Abstract
Tripartite motif (TRIM) 25 is a member of the TRIM E3 ubiquitin ligase family, which plays multiple roles in anti-tumor and antiviral defenses through various pathways. Its RBCC and SPRY/PRY domains work cooperatively for its oligomerization and subsequent activation of ligase activity. TRIM25 expression is regulated by several proteins and RNAs, and it functionally participates in the post-transcriptional and translational modification of antiviral regulators, such as RIG-I, ZAP, and avSGs. Conversely, the antiviral functions of TRIM25 are inhibited by viral proteins and RNAs through their interactions, as well as by the viral infection-mediated upregulation of certain miRNAs. Here, we review the antiviral functions of TRIM25 and highlight its significance regarding innate immunity, particularly in antiviral defense and viral immune evasion.
1. Introduction
Ubiquitination is a crucial and fundamental process of post-translational modification (PTM) consisting of the covalent attachment of ubiquitin to protein substrates mediated by a cascade of E1 ubiquitin-activating enzymes, E2 conjugating enzymes, and E3 ligase enzymes [1]. E3 ligases, or E3 ubiquitin ligases, catalyze the direct transfer of ubiquitin to substrate proteins, leading to their proteasomal degradation. E3 ligases are classified into three major types—RING (really interesting new gene), HECT (homologous to E6AP C-terminus), and RBR (RING-between-RING)—each employing distinct catalytic mechanisms for ubiquitin transfer. Tripartite motif (TRIM) proteins constitute a subset of RING-type E3 ubiquitin ligases. TRIM proteins play essential roles in various regulatory processes, such as cell signaling, carcinogenesis, protein interaction, and DNA repair [2]. Evolving from the same ancestral gene, TRIM proteins contain a highly conserved RING–B-box–coiled-coil (RBCC) domain at their N-termini [3]. However, because of evolutionary processes, the C-terminal domains of TRIM members (CⅠ-CⅣ) exhibit distinct characteristics [3]. The C-IV subfamily of TRIM proteins contains a unique PRY/SPRY (B30.2) domain at its C-termini; it is found in many other proteins, including SplA kinase and ryanodine receptors (RyRs) [4,5]. As shown in previous research, the allosteric effects of multiple TRIM protein domains and TRIM protein oligomerization are crucial for the catalytic activity of TRIM E3 ligase [6]. Furthermore, TRIM family proteins have the ability to interact with RNAs [3]. Evidence from mRNA interactome capture studies and protein–RNA crosslinking capture experiments has revealed that a variety of TRIM proteins exhibit RNA-binding capabilities [3,7,8]. TRIM25 is a member of the C-IV subfamily and was recently found to have an RNA-binding preference and functional domain through a study employing iCLIP2 [9]. During this interaction, RNA can function as a scaffold to facilitate conformational changes in TRIM family proteins and subsequent protein–protein interactions [9,10]. Basically, TRIM proteins mediate two types of polyubiquitination: K48-linked ubiquitination for proteasomal degradation and K63-linked ubiquitination for cellular signaling and cytokine synthesis [6,11,12]. Both types of ubiquitination shed light on the possibility that TRIM25 regulates antiviral defense by modulating antiviral proteins (AVPs) and virus-related components involved in innate immunity. For instance, previous studies have identified the possible viral restriction structures in TRIM25: retinoic acid inducible gene I (RIG-I) and the zinc-finger antiviral protein (ZAP) [13,14,15]. This multifaceted domain structure allows TRIM25 to engage in diverse biological activities, making it a pivotal component in the cellular response to viral infection [6,16].
Several recent reviews have focused on the roles of TRIM25 and its underlying mechanisms in cancer and inflammatory diseases [17,18,19]. TRIM25 has been shown to play critical roles in various cell death pathways, including apoptosis, pyroptosis, necroptosis, ferroptosis, and autophagy, indicating its potential as a therapeutic target in cancer [17]. However, recent advances in our understanding of the antiviral functions of TRIM25—particularly its intricate interactions with viral RNAs and proteins, as well as its regulation of antiviral responses in innate immunity—have not been comprehensively addressed in existing reviews. Therefore, we conducted this review to synthesize studies on the TRIM25–viral component interactions that regulate antiviral defense and viral immune evasion. We will discuss the basic structure of TRIM25 and its regulatory effects on immune signaling.
2. General Structure and Gene Regulation of TRIM25
2.1. Gene Regulation of TRIM25
The TRIM25 gene is located on human chromosome 17q22, composed of 25kb of genomic DNA and nine exons [20]. Functionally, the TRIM25 gene encodes TRIM25, an inducible E3 ubiquitin ligase, which is predominantly located in the cytosol and can be redistributed into the organelles within a cell following viral infection. The TRIM25 gene is a kind of interferon-stimulated gene (ISG) whose promoter can be activated by type I interferon (IFN1α and IFNβ), and its expression is induced during viral infection [19,20]. The TRIM25 gene contains interferon-stimulated response elements (ISREs) in the first intron that can recruit signal transducers and the activator of transcription 1 (STAT1), mediating IFN-stimulated TRIM25 transcription [21]. Additionally, TRIM25 was initially identified as an estrogen-responsive finger protein (EFP), which acts in an estrogen-dependent manner based on its estrogen-responsive element (ERE) at the 3′ untranslated region (3′UTR) [19,20,21]. The binding of estrogen to estrogen receptor α (ERα) and its interaction with ERE promote the activation of the proximal promoter and TRIM25 transcription [22]. Apparently, both IFNs and estrogen can induce TRIM25 mRNA transcription and subsequent protein expression. Furthermore, TRIM25 expression is negatively regulated by a myriad of microRNAs (miRNAs) [19,23,24,25,26]. These miRNAs can bind to the 3′ untranslated region (3′ UTR) of TRIM25 mRNA, inhibiting TRIM25 protein translation and promoting TRIM25 mRNA degradation, thereby downregulating TRIM25 expression [24,25]. Several studies have shown that miR-30a and miR-202-5p bind to the 3′UTR of the TRIM25 gene, negatively regulating its expression [27,28,29]. MiR-30a is upregulated by coxsackievirus B3 (CVB3), inhibiting TRIM25 expression and subsequent RIG-I ubiquitination, which facilitates CVB3 replication and enhances viral infection [27]. This suggests that the miR-30a-mediated suppression of TRIM25 and RIG-I contributes to the evasion of IFN-I-mediated antiviral responses, promoting CVB3 infection. Similarly, miR-202-5p is induced by red-spotted grouper nervous necrosis virus (RGNNV) infection. Its overexpression inhibits TRIM25-mediated RIG-I ubiquitination, impairing RIG-I-dependent innate immune responses and promoting RGNNV infection [28]. These findings advance our understanding of viral evasion mechanisms against immune responses and have clinical relevance, including therapeutic potential with respect to targeting these regulatory pathways in viral infections and related diseases.
