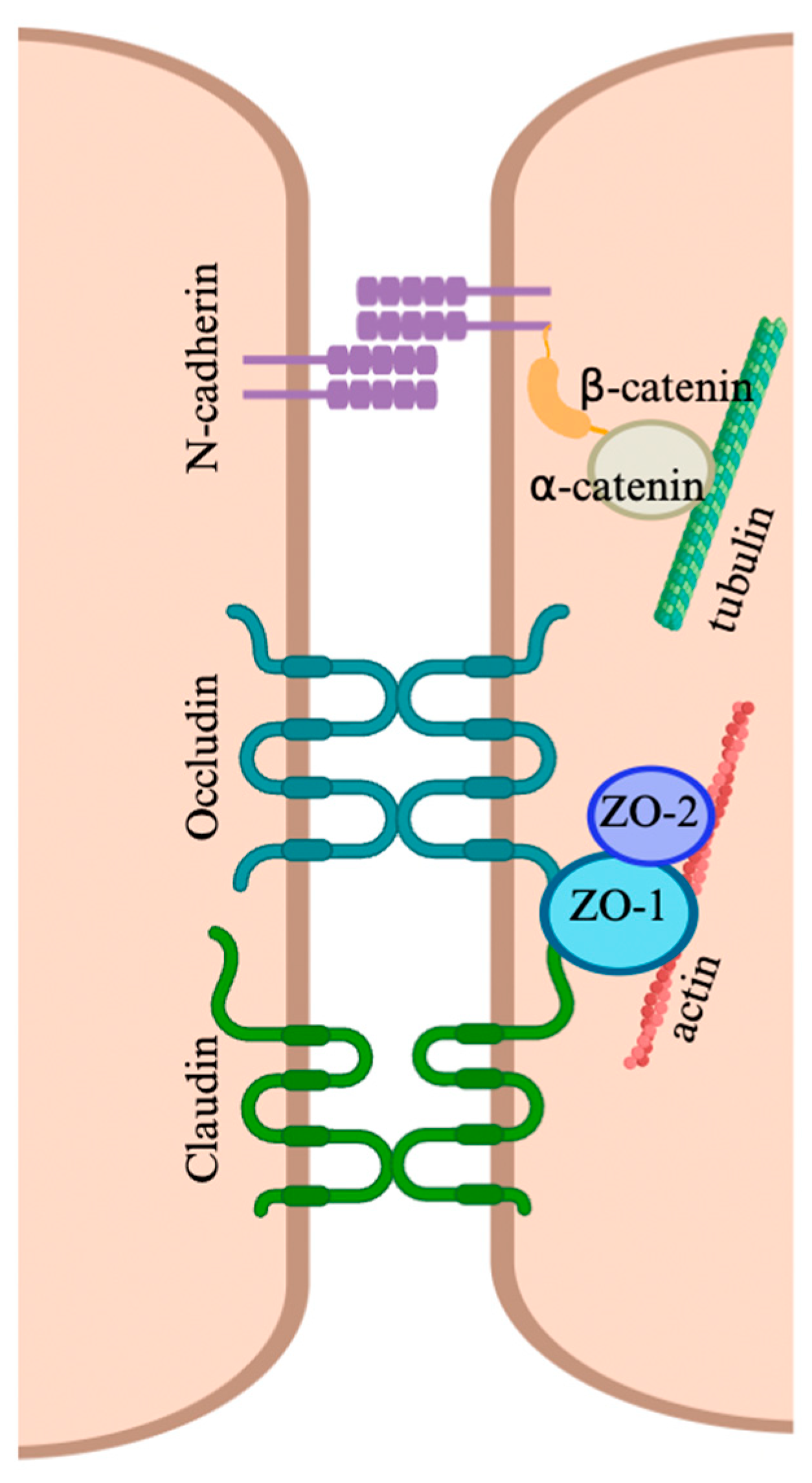
2.2. Cellular Junctions of the Blood–Testis Barrier
2.3. Permeability Regulation
3. Viral Infection of Testes
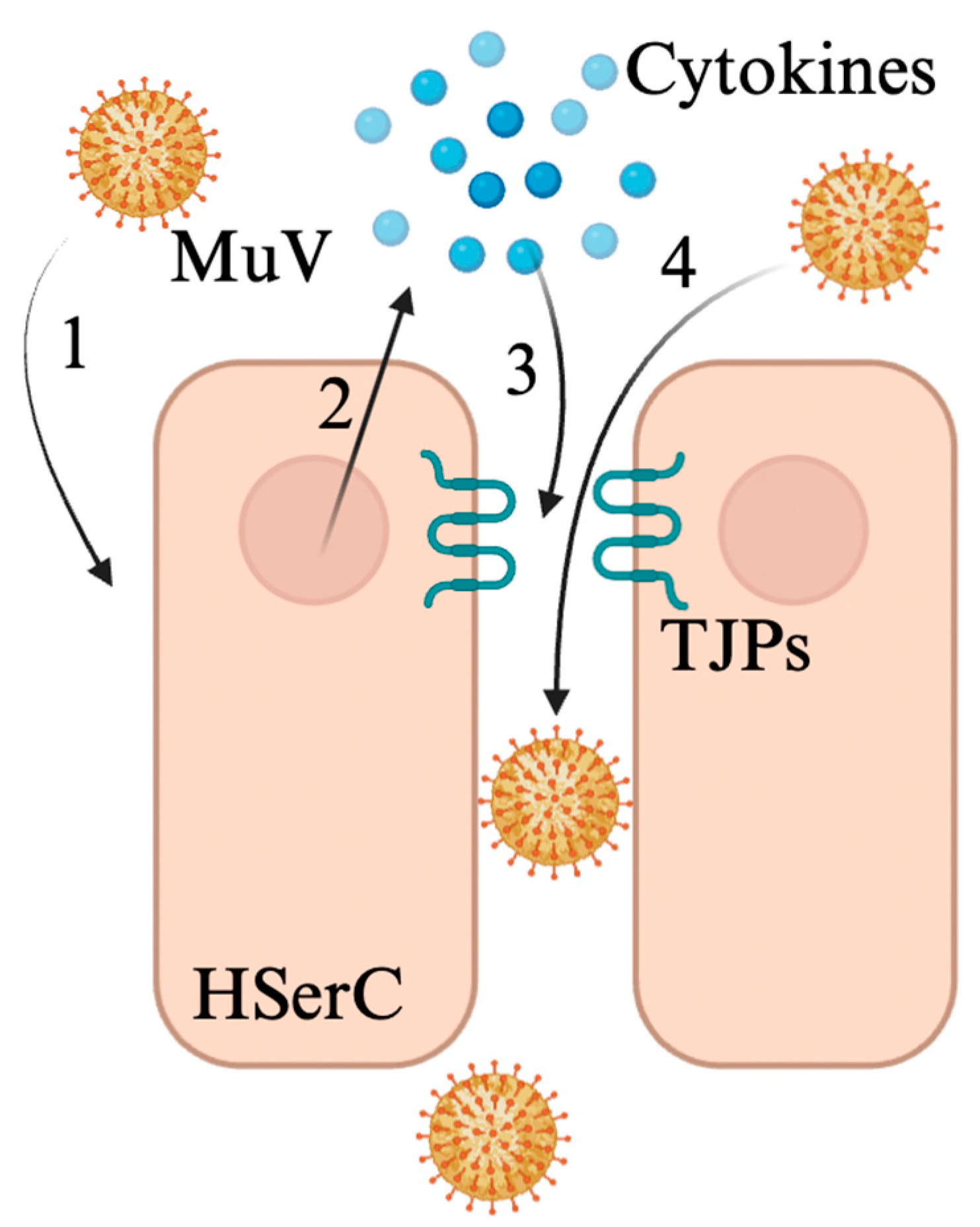
3.1. Mumps Virus
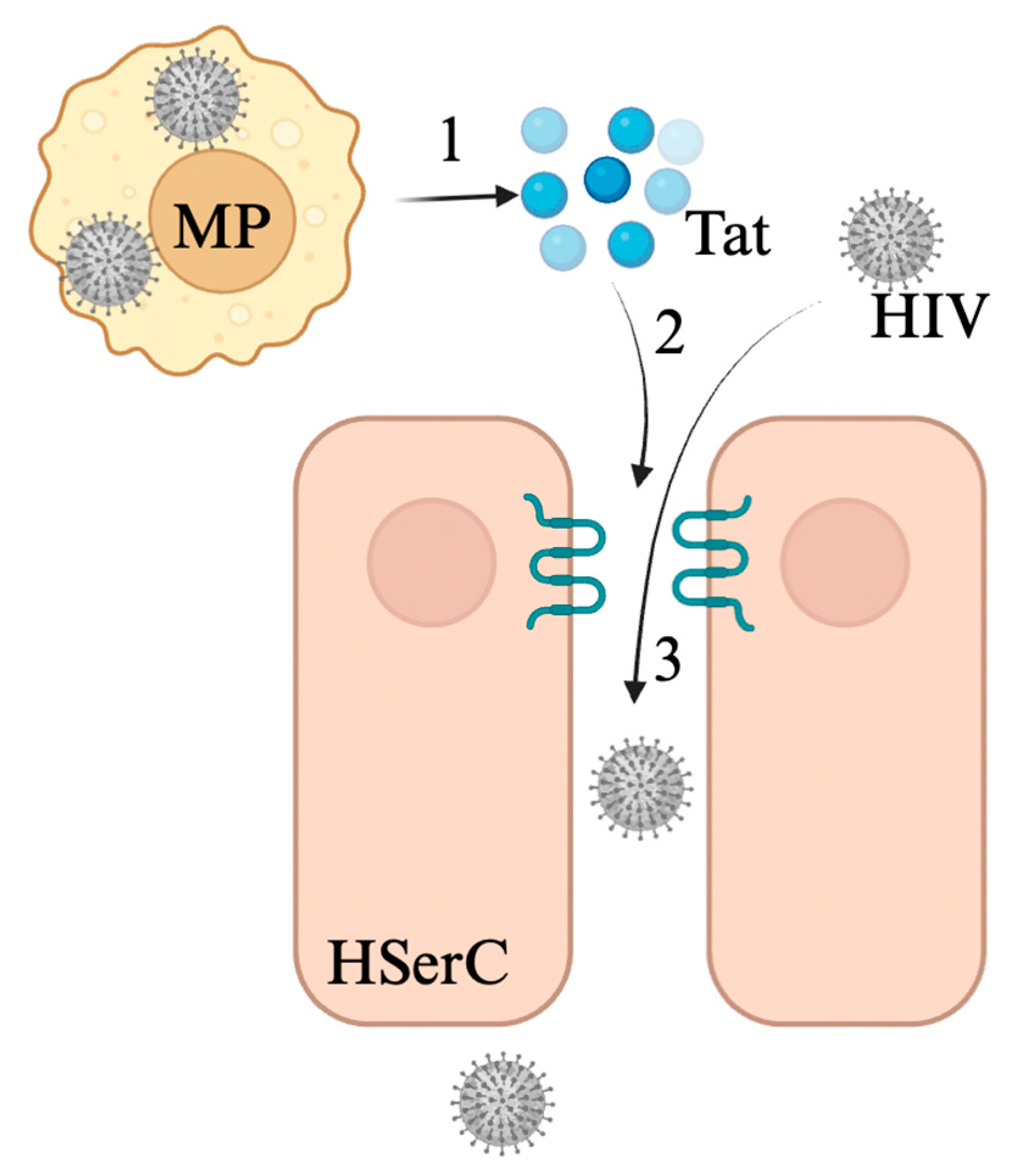
3.2. Human Immunodeficiency Virus
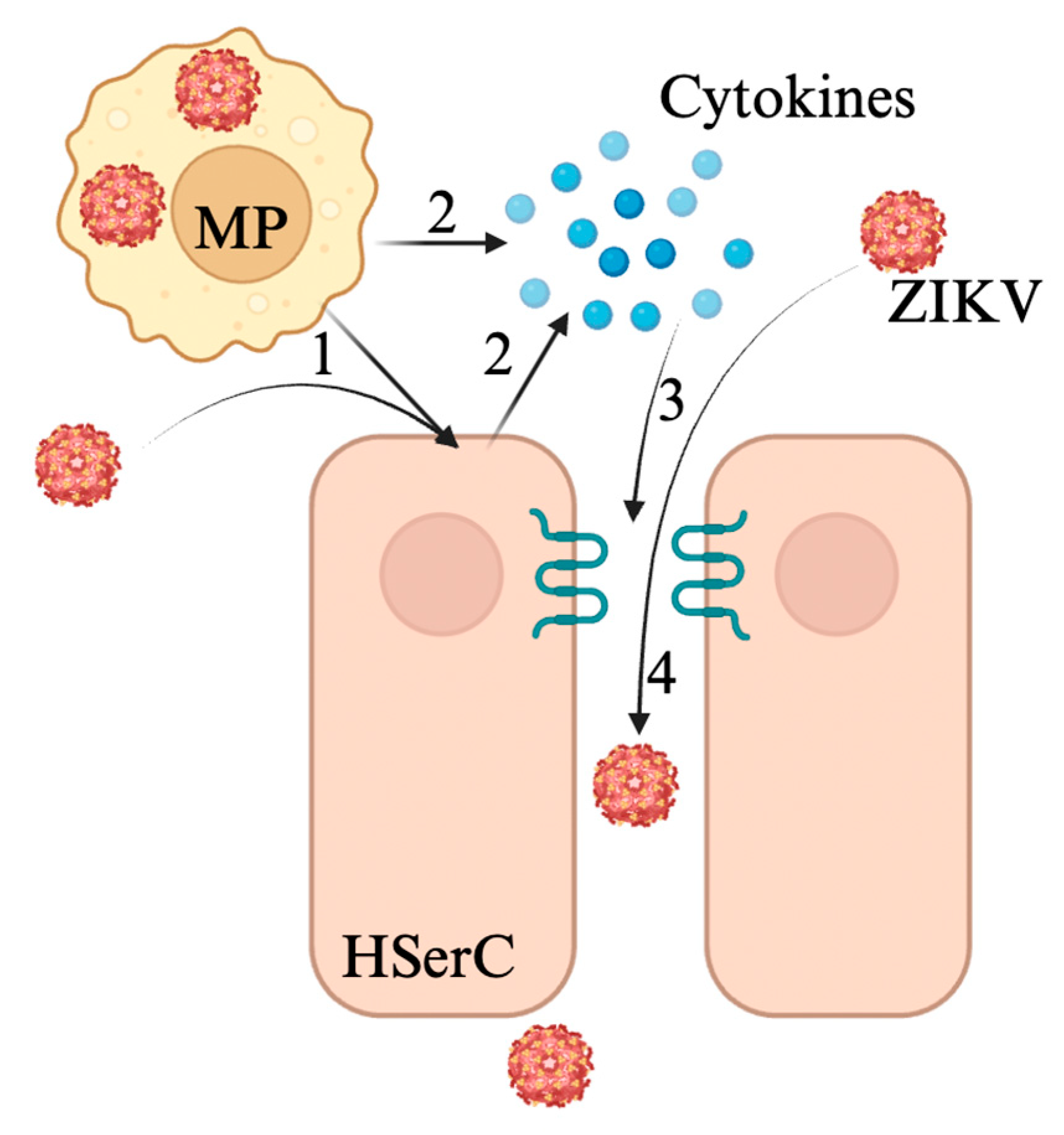
3.3. Zika Virus
3.4. Ebola and Marburg Viruses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jezek, D. Atlas on the Human Testis: Normal Morphology and Pathology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; p. 288. [Google Scholar]
- Hammar, M.; Petersson, F. Testosterone Production in Vitro in Human Testicular Tissue. Andrologia 1986, 18, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, A.; Dejucq-Rainsford, N.; Bhushan, S. Testicular macrophages: Development and function in health and disease. Trends Immunol. 2022, 43, 51–62. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Wright, K.; Verma, S.; Haynes, A.; Dufour, J.M. The Good, the Bad and the Ugly of Testicular Immune Regulation: A Delicate Balance Between Immune Function and Immune Privilege. In Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Sun, F., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 21–47, Advances in Experimental Medicine and Biology. [Google Scholar] [CrossRef]
- Akhigbe, R.E.; Dutta, S.; Hamed, M.A.; Ajayi, A.F.; Sengupta, P.; Ahmad, G. Viral Infections and Male Infertility: A Comprehensive Review of the Role of Oxidative Stress. Front. Reprod. Health 2022, 4, 782915. [Google Scholar] [CrossRef] [PubMed]
- Dejucq, N.; Jégou, B. Viruses in the Mammalian Male Genital Tract and Their Effects on the Reproductive System. Microbiol. Mol. Biol. Rev. 2001, 65, 208–231. [Google Scholar] [CrossRef]
- Garolla, A.; Pizzol, D.; Bertoldo, A.; Menegazzo, M.; Barzon, L.; Foresta, C. Sperm viral infection and male infertility: Focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J. Reprod. Immunol. 2013, 100, 20–29. [Google Scholar] [CrossRef]
- Davis, N.F.; McGuire, B.B.; Mahon, J.A.; Smyth, A.E.; O’Malley, K.J.; Fitzpatrick, J.M. The increasing incidence of mumps orchitis: A comprehensive review. BJU Int. 2010, 105, 1060–1065. [Google Scholar] [CrossRef]
- Jalal, H.; Bahadur, G.; Knowles, W.; Jin, L.; Brink, N. Mumps epididymo-orchitis with prolonged detection of virus in semen and the development of anti-sperm antibodies. J. Med. Virol. 2004, 73, 147–150. [Google Scholar] [CrossRef]