In addition, E3 ligases, such as TRIM25, are subjected to various post-translational modifications, such as ubiquitination [10], phosphorylation [30], ISGylation [31], and sumoylation [32]. The K117 of TRIM25 undergoes autoubiquitination [10]. Given that TRIM25 is an RNA-binding protein (RBP), the binding of TRIM25 to its own mRNA 3′UTR region can promote its autoubiquitination, limiting its expression [10]. Furthermore, 14-3-3, acting as an enhancer of TRIM25 binding and RIG-I ubiquitination [33], can promote TRIM25 autoubiquitination and inactivation in response to the BamH1 P fragment leftward open reading frame1 (BPLF1) protein of Epstein–Barr virus (EBV), reducing IFN production [34]. Whether RNA viruses can also interfere with the antiviral activity of TRIM25 by binding 14-3-3 remains to be explored. The linear ubiquitin chain assembly complex (LUBAC), a novel multiprotein E3 ligase complex, can add K48-linked ubiquitin chains to TRIM25, resulting in the formation of linear polyubiquitin chains [35,36]. Conversely, ubiquitin-specific protease 15 (USP15) can remove these LUBAC-mediated linear ubiquitin chains, thereby regulating the functions of TRIM25, including its antiviral activity [37]. Moreover, the TRIM25 protein can be post-translationally phosphorylated at the threonine 91, serine 97, serine 100, tyrosine 278, and threonine 427 residues, and TRIM25 tyrosine 278 phosphorylation is important for TRIM25-mediated RIG-I activation [30]. In response to the virus, TRIM25 in the cytosol can interact through its SPRY/PRY domain and be phosphorylated at the tyrosine 278 residue by c-Src tyrosine kinase, enhancing TRIM25-mediated RIG-I ubiquitination [30]. Notably, ubiquitination can be modulated by interferon-stimulated gene (ISG)15 and the small ubiquitin-like modifier (SUMO), which regulate protein expression and transportation [38]. Firstly, TRIM25 can undergo auto-ISGylation for self-modifications at lysine 177 between the RING and CC domains, enabling TRIM25 to transfer ISG15 to itself [31]. This modification requires the RING domain of TRIM25 and the support of the E2-conjugating enzymes UbcH6 and UbcH8 [31]. Originally, ISG15 should be transferred from E2 enzymes to 14-3-3σ, while TRIM25 ISGylation leads to the inhibition of its ISG15 E3 ligase activity toward 14-3-3σ [31,39]. Additionally, TRIM25 does not seem to directly interact with SUMO [32], but it is crucial for SUMO-mediated protein stabilization [32]. Specifically, TRIM25 can interact with SUMOylated proteins and promote their ISGylation, leading to TRIM25-dependent protein stabilization [32]. Furthermore, SUMO can promote the nuclear translocation of TRIM25 [32], in which TRIM25 may interact with RNAs and regulate various biological pathways.
Even though TRIM25 is involved in regulating gene and protein PTM, TRIM25 gene transcription is induced by type I interferon and estrogen, and its mRNA is targeted by a variety of miRNAs to inhibit its expression after transcription. Thus, TRIM25 expression is transcriptionally and post-transcriptionally regulated by various regulators and modifiers to maintain the balance of its biological functions.
2.2. Structural Insights into and Higher-Order Conformation of the RBCC Domain
As a highly conserved domain in the TRIM family, TRIM25 contains an RBCC domain that is composed of a RING domain, a B-box domain, and a coiled-coil (CC) domain (Figure 1A). The RING domain, known to be a zinc-finger protein, has a series of cysteine and histidine residues that coordinate two zinc ions, stabilizing the structure of this domain [40]. Functionally, the RING domain has the catalytic activity of an E3 ubiquitin ligase and is responsible for protein–protein interactions during viral restriction. The E3 ubiquitin ligase activity of the RING domain is necessary for the transfer of ubiquitin monomers from E2-conjugating enzymes to protein substrates [41]. This process is particularly important for the TRIM25-mediated antiviral responses in innate immunity. Through this catalytic activity, the RING domain interacts with RIG-I and ubiquitinates the CARD domain of RIG-I, whose feedback enhances the production of type I interferon. The B-box domain is one of the conserved domains in TRIM family proteins, and it is usually located between the RING and CC domains. There are two types of B-boxes, namely, B-box1 and B-box2, and each B-box domain is composed of approximately 50 amino acids [42]. Some TRIM family proteins have only a B-box2 domain, but TRIM25 has both B-box1 and B-box2 near its N-terminal sites [40,42]. As both the B-box and CC domains have a zinc-finger-binding ability similar to that of the RING domain, the B-box domain and CC domain enable themselves to bind zinc ions and maintain structural stability [42]. The B-box and CC domains have highly conserved motifs within the TRIM family, and the CC domain is typically positioned at the C-terminal of the B-box. Functionally, the CC domain facilitates the formation of homodimers or heterodimers in TRIM family proteins [40,41]. The CC domain of TRIM25 is the most important basic unit for its dimerization. The central region of the CC domain of TRIM25 contains numerous hydrophobic residues, which enable the antiparallel arrangement of the CC domain and subsequent tight binding of the helical CC domain through hydrophobic interactions [4], promoting TRIM25 dimer formation (Figure 1B). There is an L2 linker region between the CC and SPRY domains, and this short helical domain also undergoes self-association to form a dimer after the dimerization of CC helices [5]. TRIM family proteins usually form a conserved four-helix bundle, composed of a CC helix dimer and an L2 linker helix dimer. Given that individual TRIM proteins have unique sequences in the central region of the CC domain [5], these TRIM proteins have difficulty forming heterodimers [5]. However, the same branch of proteins with a similar central region sequence in the TRIM family can form heterodimers through CCD-mediated monomer interactions [5,43]. The specific and important roles of the central region of the CC domain in stimulating the dimerization of TRIM proteins have been demonstrated. In the proposed structure of the TRIM25 dimer, the RING and B-box domains are situated at the ends of the CC helical dimer, while the SPRY domain is in close proximity to the CC domain [12].
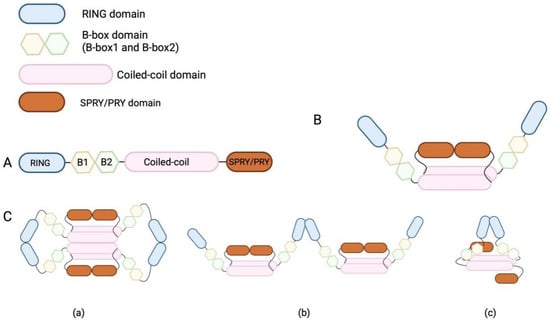
Figure 1.
Illustration of TRIM25 monomer and dimer structures and possible RING dimerization modes. (A) The structure of TRIM25, possessing a RING domain, a B-box domain, a coiled-coil domain, and a SPRY/PRY domain. (B) The structure of a TRIM25 dimer. (C) The possible modes of RING dimerization. This dimerization stabilizes the closed conformation, activates thioester intermediates, and facilitates ubiquitin transfer in chain synthesis. (Ca) The RING domains of two TRIM25 dimers interact to form a TRIM25 tetramer. (Cb) TRIM25 dimers link laterally in an end-to-end configuration for the formation of TRIM25 tetramer. (Cc) B-box domain contributes to RING dimerization by binding to the CC domain, which shortened the distance between two RING domains within a TRIM25 dimer. (This figure was created using BioRender. Liu, Q.X. https://BioRender.com/r07m967, accessed on 7 March 2025).
Several studies have extensively examined the functions of the RING domain, B-box domain, and CC domain in enhancing the catalytic activity of TRIM25. First, the CC domain of TRIM25 promotes the formation of a RING-based TRIM25 dimer through the RING domains to interact with E2 enzymes/ubiquitin and moves adjacent to the C-terminal alpha helix to complete the interaction [42,43]. Interestingly, one study has reported that the RING domain of TRIM25 is capable of mediating ubiquitination without requiring oligomerization for its activation [42], and CC domain-induced RING domain-based dimerization greatly enhances its catalytic capacity [42]. However, the RING monomer is insufficient for the formation of a polyubiquitin chain [44]. Considered to be an obligate dimer, the TRIM25 dimer undergoes further dimerization, which is believed to give rise to a closed conformation with E2/ubiquitin and facilitate further ubiquitin transfer [5,42,45]. Specifically, TRIM25 interacts with E2-conjugating enzymes through a conserved E2–E3 interface, while a RING dimer binds to the same ubiquitin, the proximal RING domain binds to the ubiquitin surface patch, and the distal RING domain interacts with ubiquitin, possibly through an acidic element outside the RING domain [5,42,45], thereby stabilizing a closed conformation, which activates ubiquitin thioester intermediates and further facilitates ubiquitin transfer [42]. Previous studies have shown that both the CC and B-box domains contribute to RING domain dimerization [40,41,42], while neither domain has an effect on the corresponding catalytic activity. Notably, it has been suggested that the B-box domain enhances RING dimerization by binding to the CC domain, thereby reducing the distance between the two RING domains in TRIM25 dimers [42] (Figure 1(Cc)). This hypothetical process, however, was deemed infeasible due to the inability of these two RING domains within TRIM25 dimers to achieve intramolecular linkage [5]. Consequently, alternative models for the RING domain association have been proposed, suggesting that TRIM25 may form higher-order aggregates [6]. For instance, the RING domains of two TRIM25 dimers can interact to form a tetramer (Figure 1(Ca)), or TRIM25 dimers can link laterally in an end-to-end configuration to form extended TRIM25 dimer chains [6] (Figure 1(Cb)). Therefore, the B-box and CC domains of TRIM25 are responsible for RING dimerization, and this dimerization greatly enhances catalytic activity during ubiquitin chain synthesis. The multiple domains of TRIM25, the structure of its dimer, and the possible mechanisms and working models of RING domain dimerization are illustrated in Figure 1.
2.3. The Structure of the SPRY/PRY Domain and Its Protein Interaction and RNA-Binding Ability
The PRY/SPRY domain is derived from the fusion of the SPRY and PRY domains [40]. It exhibits a β-sandwich structure, composed of two antiparallel β-sheets, one six-stranded sheet, and one antiparallel seven-stranded sheet [46]. Both the monomers and dimers of the PRY/SPRY domain exist at high protein concentrations, while only the monomers are present at low protein concentrations and form SPRY/PRY dimers in a concentration-dependent manner [46]. Dimerization occurs through concave contact between the C-terminal residues of one domain and the six-stranded beta-sheets of another [4,46], providing insight into PRY/SPRY’s interactions with other proteins. Specific loops and residues on the surface of the SPRY domain are crucial for recognizing and binding to target proteins. These regions can be tailored to interact with particular motifs or domains on other proteins [46]. In innate immunity, the PRY/SPRY domain of TRIM25 binds directly to the CARDs domain of RIG-I, triggering the RIG-I/IFN signaling pathway [47]. However, direct interactions between the SPRY domain and viral proteins can disrupt RIG-I ubiquitination, thus inhibiting the host immune response [19]. Additionally, the SPRY domain can interact with the M2-2 proteins of HMPV or the V proteins of paramyxovirus to inhibit the ubiquitination of RIG-I and the subsequent signaling pathway [48,49,50]. Moreover, a 39-amino-acid stretch in the C-terminal PRY/SPRY domain of TRIM25 can bind to RNA or RNA viruses to facilitate this domain’s interaction with AVPs, such as RIG-I [10,11]. Besides the PRY/SPRY domain, the L2 linker between the CC and SPRY domains contains a lysine-rich region of the 7K motif for RNA binding [12]. However, the 7K motif itself does not exist independently as an RNA-binding element. A study has shown that the 7K motif and SPRY domains are required for structural adaption in RNA binding [12]. Previous studies have revealed that the 7K motif in TRIM25 is critical for RNA virus infection-induced IFN production because its mutation significantly decreases the RNA-binding capacity of TRIM25 [10,12]. However, the binding of WT TRIM25 to RNA significantly enhances TRIM25-mediated RIG-I ubiquitination and IFN production [12]. Although the interaction between TRIM25 and RIG-I and the activation of downstream signaling pathways do not necessarily involve the binding of TRIM25 to the same RNA [12], this RNA-binding process is critical for the E3 ligase activity of TRIM25.
3. Biological Functions of TRIM25
3.1. Basic Functions of TRIM25
TRIM25 can directly or indirectly regulate various basic biological processes, such as cell proliferation, the cell cycle, protein stabilization, and mRNA translocation. These basic biological processes are critical in cancer development, inflammation, and innate immunity, and most of them are related to the E3 ligase activity and RNA-binding ability of TRIM25.