- Anderson, J.A.; Ping, L.H.; Dibben, O.; Jabara, C.B.; Arney, L.; Kincer, L.; Tang, Y.; Hobbs, M.; Hoffman, I.; Kazembe, P.; et al. HIV-1 Populations in Semen Arise through Multiple Mechanisms. Douek DC, editor. PLoS Pathog. 2010, 6, e1001053. [Google Scholar] [CrossRef]
- Darcis, G.; Coombs, R.W.; Van Lint, C. Exploring the anatomical HIV reservoirs: Role of the testicular tissue. AIDS 2016, 30, 2891–2893. [Google Scholar] [CrossRef]
- Le Tortorec, A.; Dejucq-Rainsford, N. HIV infection of the male genital tract—Consequences for sexual transmission and reproduction. Int. J. Androl. 2010, 33, e98–e108. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, M.M.; Nuovo, G.J.; Khalife, G. HIV in testis: Quantitative histology and HIV localization in germ cells. J. Reprod. Immunol. 1998, 41, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.A. Marburg Virus Disease. Clinical Syndrome. In Marburg Virus Disease; Martini, G.A., Siegert, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1971; pp. 1–9. [Google Scholar] [CrossRef]
- Brainard, J.; Pond, K.; Hooper, L.; Edmunds, K.; Hunter, P. Presence and Persistence of Ebola or Marburg Virus in Patients and Survivors: A Rapid Systematic Review. PLoS Neglected Trop. Dis. 2016, 10, e0004475. [Google Scholar] [CrossRef]
- Brainard, J.; Hooper, L.; Pond, K.; Edmunds, K.; Hunter, P.R. Risk factors for transmission of Ebola or Marburg virus disease: A systematic review and meta-analysis. Int. J. Epidemiol. 2016, 45, 102–116. [Google Scholar] [CrossRef]
- Crozier, I. Ebola Virus RNA in the Semen of Male Survivors of Ebola Virus Disease: The Uncertain Gravitas of a Privileged Persistence. J. Infect. Dis. 2016, 214, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.; Davies-Wayne, G.J.; Cordier-Lasalle, T.; Blackley, D.J.; Laney, A.S.; Williams, D.E.; Shinde, S.A.; Badio, M.; Lo, T.; Mate, S.E.; et al. Possible Sexual Transmission of Ebola Virus—Liberia, 2015. MMWR Morb Mortal Wkly Rep. 2015, 64, 479–481. [Google Scholar]
- Mate, S.E.; Kugelman, J.R.; Nyenswah, T.G.; Ladner, J.T.; Wiley, M.R.; Cordier-Lassalle, T.; Christie, A.; Schroth, G.P.; Gross, S.M.; Davies-Wayne, G.J.; et al. Molecular Evidence of Sexual Transmission of Ebola Virus. New Engl. J. Med. 2015, 373, 2448–2454. [Google Scholar] [CrossRef]