Recent studies have revealed novel mechanisms by which TRIM25 regulates cancer cell growth and migration based on its catalytic activity [20,22,51]. TRIM25 can modify protein targets through K63- or K48-linked polyubiquitination [11]. K63-linked polyubiquitination can activate multiple signaling pathways, promoting protein localization or modulating protein–protein interaction [22]. In contrast, K48-linked polyubiquitination can mediate protein degradation [11]. Interestingly, upregulated TRIM25 expression is usually detected in many types of tumor cells, especially in hormone-responsive tumors, such as breast cancer, prostate cancer, ovarian cancer, and endometrial cancer, because of its estrogen-responsive characteristics [18]. Upregulated TRIM25 expression is also detected in lung cancer, gastric cancer, and hepatocellular carcinoma [19]. TRIM25 recognizes 14-3-3σ and promotes its degradation to reduce its activity in breast and endometrial cancer [17,19,50], promoting cancer cell survival and proliferation. In particular, the degradation of 14-3-3σ induced by TRIM25 limits Mdm2 autoubiquitination and degradation, thereby destabilizing p53, attenuating p53-dependent tumor-suppressive signaling [17] (Figure 2(Bb)). Additionally, TRIM25 can target Keap1, a regulatory protein of Nrf2, and the proteasomal degradation of Keap1 increases the nuclear localization of Nrf2 to initiate anti-oxidative responsive gene expression [52] (Figure 2(Ba)). Furthermore, TRIM25 enhances tumor cell survival, as treatment with N6F11 to target TRIM25-mediated GPX4 degradation selectively induces cancer cell ferroptosis [17,53,54,55] (Figure 2(Bc)). Moreover, TRIM25 can target the phosphatase and tensin homolog (PTEN) by mediating its K48- and K63-linked polyubiquitination to enhance PI3K/AKT signaling and cancer cell growth [17,56,57]. Interestingly, TRIM25 can mediate breast cancer cell autophagy via UBC12-enhanced TRIM25 neddylation, which increases transcription factor EB (TFEB) K63-linked polyubiquitination and nuclear translocation and thus activates autophagy-related gene expression, leading to drug resistance [17,58] (Figure 2(Bd)). The specific mechanisms are shown in Figure 2B.
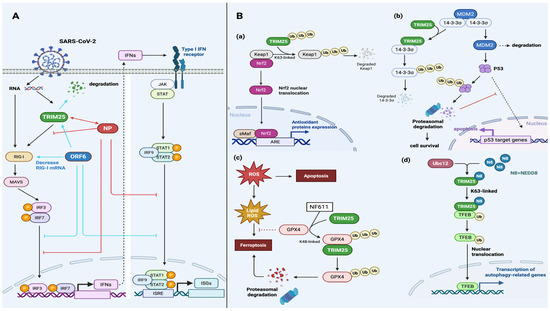
Figure 2.
Biological functions of TRIM25 in SARS-CoV-2 infection and cancer cell survival. (A) SARS-CoV-2 proteins participate in viral immune evasion by interfering with antiviral signaling. SARS-CoV-2 viral RNA can activate TRIM25 and RIG-I for the subsequent aggregation of MAVS, which regulate IRF3/7 and IFN production. The IFNs produced bind to a type I IFN receptor to activate the JAK/STAT signaling pathway, leading to the production of ISGs. Both the SARS-CoV-2 nucleocapsid protein (NP) and ORF6 proteins influence the nuclear translocation of IRF3/7 and STAT. The NP recruits G3BP2 and TRIM25, while ORF6 mediates TRIM25 degradation, limiting TRIM25-mediated RIG-I ubiquitination. (B) TRIM25-mediated ubiquitination regulates multiple biological pathways. (Ba) TRIM25 can lead to the degradation of Keap1, resulting in the nuclear translocation of Nrf2 and the production of antioxidant proteins. (Bb) 14-3-3σ can be degraded by TRIM25-mediated ubiquitination, which contributes to the degradation of Mdm2 and p53. (Bc) The inhibitory effect of GPX4 on ferroptosis is negated by the participation of TRIM25 in the treatment of N6F11. (Bd) Neddylated TRIM25 can induce the nuclear translocation of TEFB, which is responsible for the transcription of autophagy-related genes. (This figure was created using BioRender. Liu, Q.X. https://BioRender.com/zi5oxce, accessed on 7 May 2025).
Notably, the role the binding of TRIM25 to mRNAs plays has been described in regard to both cancer therapy and antiviral responses. A recent study has shown that TRIM25 can bind to caspase-7 mRNA to attenuate caspase-7 expression by reducing its mRNA stability in colon cancer cells [59]. Furthermore, TRIM25 can bind to the 5′ UTR of caspase-2 mRNA to inhibit its translation, but this binding does not interfere with the overall structure and integrity of caspase-2 mRNA [60]. Interestingly, TRIM25 expression is negatively targeted by miRNAs, which bind to the 3′ UTR of TRIM25 mRNA to inhibit its translation and promote its degradation [24,25]. In acute myeloid leukemia (AML), TRIM25 can promote tumor cell proliferation and migration, and its expression is negatively regulated by miRNA-137 [23]. Similarly, miR-365 can also inhibit the expression of TRIM25 in NSCLC [61].
The ubiquitination activity of TRIM25 enhances tumor cell survival and modifies regulatory proteins in innate immunity, e.g., the activation of RIG-I and TRAF proteins [62,63,64]. Based on its transcriptional and post-transcriptional regulatory mechanisms, TRIM25 serves as an oncogenic factor promoting the proliferation and migration of cancer cells. Furthermore, TRIM25 can bind viral RNAs to regulate the defense against viral infection in innate immunity.