- Counotte, M.J.; Kim, C.R.; Wang, J.; Bernstein, K.; Deal, C.D.; Broutet, N.J.N.; Low, N. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med. 2018, 15, e1002611. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Yazdanpanah, Y.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. New Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Suberbielle, E.; Chapuy-Regaud, S.; Mengelle, C.; Bujan, L.; Marchou, B.; Delobel, P.; Gonzalez-Dunia, D.; E Malnou, C.; Izopet, J.; et al. Zika virus in semen and spermatozoa. Lancet Infect. Dis. 2016, 16, 1106–1107. [Google Scholar] [CrossRef]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.M. Potential Sexual Transmission of Zika Virus. Emerg Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Burki, T.K. Post-Ebola syndrome. Lancet Infect. Dis. 2016, 16, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.A.I.I.; Wohl, D.A. Confronting Ebola as a Sexually Transmitted Infection. Clin. Infect. Dis. 2016, 62, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Schindell, B.G.; Webb, A.L.; Kindrachuk, J. Persistence and Sexual Transmission of Filoviruses. Viruses 2018, 10, 683. [Google Scholar] [CrossRef]
- Yang, W.; Wu, Y.H.; Liu, S.Q.; Sheng, Z.Y.; Zhen, Z.D.; Gao, R.Q.; Cui, X.Y.; Fan, D.Y.; Qin, Z.H.; Zheng, A.H.; et al. S100A4+ macrophages facilitate zika virus invasion and persistence in the seminiferous tubules via interferon-gamma mediation. PLOS Pathog. 2020, 16, e1009019. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, X.; Gao, Y.; Liu, W.; Wang, F.; Gong, M.; Chen, R.; Yu, X.; Zhang, W.; Gao, B.; et al. Mumps virus infection disrupts blood-testis barrier through the induction of TNF-α in Sertoli cells. FASEB J. 2019, 33, 12528–12540. [Google Scholar] [CrossRef]
- Wang, F.; Chen, R.; Jiang, Q.; Wu, H.; Gong, M.; Liu, W.; Yu, X.; Zhang, W.; Han, R.; Liu, A.; et al. Roles of Sialic Acid, AXL, and MER Receptor Tyrosine Kinases in Mumps Virus Infection of Mouse Sertoli and Leydig Cells. Front Microbiol. 2020, 11, 1292. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, F.; Shi, L.; Zhao, X.; Gong, M.; Liu, W.; Song, C.; Li, Q.; Chen, Y.; Wu, H.; et al. C-X-C motif chemokine ligand 10 produced by mouse Sertoli cells in response to mumps virus infection induces male germ cell apoptosis. Cell Death Dis. 2017, 8, e3146. [Google Scholar] [CrossRef]
- Wu, S.; Frank, I.; Derby, N.; Martinelli, E.; Cheng, C.Y. HIV-1 Establishes a Sanctuary Site in the Testis by Permeating the BTB Through Changes in Cytoskeletal Organization. Endocrinology 2021, 162, bqab156. [Google Scholar] [CrossRef]