3.2. TRIM25 Is Targeted to Inhibit RIG-I Signaling
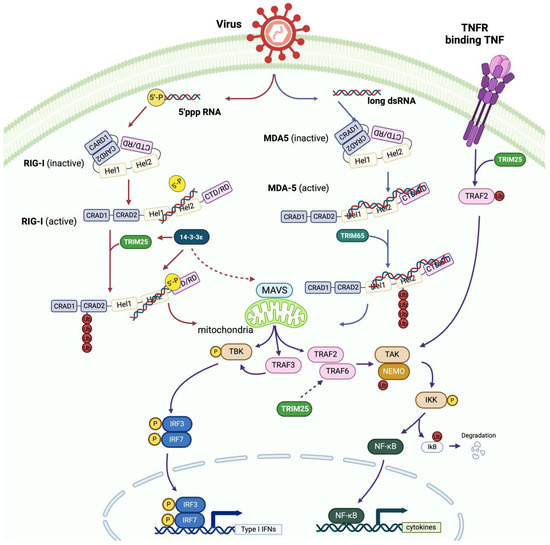
SARS-CoV-2 is a type of single-stranded positive-sense RNA virus [65]. The 5’ end of its viral genome has reading frames of 1a and 1b encoding 16 nonstructural proteins (NSP1–16), while the 3’ end of the viral genome contains multiple subgenomic RNAs for the expression of four structural proteins, namely, spike (S), envelope (E), membrane (M), and nucleocapsid proteins, as well as several accessory proteins, including ORF6 [65,66]. Previous studies have reported that nucleocapsid proteins (NPs), NSPs, and many SARS-CoV-2 accessory proteins have antagonistic effects on IFN responses [65,67,68,69,70,71,72]. The detailed mechanism underlying interactions between TRIM25 and virus accessory proteins (especially SARS-CoV-2) will be discussed in the following section (Table 1).

Table 1.
The interaction between TRIM25 and viral components.
SARS-CoV-2 infection can upregulate TRIM25 expression, which activates RIG-I and ZAP through ubiquitination and thus induces IFN production [67,82,83]. A study has reported that upregulated TRIM25 expression can limit SARS-CoV-2 replication [84], suggesting that upregulated TRIM25 expression is a viral-infection-induced compensative antiviral response. During SARS-CoV and MERS-CoV infection, the viral NP can inhibit the innate immune system and the antiviral activity of TRIM25 to promote viral invasion [62,67]. In fact, the SARS-CoV-2 NP can interact with TRIM25 at its N-terminal aa 1-360 and aa 1-175, C-terminal aa 361-419, and intermediate region aa 252-360, allowing it to interact with the SPRY and RING domains of TRIM25 to inhibit RIG-I polyubiquitination and IFN production because the direct binding of viral NPs to TRIM25 inhibits the binding of TRIM25 to RIG-I [70]. Interestingly, co-immunoprecipitation revealed that the viral NP, together with TRIM25 and RIG-I, formed a protein complex in epithelial cells [68]. The protein complex did not affect TRIM25 aggregation, but it did reduce RIG-I ubiquitination, impairing IRF activation [68]. It is likely that the viral NP mainly acts as an interposing element between TRIM25 and RIG-I, impeding their interaction to diminish IFN production [68]. In addition, the viral NP can recruit TRIM25 and G3BP2, enhancing their interactions, which, in turn, interfere with RIG-I signaling [73]. Consistently, the viral NP interacts with G3BP1 to impede the formation of stress granules (SGs) [72], wherein TRIM25 co-condensates with G3BP1 to enhance the polyubiquitination of RIG-I and other antiviral proteins [74]. The SARS-CoV-2 NSP5 and NP counteract antiviral SG formation by inhibiting virus-induced IFN production and RIG-I-mediated antiviral immunity [72]. Specifically, the viral NP dose-dependently attenuates TRIM25-mediated RIG-I antiviral signaling [68,70]. Low-dose NPs inhibit RIG-I ubiquitination by hampering the interaction between TRIM25 and RIG-I [68,70], reducing IFN-I production, while high-dose NPs enhance the phosphorylation of STAT1 and STAT2 and their nuclear translocation to induce ISG translation for antiviral defense [70].
Notably, the SARS-CoV-2 ORF6 protein can inhibit the antiviral effect of IFNs through a variety of mechanisms. Firstly, ORF6 can significantly reduce the levels of RIG-I ubiquitination by decreasing RIG-I mRNA levels by 50%, targeting TRIM25-related proteasomal degradation and inhibiting RIG-I activation and IFN production [65]. Secondly, ORF6 can directly inhibit the nuclear translocation of IRF3/7 and STAT1 for the induction of IFN and ISG activity and the triggering of the IFN-mediated signaling pathway [65].
Various types of viruses interact with TRIM25 to inhibit TRIM25-mediated RIG-I ubiquitination. For example, the NS1 protein of influenza A virus (NS1-A) can interact with the CC domain of TRIM25 to form stable complexes, preventing TRIM25-mediated RIG-I ubiquitination [76,77], or directly interact with RIG-I to inactivate IRF-3 and other transcription factors, inhibiting IFN transcription [85]. In contrast, through its RBD domain, the NS1 protein of influenza B virus (NS1-B) interacts with TRIM25, but not with RIG-I, to block the inhibition of the NS1-B CTD region upon RIG-I ubiquitination, enhancing IFN and inflammatory cytokine production in lung epithelial cells [78]. These two different effects of NS1 proteins from two types of influenza viruses on RIG-I signaling highlight the various pathogens and antiviral responses involved in early infection with influenza A and B virus.
Accordingly, the NP, NSP5, and ORF6 of SARS-CoV-2 virus interact with TRIM25 to inhibit RIG-I/IFN signaling, forming ternary complexes or degrading TRIM25. The specific signaling pathways and consequences of NP and ORF6 interference with antiviral processes are shown in Figure 2A. NS1-A and NS1-B proteins can interact with TRIM25, and both can inhibit or enhance RIG-1 ubiquitination and IFN production through various mechanisms.
3.3. TRIM25 Regulates Viral RNAs for Immune Evasion
A previous study has shown that TRIM25 may prefer to bind to dsRNA or ssRNA instead of DNA [12]. TRIM25 can bind to RNA through its SPRY domain and 7K motif [74]. After binding to RNA, TRIM25 can more effectively interact with RIG-I and act as a ubiquitin ligase for RIG-I ubiquitination [12]. Furthermore, PR-2B, an emerging viral clade of Dengue virus serotype 2 (DENV-2), can produce high levels of subgenomic flavivirus RNA (sfRNA), which sequence-dependently binds to TRIM25 and prevents its deubiquitination, inhibiting RIG-I-induced IFN production [81]. The PR-2B clade replaced the PR-1 clade during an epidemic lasting from 1995 to 2007 [86], and this process was accompanied by significant changes in the sequences of its 3’ UTR [86]. The increased expression of this sfRNA in viral genes is thought to promote viral replication and transmission and inhibit the action of TRIM25 [81]. The relatively low level of gRNA in this case may stimulate RIG-I and MDA5 in a moderate way, diminishing IFN production [81]. As a result, these would increase virus replication and blood viral concentrations, promoting DENV transmission between humans and mosquitoes [81].