- Siemann, D.N.; Strange, D.P.; Maharaj, P.N.; Shi, P.Y.; Verma, S. Zika Virus Infects Human Sertoli Cells and Modulates the Integrity of the In Vitro Blood-Testis Barrier Model. J. Virol. 2017, 91, e00623-17. [Google Scholar] [CrossRef]
- Nie, Y.; Hui, L.; Guo, M.; Yang, W.; Huang, R.; Chen, J.; Wen, X.; Zhao, M.; Wu, Y. Rearrangement of Actin Cytoskeleton by Zika Virus Infection Facilitates Blood–Testis Barrier Hyperpermeability. Virol Sin. 2021, 36, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Coffin, K.M.; Liu, J.; Warren, T.K.; Blancett, C.D.; Kuehl, K.A.; Nichols, D.K.; Bearss, J.J.; Schellhase, C.W.; Retterer, C.J.; Weidner, J.M.; et al. Persistent Marburg Virus Infection in the Testes of Nonhuman Primate Survivors. Cell Host Microbe 2018, 24, 405–416.e3. [Google Scholar] [CrossRef] [PubMed]
- Edidin, M.; Johnson, M.H. Immunobiology of Gametes; Edidin, M., Johnson, M.H., Eds.; Cambridge University Press: Cambridge, UK, 1977; p. 328. [Google Scholar]
- Tung, K.S.K.; Unanue, E.R.; Dixon, F.J. Immunological Events Associated with Immunization by Sperm in Incomplete Freund’s Adjuvant. Int. Arch. Allergy Appl. Immunol. 2009, 41, 565–574. [Google Scholar] [CrossRef]
- Bobzien, B.; Yasunami, Y.; Majercik, M.; Lacy, P.E.; Davie, J.M. Intratesticular Transplants of Islet Xenografts (Rat to Mouse). Diabetes 1983, 32, 213–216. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Washburn, R.L.; Hibler, T.; Kaur, G.; Dufour, J.M. Sertoli Cell Immune Regulation: A Double-Edged Sword. Front. Immunol. 2022, 13, 913502. [Google Scholar] [CrossRef]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef] [PubMed]
- Chiquoine, A.D. Observations on the early events of cadmium necrosis of the testis. Anat. Rec. 1964, 149, 23–35. [Google Scholar] [CrossRef]
- Kormano, M. Dye permeability and alkaline phosphatase activity of testicular capillaries in the postnatal rat. Histochemie 1967, 9, 327–338. [Google Scholar] [CrossRef]
- Lie, P.P.Y.; Cheng, C.Y.; Mruk, D.D. Chapter five—The Biology of the Desmosome-Like Junction: A Versatile Anchoring Junction and Signal Transducer in the Seminiferous Epithelium. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 223–269. Available online: https://www.sciencedirect.com/science/article/pii/B9780123858597000057 (accessed on 1 December 2024).
- Pointis, G.; Fiorini, C.; Defamie, N.; Segretain, D. Gap junctional communication in the male reproductive system. Biochim. Et Biophys. Acta (BBA) Biomembr. 2005, 1719, 102–116. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Cell–cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol. Metab. 2004, 15, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Gilula, N.B.; Fawcett, D.W.; Aoki, A. The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev. Biol. 1976, 50, 142–168. [Google Scholar] [CrossRef] [PubMed]
- Djakiew, D.; Dym, M. Pachytene Spermatocyte Proteins Influence Sertoli Cell Function1. Biol. Reprod. 1988, 39, 1193–1205. [Google Scholar] [CrossRef]
- Hadley, M.A.; Byers, S.W.; Suárez-Quian, C.A.; Kleinman, H.K.; Dym, M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985, 101, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Lie, P.P.Y.; Chan, A.Y.N.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Restricted Arp3 expression in the testis prevents blood–testis barrier disruption during junction restructuring at spermatogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 11411–11416. [Google Scholar] [CrossRef]
- Gye, M.C. Expression of Claudin-1 in Mouse Testis. Arch. Androl. 2003, 49, 271–279. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, Y.; Wang, X.X.; Jin, C.; Wang, Y.Q.; Sun, T.C.; Li, J.; Tang, J.X.; Batool, A.; Deng, S.L.; et al. Regulation of blood–testis barrier assembly in vivo by germ cells. FASEB J. 2018, 32, 1653–1664. [Google Scholar] [CrossRef]
- Papadopoulos, D.; Dietze, R.; Shihan, M.; Kirch, U.; Scheiner-Bobis, G. Dehydroepiandrosterone Sulfate Stimulates Expression of Blood-Testis-Barrier Proteins Claudin-3 and -5 and Tight Junction Formation via a Gnα11-Coupled Receptor in Sertoli Cells. PLoS ONE 2016, 11, e0150143. [Google Scholar] [CrossRef]
- Fink, C.; Weigel, R.; Fink, L.; Wilhelm, J.; Kliesch, S.; Zeiler, M.; Bergmann, M.; Brehm, R. Claudin-11 is over-expressed and dislocated from the blood–testis barrier in Sertoli cells associated with testicular intraepithelial neoplasia in men. Histochem Cell Biol. 2009, 131, 755–764. [Google Scholar] [CrossRef]
- Lui, W.Y.; Mruk, D.; Lee, W.M.; Cheng, C.Y. Sertoli Cell Tight Junction Dynamics: Their Regulation During Spermatogenesis1. Biol. Reprod. 2003, 68, 1087–1097. [Google Scholar] [CrossRef]
- McCabe, M.J.; Foo, C.F.; Dinger, M.E.; Smooker, P.M.; Stanton, P.G. Claudin-11 and occludin are major contributors to Sertoli cell tight junction function, in vitro. Asian J. Androl. 2016, 18, 620. [Google Scholar]
- Pérez, C.V.; Sobarzo, C.M.; Jacobo, P.V.; Pellizzari, E.H.; Cigorraga, S.B.; Denduchis, B.; Lustig, L. Loss of Occludin Expression and Impairment of Blood-Testis Barrier Permeability in Rats with Autoimmune Orchitis: Effect of Interleukin 6 on Sertoli Cell Tight Junctions. Biol. Reprod. 2012, 87, 1–12. [Google Scholar] [CrossRef]
- Wong, E.W.P.; Cheng, C.Y. Chapter 7 Polarity Proteins and Cell–Cell Interactions in the Testis. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2009; Volume 278, pp. 309–353. Available online: https://www.sciencedirect.com/science/article/pii/S1937644809780074 (accessed on 19 January 2025).
- Chakraborty, P.; William Buaas, F.; Sharma, M.; Smith, B.E.; Greenlee, A.R.; Eacker, S.M.; Braun, R.E. Androgen-Dependent Sertoli Cell Tight Junction Remodeling Is Mediated by Multiple Tight Junction Components. Mol. Endocrinol. 2014, 28, 1055–1072. [Google Scholar] [CrossRef]
- Erkanlı Şentürk, G.; Ersoy Canillioĝlu, Y.; Umay, C.; Demiralp-Eksioglu, E.; Ercan, F. Distribution of Zonula Occludens-1 and Occludin and alterations of testicular morphology after in utero radiation and postnatal hyperthermia in rats. Int. J. Exp. Pathol. 2012, 93, 438–449. [Google Scholar] [CrossRef]
- Xu, J.; Anuar, F.; Mohamed Ali, S.; Ng, M.Y.; Phua, D.C.Y.; Hunziker, W. Zona Occludens-2 Is Critical for Blood–Testis Barrier Integrity and Male Fertility. MBoC 2009, 20, 4268–4277. [Google Scholar] [CrossRef] [PubMed]
- Byers, S.; Graham, R.; Dai, H.N.; Hoxter, B. Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am. J. Anat. 1991, 191, 35–47. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Campbell, R.; Song, J.; Massachi, S.; Razi, M.; Chiu, R.; Berenson, J.; O Yang, O.; Chen, I.S.; et al. Involvement of claudin-7 in HIV infection of CD4(-) cells. Retrovirology 2005, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Katahira, J.; Horiguchi, Y.; Sonoda, N.; Furuse, M.; Tsukita, S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000, 476, 258–261. [Google Scholar] [CrossRef]
- McCarthy, K.M.; Skare, I.B.; Stankewich, M.C.; Furuse, M.; Tsukita, S.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Occludin is a functional component of the tight junction. J. Cell Sci. 1996, 109, 2287–2298. [Google Scholar] [CrossRef]
- Morrow, C.M.K.; Mruk, D.; Cheng, C.Y.; Hess, R.A. Claudin and occludin expression and function in the seminiferous epithelium. Philos. Trans. R Soc. Lond. B Biol. Sci. 2010, 365, 1679–1696. [Google Scholar] [CrossRef]
- Mariano, C.; Sasaki, H.; Brites, D.; Brito, M.A. A look at tricellulin and its role in tight junction formation and maintenance. Eur. J. Cell Biol. 2011, 90, 787–796. [Google Scholar] [CrossRef]
- Vitale, R.; Fawcett, D.W.; Dym, M. The normal development of the blood-testis barrier and the effects of clomiphene and estrogen treatment. Anat. Rec. 1973, 176, 333–344. [Google Scholar] [CrossRef]
- McCabe, M.J.; Tarulli, G.A.; Laven-Law, G.; Matthiesson, K.L.; Meachem, S.J.; McLachlan, R.I.; Dinger, M.; Stanton, P. Gonadotropin suppression in men leads to a reduction in claudin-11 at the Sertoli cell tight junction. Hum. Reprod. 2016, 31, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Lui Wyee Lee, W.M.; Cheng, C.Y. Transforming Growth Factor β3 Regulates the Dynamics of Sertoli Cell Tight Junctions Via the p38 Mitogen-Activated Protein Kinase Pathway. Biol. Reprod. 2003, 68, 1597–1612. [Google Scholar]
- Hogarth, C.A.; Arnold, S.; Kent, T.; Mitchell, D.; Isoherranen, N.; Griswold, M.D. Processive Pulses of Retinoic Acid Propel Asynchronous and Continuous Murine Sperm Production. Biol. Reprod. 2015, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Holdcraft, R.W.; Shima, J.E.; Griswold, M.D.; Braun, R.E. Androgens regulate the permeability of the blood–testis barrier. Proc. Natl. Acad. Sci. USA 2005, 102, 16696–16700. [Google Scholar] [CrossRef]
- Li, M.W.M.; Xia, W.; Mruk, D.D.; Wang, C.Q.F.; Yan, H.H.N.; Siu, M.K.Y.; Lui, W.-Y.; Lee, W.M.; Cheng, C.Y. Tumor Necrosis Factor α Reversibly Disrupts the Blood–Testis Barrier and Impairs Sertoli–Germ Cell Adhesion in the Seminiferous Epithelium of Adult Rat Testes. 2006. Available online: https://joe.bioscientifica.com/view/journals/joe/190/2/1900313.xml (accessed on 5 May 2024).