The DENV-2 PR-2B clade can inhibit the RIG-I-dependent antiviral pathway. However, its sfRNA interacts with TRIM25 to inhibit virus replication and spread through a RIG-I-independent pathway. A previous study has shown that TRIM25, even in the case of an RNA-binding deficient TRIM25 mutant, can interact with IAV RNA, indicating that TRIM25 binds to viral RNA in a novel manner and is crucial for its antiviral activity [75]. TRIM25 binds to positive-strand IAV RNA to promote RNA instability and degradation, inhibiting IAV replication and assembly [75].
TRIM25’s RNA-binding ability is critical to its capacity to restrict viral replication and spread through RIG-I-dependent or -independent pathways. Further investigations are necessary to explore the novel RNA-binding mode of TRIM25 in the absence of currently known RBD.
5. Discussion
In this review, we have discussed the crucial role of TRIM25 in defending against viral evasion via its E3 ubiquitin ligase activity, which contributes to the RIG-I-related antiviral effect and its RNA-binding capacity to inhibit viral replication. A recent study has shown that TRIM25 can ubiquitinate RIG-I to induce the production of IFNs as well as regulate TRAF6 and IKK activities and MDA5/MAVS downstream signaling [94]. Furthermore, both TRIM25 and ZAP prefer to recognize CpG-rich sequences, which triggers TRIM25 to use RNA as a platform to modify ZAP via K48- and K63-linked ubiquitination [13,82]. Additionally, TRIM25 interacts with the NP of EBOV to expose its CpG-rich sequences to ZAP [79]. It is unknown whether TRIM25 can interact with the NPs of other viruses to enhance ZAP antiviral activity and how TRIM25 activates ZAP. It is possible that the binding of TRIM25 to viral RNA leads to TRIM25 conformational changes that enhance TRIM25-mediated ZAP ubiquitination. Apparently, the activation of antiviral proteins by TRIM25 depends on its E3 ligase activity and RNA-binding ability. Inhibiting TRIM25’s dimerization, its catalytic activity, and its interaction with RIG-I will attenuate its antiviral activity and thus promote viral immune evasion. However, TRIM25 can also directly inhibit the expression of viral RNA by degrading it [75]. Even without ubiquitinating NPs in vRNPs, TRIM25 can bind to NPs and RNA to inhibit RNA elongation, thus inhibiting viral replication and strengthening the innate immunity [101].
Thus, TRIM25 can bind to viral RNA, inhibit RNA elongation [101] and viral replication [102], and mediate RNA degradation [75], limiting viral spreading. TRIM25 serves as an enhancer of ubiquitination-mediated RIG-I activation and participates in SG formation and ZAP antiviral processes. Additionally, various viral components interact with the CC and SPRY/PRY domains of TRIM25 to inhibit its conformational changes and antiviral protein activation, facilitating viral immune evasion [62,85,94]. Furthermore, some viral proteins recruit regulatory proteins to inhibit the subsequent activity of TRIM25 [73]. Moreover, viral RNA binds to TRIM25 to suppress its deubiquitination and inhibit the activation of TRIM25 E3 ligase activity [81].
Despite advances in our understanding of the critical roles of TRIM25 in antiviral defense and viral immune evasion, limitations remain that necessitate further investigation. We propose several future directions and perspectives for research in this specific area. First, an in-depth exploration of the regulatory mechanisms governing TRIM25 expression is needed. For instance, elucidating how viral proteins and RNAs modulate TRIM25 levels (i.e., by interacting with host miRNAs) [27,28] may provide valuable insights into the conditions that promote or inhibit its activity and may help to identify new therapeutic targets. Second, delineating the molecular pathways through which viral proteins inhibit the antiviral functions of TRIM25 will allow researchers to identify potential therapeutic targets, thus paving the way for the development of antiviral agents that either enhance RIM25 activity or counteract viral evasion mechanisms. Third, there is currently no information on how TRIM25 binds to RNA viruses, independent of its known RNA-binding motif, given that this action may initiate viral mRNA degradation. In fact, TRIM25 overexpression is associated with decreases in viral replication. Hence, interference with TRIM25 expression is also a common strategy for viral evasion. Therefore, TRIM25 may serve as a suitable therapeutic target for enhancing antiviral responses to limit viral spread. Finally, future studies should consider the implication of TRIM25 in the context of emerging viral pathogens and the evolving landscape of viral immune evasion strategies. As new viruses emerge, understanding the role of TRIM25 in antiviral responses will be critical for developing effective vaccines and therapeutics.
Author Contributions
Conceptualization, Q.L. and Z.X.; writing—original draft preparation, Q.L.; writing—review and editing, Q.L., S.P., J.W. and Z.X.; supervision, Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the National Natural Science Foundation of China (41966006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The figures were created using Biorender.com.
Conflicts of Interest
The authors declare there are no conflicts of interest.