- Rival, C.; Theas, M.S.; Guazzone, V.A.; Lustig, L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J. Reprod. Immunol. 2006, 70, 43–58. [Google Scholar] [CrossRef]
- Pérez, C.V.; Pellizzari, E.H.; Cigorraga, S.B.; Galardo, M.N.; Naito, M.; Lustig, L.; Jacobo, P.V. IL17A impairs blood–testis barrier integrity and induces testicular inflammation. Cell Tissue Res. 2014, 358, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, Y.; Wang, G.; Liu, Z.; Liu, L.; Sun, F. Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci Rep. 2014, 4, 4260. [Google Scholar] [CrossRef]
- Rubin, S.; Eckhaus, M.; Rennick, L.J.; Bamford, C.G.; Duprex, W.P. Molecular biology, pathogenesis and pathology of mumps virus. J. Pathol. 2015, 235, 242–252. [Google Scholar] [CrossRef]
- Eagles, A.Y. Analysis of a four year epidemic of mumps. Arch. Intern. Med. 1947, 80, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Philip, B.N.; Reinhard, K.R.; Lackman, D.B. Observations on a mumps epidemic in a “virgin” population. Am. J. Epidemiol. 1959, 69, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Candel, S. Epididymitis in mumps, including orchitis: Further clinical studies and comments. Ann. Intern. Med. 1951, 34, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Shulman, A.; Shohat, B.; Gillis, D.; Yavetz, H.; Homonnai, Z.T.; Paz, G. Mumps orchitis among soldiers: Frequency, effect on sperm quality, and sperm antibodies. Fertil Steril. 1992, 57, 1344–1346. [Google Scholar] [CrossRef]
- Choi, K.M. Reemergence of mumps. Korean J. Pediatr. 2010, 53, 623–628. [Google Scholar] [CrossRef]
- Bjorvatn, B. Mumps Virus Recovered from Testicles by Fine-Needle Aspiration Biopsy in Cases of Mumps Orchitis. Scand. J. Infect. Dis. 1973, 5, 3–5. [Google Scholar] [CrossRef]
- Adamopoulos, D.A.; Lawrence, D.M.; Vassilopoulos, P.; Contoyiannis, P.A.; Swyer, G.I.M. Pituitary-testicular interrelationships in mumps orchitis and other viral infections. Br. Med. J. 1978, 1, 1177–1180. [Google Scholar] [CrossRef]
- Yan, H.H.N.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008, 22, 1945–1959. [Google Scholar] [CrossRef]
- Levy, J.A. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 1993, 57, 183–289. [Google Scholar] [CrossRef]
- Pantaleo, G.; Graziosi, C.; Fauci, A.S. The Immunopathogenesis of Human Immunodeficiency Virus Infection. New Engl. J. Med. 1993, 328, 327–335. [Google Scholar]
- Sabin, C.A.; Devereux, H.; Phillips, A.N.; Hill, A.; Janossy, G.; Lee, C.A.; Loveday, C. Course of Viral Load Throughout HIV-1 Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2000, 23, 172. [Google Scholar]
- Volberding, P.A.; Deeks, S.G. Antiretroviral therapy and management of HIV infection. Lancet 2010, 376, 49–62. [Google Scholar] [CrossRef]
- De Paepe, M.E.; Waxman, M. Testicular atrophy in AIDS: A study of 57 autopsy cases. Hum. Pathol. 1989, 20, 210–214. [Google Scholar] [CrossRef]
- Rogers, C.; Klatt, E.C. Pathology of the testis in acquired immunodeficiency syndrome. Histopathology 1988, 12, 659–665. [Google Scholar] [CrossRef]
- Jenabian, M.A.; Costiniuk, C.T.; Mehraj, V.; Ghazawi, F.M.; Fromentin, R.; Brousseau, J.; Brassard, P.; Bélanger, M.; Ancuta, P.; Bendayan, R.; et al. Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS 2016, 30, 2777–2786. [Google Scholar] [CrossRef]
- Meltzer, M.S.; Nakamura, M.; Hansen, B.D.; Turpin, J.A.; Kalter, D.C.; Gendelman, H.E. Macrophages as Susceptible Targets for HIV Infection, Persistent Viral Reservoirs in Tissue, and Key Immunoregulatory Cells that Control Levels of Virus Replication and Extent of Disease. AIDS Res. Hum. Retroviruses 1990, 6, 967–971. [Google Scholar] [CrossRef]
- Moreno-Fernandez, M.E.; Zapata, W.; Blackard, J.T.; Franchini, G.; Chougnet, C.A. Human Regulatory T Cells Are Targets for Human Immunodeficiency Virus (HIV) Infection, and Their Susceptibility Differs Depending on the HIV Type 1 Strain. J. Virol. 2009, 83, 12925–12933. [Google Scholar] [CrossRef]
- Al-Jabri, A.A. How does HIV-1 infect a susceptible human cell? J. Sci. Res. Med. Sci. 2003, 5, 31–44. [Google Scholar]
- Banks, W.A.; Robinson, S.M.; Nath, A. Permeability of the blood–brain barrier to HIV-1 Tat. Exp. Neurol. 2005, 193, 218–227. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, M.; Liang, Y.; Zhang, Y. Disruption of blood–brain barrier: Effects of HIV Tat on brain microvascular endothelial cells and tight junction proteins. J. Neurovirol. 2023, 29, 658–668. [Google Scholar] [CrossRef]
- Xia, W.; Wong, E.W.P.; Mruk, D.D.; Cheng, C.Y. TGF-β3 and TNFα perturb blood–testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009, 327, 48–61. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, M.; Dhull, D.; Sharma, Y.; Kaushik, S.; Kaushik, S. Zika virus: An emerging challenge to public health worldwide. Can. J. Microbiol. 2020, 66, 87–98. [Google Scholar] [CrossRef]
- Hennessey, M. Zika Virus Spreads to New Areas—Region of the Americas, May 2015–January 2016. MMWR Morb. Mortal Wkly. Rep. 2016, 65. Available online: https://www.cdc.gov/mmwr/volumes/65/wr/mm6503e1.htm (accessed on 20 December 2024). [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Cerbino-Neto, J.; Mesquita, E.C.; Souza, T.M.L.; Parreira, V.; Wittlin, B.B.; Durovni, B.; Lemos, M.C.F.; Vizzoni, A.; Filippis, A.M.B.; Sampaio, S.A.; et al. Clinical Manifestations of Zika Virus Infection, Rio de Janeiro, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1318–1320. [Google Scholar] [CrossRef]
- Calvet, G.A.; Santos FBdos Sequeira, P.C. Zika virus infection: Epidemiology, clinical manifestations and diagnosis. Curr. Opin. Infect. Dis. 2016, 29, 459. [Google Scholar] [CrossRef]
- Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians | Infectious Diseases|JAMA Pediatrics|JAMA Network. 2024. Available online: https://jamanetwork.com/journals/jamapediatrics/article-abstract/2579543 (accessed on 1 November 2024).
- Zorrilla, C.D.; García García, I.; García Fragoso, L.; De La Vega, A. Zika Virus Infection in Pregnancy: Maternal, Fetal, and Neonatal Considerations. J. Infect. Dis. 2017, 216, S891–S896. [Google Scholar] [CrossRef]
- Foy, B.D.; Kobylinski, K.C.; Foy, J.L.C.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable Non–Vector-borne Transmission of Zika Virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef]
- Arsuaga, M.; Bujalance, S.G.; Díaz-Menéndez, M.; Vázquez, A.; Arribas, J.R. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect. Dis. 2016, 16, 1107. [Google Scholar] [CrossRef]
- Mead, P.S.; Duggal, N.K.; Hook, S.A.; Delorey, M.; Fischer, M.; Olzenak McGuire, D.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika Virus Shedding in Semen of Symptomatic Infected Men. New Engl. J. Med. 2018, 378, 1377–1385. [Google Scholar] [CrossRef]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef]
- Tripathi, S.; Balasubramaniam, V.R.M.T.; Brown, J.A.; Mena, I.; Grant, A.; Bardina, S.V.; Maringer, K.; Schwarz, M.C.; Maestre, A.M.; Sourisseau, M.; et al. A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017, 13, e1006258. [Google Scholar] [CrossRef]
- Schmitt, K.; Curlin, J.Z.; Remling-Mulder, L.; Aboellail, T.; Akkina, R. Zika virus induced microcephaly and aberrant hematopoietic cell differentiation modeled in novel neonatal humanized mice. Front. Immunol. 2023, 14, 1060959. [Google Scholar] [CrossRef]
- Kurscheidt, F.A.; Mesquita, C.S.S.; Damke, G.M.Z.F.; Damke, E.; Carvalho ARBde, A.; Suehiro, T.T.; Teixeira, J.J.V.; da Silva, V.R.S.; Souza, R.P.; Consolaro, M.E.L. Persistence and clinical relevance of Zika virus in the male genital tract. Nat. Rev. Urol. 2019, 16, 211–230. [Google Scholar] [CrossRef]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–542. [Google Scholar] [CrossRef]
- Campos, R.K.; Liang, Y.; Azar, S.R.; Ly, J.; Camargos, V.N.; Hager-Soto, E.E.; Eyzaguirre, E.; Sun, J.; Rossi, S.L. CD8+ T cells promote ZIKV clearance and mitigate testicular damage in mice. NPJ Viruses 2024, 2, 1–10. [Google Scholar] [CrossRef]
- Strange, D.P.; Jiyarom, B.; Pourhabibi Zarandi, N.; Xie, X.; Baker, C.; Sadri-Ardekani, H.; Shi, P.Y.; Verma, S. Axl Promotes Zika Virus Entry and Modulates the Antiviral State of Human Sertoli Cells. mBio 2019, 10, e01372-19. [Google Scholar] [CrossRef]
- Hui, L.; Nie, Y.; Li, S.; Guo, M.; Yang, W.; Huang, R.; Chen, J.; Liu, Y.; Lu, X.; Chen, Z.; et al. Matrix metalloproteinase 9 facilitates Zika virus invasion of the testis by modulating the integrity of the blood-testis barrier. PLoS Pathog. 2020, 16, e1008509. [Google Scholar] [CrossRef]
- Slenczka, W.; Klenk, H.D. Forty Years of Marburg Virus. J. Infect. Dis. 2007, 196 (Suppl. 2), S131–S135. [Google Scholar] [CrossRef]
- Coltart, C.E.M.; Lindsey, B.; Ghinai, I.; Johnson, A.M.; Heymann, D.L. The Ebola outbreak, 2013–2016: Old lessons for new epidemics. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160297. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Hensley, L.E.; Larsen, T.; Young, H.A.; Reed, D.S.; Geisbert, J.B.; Scott, D.P.; Kagan, E.; Jahrling, P.B.; Davis, K.J. Pathogenesis of Ebola Hemorrhagic Fever in Cynomolgus Macaques: Evidence that Dendritic Cells Are Early and Sustained Targets of Infection. Am. J. Pathol. 2003, 163, 2347–2370. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Young, H.A.; Jahrling, P.B.; Davis, K.J.; Kagan, E.; Hensley, L.E. Mechanisms Underlying Coagulation Abnormalities in Ebola Hemorrhagic Fever: Overexpression of Tissue Factor in Primate Monocytes/Macrophages Is a Key Event. J. Infect. Dis. 2003, 188, 1618–1629. [Google Scholar] [CrossRef]
- Bray, M.; Geisbert, T.W. Ebola virus: The role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int. J. Biochem. Cell Biol. 2005, 37, 1560–1566. [Google Scholar] [CrossRef]
- Davis, K.; Anderson, A.; Geisbert, T.; Steele, K.; Geisbert, J.; Vogel, P.; Connolly, B.M.; Huggins, J.W.; Jahrling, P.B.; Jaax, N.K. Pathology of experimental Ebola virus infection in African green monkeys. Arch. Pathol. Lab. Med. 1997, 121, 805–819. [Google Scholar]
- Abir, M.H.; Rahman, T.; Das, A.; Etu, S.N.; Nafiz, I.H.; Rakib, A.; Mitra, S.; Bin Emran, T.; Dhama, K.; Islam, A.; et al. Pathogenicity and virulence of Marburg virus. Virulence 2022, 13, 609–633. [Google Scholar] [CrossRef]
- Bosio, C.M.; Aman, M.J.; Grogan, C.; Hogan, R.; Ruthel, G.; Negley, D.; Mohamadzadeh, M.; Bavari, S.; Schmaljohn, A. Ebola and Marburg Viruses Replicate in Monocyte-Derived Dendritic Cells without Inducing the Production of Cytokines and Full Maturation. J. Infect. Dis. 2003, 188, 1630–1638. [Google Scholar] [CrossRef]
- Lanini, S.; Portella, G.; Vairo, F.; Kobinger, G.P.; Pesenti, A.; Langer, M.; Kabia, S.; Brogiato, G.; Amone, J.; Castilletti, C.; et al. Blood kinetics of Ebola virus in survivors and nonsurvivors. J. Clin. Investig. 2015, 125, 4692–4698. [Google Scholar] [CrossRef]
- Smither, S.J.; O’Brien, L.M.; Eastaugh, L.; Woolley, T.; Lever, M.; Fletcher, T.; Parmar, K.; Hunt, B.J.; Watts, S.; Kirkman, E. Haemostatic Changes in Five Patients Infected with Ebola Virus. Viruses 2019, 11, 647. [Google Scholar] [CrossRef]
- Lamontagne, F.; Fowler, R.A.; Adhikari, N.K.; Murthy, S.; Brett-Major, D.M.; Jacobs, M.; Uyeki, T.M.; Vallenas, C.; Norris, S.L.; Fischer, W.A., 2nd; et al. Evidence-based guidelines for supportive care of patients with Ebola virus disease. Lancet 2018, 391, 700–708. [Google Scholar] [CrossRef]