References
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.P.; Haubrich, K.; Perez-Borrajero, C.; Hennig, J. Emerging RNA-binding roles in the TRIM family of ubiquitin ligases. Biol. Chem. 2019, 400, 1443–1464. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Wu, W.; Zhuo, W.; Liu, W.; Zhang, Y.; Cheng, M.; Chen, Y.G.; Gao, N.; Yu, H.; et al. Structural insights into the TRIM family of ubiquitin E3 ligases. Cell Res. 2014, 24, 762–765. [Google Scholar] [CrossRef]
- Sanchez, J.G.; Okreglicka, K.; Chandrasekaran, V.; Welker, J.M.; Sundquist, W.I.; Pornillos, O. The tripartite motif coiled-coil is an elongated antiparallel hairpin dimer. Proc. Natl. Acad. Sci. USA 2014, 111, 2494–2499. [Google Scholar] [CrossRef]
- Choudhury, N.R.; Heikel, G.; Michlewski, G. TRIM25 and its emerging RNA-binding roles in antiviral defense. Wiley Interdiscip. Rev. RNA 2020, 11, e1588. [Google Scholar] [CrossRef]
- Baltz, A.G.; Munschauer, M.; Schwanhäusser, B.; Vasile, A.; Murakawa, Y.; Schueler, M.; Youngs, N.; Penfold-Brown, D.; Drew, K.; Milek, M.; et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 2012, 46, 674–690. [Google Scholar] [CrossRef]
- Trendel, J.; Schwarzl, T.; Horos, R.; Prakash, A.; Bateman, A.; Hentze, M.W.; Krijgsveld, J. The Human RNA-Binding Proteome and Its Dynamics during Translational Arrest. Cell 2019, 176, 391–403.e319. [Google Scholar] [CrossRef]
- Álvarez, L.; Haubrich, K.; Iselin, L.; Gillioz, L.; Ruscica, V.; Lapouge, K.; Augsten, S.; Huppertz, I.; Choudhury, N.R.; Simon, B.; et al. The molecular dissection of TRIM25’s RNA-binding mechanism provides key insights into its antiviral activity. Nat. Commun. 2024, 15, 8485. [Google Scholar] [CrossRef]
- Choudhury, N.R.; Heikel, G.; Trubitsyna, M.; Kubik, P.; Nowak, J.S.; Webb, S.; Granneman, S.; Spanos, C.; Rappsilber, J.; Castello, A.; et al. RNA-binding activity of TRIM25 is mediated by its PRY/SPRY domain and is required for ubiquitination. BMC Biol. 2017, 15, 105. [Google Scholar] [CrossRef]
- Martín-Vicente, M.; Medrano, L.M.; Resino, S.; García-Sastre, A.; Martínez, I. TRIM25 in the Regulation of the Antiviral Innate Immunity. Front. Immunol. 2017, 8, 1187. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.G.; Sparrer, K.M.J.; Chiang, C.; Reis, R.A.; Chiang, J.J.; Zurenski, M.A.; Wan, Y.; Gack, M.U.; Pornillos, O. TRIM25 Binds RNA to Modulate Cellular Anti-viral Defense. J. Mol. Biol. 2018, 430, 5280–5293. [Google Scholar] [CrossRef]
- Cagliani, R.; Forni, D.; Mozzi, A.; Fuchs, R.; Hagai, T.; Sironi, M. Evolutionary analysis of ZAP and its cofactors identifies intrinsically disordered regions as central elements in host-pathogen interactions. Comput. Struct. Biotechnol. J. 2024, 23, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.A.; Meagher, J.L.; Takata, M.A.; Gonçalves-Carneiro, D.; Yeoh, Z.C.; Ohi, M.D.; Smith, J.L.; Bieniasz, P.D. Functional anatomy of zinc finger antiviral protein complexes. Nat. Commun. 2024, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Tian, X.; Kong, C.; Hong, W.; Xiao, T.; Wang, S.; Wei, Z.; Su, Z.; Ren, H.; et al. The structural basis of TRIM25-mediated regulation of RIG-I. J. Biol. Chem. 2025, 301, 108367. [Google Scholar] [CrossRef]
- Suleman, M.; Sayaf, A.M.; Khan, A.; Khan, S.A.; Albekairi, N.A.; Alshammari, A.; Agouni, A.; Yassine, H.M.; Crovella, S. Molecular screening of phytocompounds targeting the interface between influenza A NS1 and TRIM25 to enhance host immune responses. J. Infect. Public Health 2024, 17, 102448. [Google Scholar] [CrossRef]
- Eberhardt, W.; Nasrullah, U.; Pfeilschifter, J. TRIM25: A Global Player of Cell Death Pathways and Promising Target of Tumor-Sensitizing Therapies. Cells 2025, 14, 65. [Google Scholar] [CrossRef]
- Chiang, C.; Yap, B.K. TRIM25, TRIM28 and TRIM59 and Their Protein Partners in Cancer Signaling Crosstalk: Potential Novel Therapeutic Targets for Cancer. Curr. Issues Mol. Biol. 2024, 46, 10745–10761. [Google Scholar] [CrossRef]
- Rahimi-Tesiye, M.; Zaersabet, M.; Salehiyeh, S.; Jafari, S.Z. The role of TRIM25 in the occurrence and development of cancers and inflammatory diseases. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188954. [Google Scholar] [CrossRef]
- Tecalco-Cruz, A.C.; Abraham-Juarez, M.J.; Solleiro-Villavicencio, H.; Ramirez-Jarquin, J.O. TRIM25: A central factor in breast cancer. World J. Clin. Oncol. 2021, 12, 646–655. [Google Scholar] [CrossRef]
- Nakasato, N.; Ikeda, K.; Urano, T.; Horie-Inoue, K.; Takeda, S.; Inoue, S. A ubiquitin E3 ligase Efp is up-regulated by interferons and conjugated with ISG15. Biochem. Biophys. Res. Commun. 2006, 351, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Heikel, G.; Choudhury, N.R.; Michlewski, G. The role of Trim25 in development, disease and RNA metabolism. Biochem. Soc. Trans. 2016, 44, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, B.S.; Yang, Y.; Li, Y.; Lv, J.L.; Cheng, Y. TRIM25 contributes to the malignancy of acute myeloid leukemia and is negatively regulated by microRNA-137. Open Med. 2021, 16, 95–103. [Google Scholar] [CrossRef]
- Lu, L.F.; Gasteiger, G.; Yu, I.S.; Chaudhry, A.; Hsin, J.P.; Lu, Y.; Bos, P.D.; Lin, L.L.; Zawislak, C.L.; Cho, S.; et al. A Single miRNA-mRNA Interaction Affects the Immune Response in a Context- and Cell-Type-Specific Manner. Immunity 2015, 43, 52–64. [Google Scholar] [CrossRef]
- Wang, J.; Yin, G.; Bian, H.; Yang, J.; Zhou, P.; Yan, K.; Liu, C.; Chen, P.; Zhu, J.; Li, Z.; et al. LncRNA XIST upregulates TRIM25 via negatively regulating miR-192 in hepatitis B virus-related hepatocellular carcinoma. Mol. Med. 2021, 27, 41. [Google Scholar] [CrossRef]
- Vuillier, F.; Li, Z.; Black, I.; Cruciani, M.; Rubino, E.; Michel, F.; Pellegrini, S. IFN-I inducible miR-3614-5p targets ADAR1 isoforms and fine tunes innate immune activation. Front. Immunol. 2022, 13, 939907. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Li, L.; Li, X.; Shen, L.; Gong, J.; Zhang, R. MicroRNA-30a Modulates Type I Interferon Responses to Facilitate Coxsackievirus B3 Replication Via Targeting Tripartite Motif Protein 25. Front. Immunol. 2020, 11, 603437. [Google Scholar] [CrossRef]