- Piszczatoski, C.R.; Gums, J.G. Ervebo (Ebola Zaire Vaccine, Live/rVSVΔG-ZEBOV-GP): The First Licensed Vaccine for the Prevention of Ebola Virus Disease. J. Pharm. Technol. 2020, 36, 243–250. [Google Scholar] [CrossRef]
- Malik, S.; Kishore, S.; Nag, S.; Dhasmana, A.; Preetam, S.; Mitra, O.; León-Figueroa, D.A.; Mohanty, A.; Chattu, V.K.; Assefi, M.; et al. Ebola Virus Disease Vaccines: Development, Current Perspectives & Challenges. Vaccines 2023, 11, 268. [Google Scholar] [CrossRef]
- Choi, E.M.L.; Lacarra, B.; Afolabi, M.O.; Ale, B.M.; Baiden, F.; Bétard, C.; Foster, J.; Hamzé, B.; Schwimmer, C.; Manno, D.; et al. Safety and immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in infants: A phase 2, randomised, double-blind, active-controlled trial in Guinea and Sierra Leone. Lancet Glob. Health 2023, 11, e1743-52. [Google Scholar] [CrossRef]
- Martini, G.A. Marburg virus disease. Postgrad. Med. J. 1973, 49, 542–546. [Google Scholar] [CrossRef]
- Martini, G.A.; Schmidt, H.A. Spermatogene Übertragung des „Virus Marburg“. Klin. Wochenschr. 1968, 46, 398–400. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; De Roo, A.; Guimard, Y.; Trappier, S.G.; Sanchez, A.; Bressler, D.; Williams, A.J.; Rowe, A.K.; Bertolli, J.; Khan, A.S.; et al. Persistence and Genetic Stability of Ebola Virus during the Outbreak in Kikwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 1999, 179 (Suppl. 1), S170–S176. [Google Scholar] [CrossRef]
- Rowe, A.K.; Bertolli, J.; Khan, A.S.; Mukunu, R.; Muyembe-Tamfum, J.J.; Bressler, D.; Williams, A.J.; Peters, C.J.; Rodriguez, L.; Feldmann, H.; et al. Clinical, Virologic, and Immunologic Follow-Up of Convalescent Ebola Hemorrhagic Fever Patients and Their Household Contacts, Kikwit, Democratic Republic of the Congo. J. Infect. Dis. 1999, 179 (Suppl. 1), S28–S35. [Google Scholar] [CrossRef]
- Martines, R.B.; Ng, D.L.; Greer, P.W.; Rollin, P.E.; Zaki, S.R. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J. Pathol. 2015, 235, 153–174. [Google Scholar] [CrossRef]
- Perry, D.L.; Huzella, L.M.; Bernbaum, J.G.; Holbrook, M.R.; Jahrling, P.B.; Hagen, K.R.; Schnell, M.J.; Johnson, R.F. Ebola Virus Localization in the Macaque Reproductive Tract during Acute Ebola Virus Disease. Am. J. Pathol. 2018, 188, 550–558. [Google Scholar] [CrossRef]
- Cooper, T.K.; Sword, J.; Johnson, J.C.; Bonilla, A.; Hart, R.; Liu, D.X.; Bernbaum, J.G.; Cooper, K.; Jahrling, P.B.; E Hensley, L. New Insights Into Marburg Virus Disease Pathogenesis in the Rhesus Macaque Model. J. Infect. Dis. 2018, 218 (Suppl. 5), S423–S433. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M.; Takada, A.; Ebihara, H.; Neumann, G.; Fujioka, K.; Irimura, T.; Jones, S.; Feldmann, H.; Kawaoka, Y. Tyro3 Family-Mediated Cell Entry of Ebola and Marburg Viruses. J. Virol. 2006, 80, 10109–10116. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.S.; Smart, G.; Rhoderick, J.F.; O’Donnell, K.L.; Rosenke, R.; Schäfer, A.; Marzi, A. Establishing a Mouse Model for Sexual Transmission and Male Reproductive Tract Persistence of Ebola virus. J. Infect. Dis. 2023, 228, S554–S558. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.L.; Schindell, B.G.; Soule, G.; Siddik, A.B.; Abrenica, B.; Memon, H.; Su, R.-C.; Safronetz, D.; Kindrachuk, J. Characterizing changes in transcriptome and kinome responses in testicular cells during infection by Ebola virus. NPJ Viruses 2024, 2, 12. [Google Scholar] [CrossRef]
- Campos, R.K.; Camargos, V.N.; Azar, S.R.; Haines, C.A.; Eyzaguirre, E.J.; Rossi, S.L. SARS-CoV-2 Infects Hamster Testes. Microorganisms 2021, 9, 1318. [Google Scholar] [CrossRef]
- Costa, G.M.J.; Lacerda, S.M.S.N.; Figueiredo, A.F.A.; Wnuk, N.T.; Brener, M.R.G.; Andrade, L.M.; Campolina-Silva, G.H.; Kauffmann-Zeh, A.; Pacifico, L.G.G.; Versiani, A.F.; et al. High SARS-CoV-2 tropism and activation of immune cells in the testes of non-vaccinated deceased COVID-19 patients. BMC Biol. 2023, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.H.; Spera, K.M.; Ryan, S.L.; Helgager, J.; Andrici, J.; Zaki, S.R.; Vaitkevicius, H.; Leon, K.E.; Wilson, M.R.; DeRisi, J.L.; et al. Fatal Powassan Encephalitis (Deer Tick Virus, Lineage II) in a Patient With Fever and Orchitis Receiving Rituximab. JAMA Neurol. 2018, 75, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Castilletti, C.; Huits, R.; Mantovani, R.P.; Accordini, S.; Alladio, F.; Gobbi, F. Replication-Competent Oropouche Virus in Semen of Traveler Returning to Italy from Cuba, 2024. Emerg. Infect. Dis. 2024, 30, 2684–2686. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Post-Ebolavirus disease syndrome: What do we know? Expert Rev. Anti-Infect. Ther. 2015, 13, 1185–1187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hager-Soto, E.E.; Freiberg, A.N.; Rossi, S.L. Viral Disruption of Blood–Testis Barrier Precedes Testicular Infection. Viruses 2025, 17, 747. https://doi.org/10.3390/v17060747
Hager-Soto EE, Freiberg AN, Rossi SL. Viral Disruption of Blood–Testis Barrier Precedes Testicular Infection. Viruses. 2025; 17(6):747. https://doi.org/10.3390/v17060747
Chicago/Turabian StyleHager-Soto, E. Eldridge, Alexander N. Freiberg, and Shannan L. Rossi. 2025. "Viral Disruption of Blood–Testis Barrier Precedes Testicular Infection" Viruses 17, no. 6: 747. https://doi.org/10.3390/v17060747
APA StyleHager-Soto, E. E., Freiberg, A. N., & Rossi, S. L. (2025). Viral Disruption of Blood–Testis Barrier Precedes Testicular Infection. Viruses, 17(6), 747. https://doi.org/10.3390/v17060747