- Liu, W.; Jin, Y.; Zhang, W.; Xiang, Y.; Jia, P.; Yi, M.; Jia, K. MiR-202-5p Inhibits RIG-I-Dependent Innate Immune Responses to RGNNV Infection by Targeting TRIM25 to Mediate RIG-I Ubiquitination. Viruses 2020, 12, 261. [Google Scholar] [CrossRef]
- Chen, D.; Ji, Q.; Liu, J.; Cheng, F.; Zheng, J.; Ma, Y.; He, Y.; Zhang, J.; Song, T. MicroRNAs in the Regulation of RIG-I-like Receptor Signaling Pathway: Possible Strategy for Viral Infection and Cancer. Biomolecules 2023, 13, 1344. [Google Scholar] [CrossRef]
- Lee, N.R.; Choi, J.Y.; Yoon, I.H.; Lee, J.K.; Inn, K.S. Positive regulatory role of c-Src-mediated TRIM25 tyrosine phosphorylation on RIG-I ubiquitination and RIG-I-mediated antiviral signaling pathway. Cell Immunol. 2018, 332, 94–100. [Google Scholar] [CrossRef]
- Zou, W.; Wang, J.; Zhang, D.E. Negative regulation of ISG15 E3 ligase EFP through its autoISGylation. Biochem. Biophys. Res. Commun. 2007, 354, 321–327. [Google Scholar] [CrossRef] [PubMed]
- El-Asmi, F.; McManus, F.P.; Thibault, P.; Chelbi-Alix, M.K. Interferon, restriction factors and SUMO pathways. Cytokine Growth Factor. Rev. 2020, 55, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Loo, Y.M.; Horner, S.M.; Zornetzer, G.A.; Katze, M.G.; Gale, M., Jr. The mitochondrial targeting chaperone 14-3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe 2012, 11, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Yla-Anttila, P.; Sandalova, T.; Sun, R.; Achour, A.; Masucci, M.G. 14-3-3 scaffold proteins mediate the inactivation of trim25 and inhibition of the type I interferon response by herpesvirus deconjugases. PLoS Pathog. 2019, 15, e1008146. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Shi, X.H.; Chang, X.; Han, Y.; Liu, C.; Jiang, Z.; Yang, X. The mechanism of linear ubiquitination in regulating cell death and correlative diseases. Cell Death Dis. 2023, 14, 659. [Google Scholar] [CrossRef]
- Shibata, Y.; Komander, D. LUBAC. Curr. Biol. 2022, 32, R506–R508. [Google Scholar] [CrossRef]
- Oshiumi, H. Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses. Front. Immunol. 2020, 11, 1296. [Google Scholar] [CrossRef]
- Oudshoorn, D.; Versteeg, G.A.; Kikkert, M. Regulation of the innate immune system by ubiquitin and ubiquitin-like modifiers. Cytokine Growth Factor. Rev. 2012, 23, 273–282. [Google Scholar] [CrossRef]
- Horie-Inoue, K.; Inoue, S. Epigenetic and proteolytic inactivation of 14-3-3sigma in breast and prostate cancers. Semin. Cancer Biol. 2006, 16, 235–239. [Google Scholar] [CrossRef]
- Ozato, K.; Shin, D.M.; Chang, T.H.; Morse, H.C., 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef]
- Koepke, L.; Gack, M.U.; Sparrer, K.M. The antiviral activities of TRIM proteins. Curr. Opin. Microbiol. 2021, 59, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, M.G.; Esposito, D.; Christodoulou, E.; Taylor, I.A.; Rittinger, K. Functional role of TRIM E3 ligase oligomerization and regulation of catalytic activity. EMBO J. 2016, 35, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.G.; Chiang, J.J.; Sparrer, K.M.J.; Alam, S.L.; Chi, M.; Roganowicz, M.D.; Sankaran, B.; Gack, M.U.; Pornillos, O. Mechanism of TRIM25 Catalytic Activation in the Antiviral RIG-I Pathway. Cell Rep. 2016, 16, 1315–1325. [Google Scholar] [CrossRef]
- Streich, F.C., Jr.; Ronchi, V.P.; Connick, J.P.; Haas, A.L. Tripartite motif ligases catalyze polyubiquitin chain formation through a cooperative allosteric mechanism. J. Biol. Chem. 2013, 288, 8209–8221. [Google Scholar] [CrossRef]
- Dou, H.; Buetow, L.; Sibbet, G.J.; Cameron, K.; Huang, D.T. Essentiality of a non-RING element in priming donor ubiquitin for catalysis by a monomeric E3. Nat. Struct. Mol. Biol. 2013, 20, 982–986. [Google Scholar] [CrossRef]
- Grütter, C.; Briand, C.; Capitani, G.; Mittl, P.R.; Papin, S.; Tschopp, J.; Grütter, M.G. Structure of the PRYSPRY-domain: Implications for autoinflammatory diseases. FEBS Lett. 2006, 580, 99–106. [Google Scholar] [CrossRef]
- D’Cruz, A.A.; Babon, J.J.; Norton, R.S.; Nicola, N.A.; Nicholson, S.E. Structure and function of the SPRY/B30.2 domain proteins involved in innate immunity. Protein Sci. 2013, 22, 1–10. [Google Scholar] [CrossRef]
- Sanchez-Aparicio, M.T.; Feinman, L.J.; Garcia-Sastre, A.; Shaw, M.L. Paramyxovirus V Proteins Interact with the RIG-I/TRIM25 Regulatory Complex and Inhibit RIG-I Signaling. J. Virol. 2018, 92, 10-1128. [Google Scholar] [CrossRef]
- Morita, N.; Tanaka, Y.; Odkhuu, E.; Naiki, Y.; Komatsu, T.; Koide, N. Sendai virus V protein decreases nitric oxide production by inhibiting RIG-I signaling in infected RAW264.7 macrophages. Microbes Infect. 2020, 22, 322–330. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morita, N.; Kitagawa, Y.; Gotoh, B.; Komatsu, T. Human metapneumovirus M2-2 protein inhibits RIG-I signaling by preventing TRIM25-mediated RIG-I ubiquitination. Front. Immunol. 2022, 13, 970750. [Google Scholar] [CrossRef]
- Urano, T.; Saito, T.; Tsukui, T.; Fujita, M.; Hosoi, T.; Muramatsu, M.; Ouchi, Y.; Inoue, S. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature 2002, 417, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tao, S.; Liao, L.; Li, Y.; Li, H.; Li, Z.; Lin, L.; Wan, X.; Yang, X.; Chen, L. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat. Commun. 2020, 11, 348. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Kang, R.; Tang, D. Cell type-specific induction of ferroptosis to boost antitumor immunity. Oncoimmunology 2023, 12, 2282252. [Google Scholar] [CrossRef]
- Zou, J.; Wang, L.; Tang, H.; Liu, X.; Peng, F.; Peng, C. Ferroptosis in Non-Small Cell Lung Cancer: Progression and Therapeutic Potential on It. Int. J. Mol. Sci. 2021, 22, 13335. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef]
- He, Y.M.; Zhou, X.M.; Jiang, S.Y.; Zhang, Z.B.; Cao, B.Y.; Liu, J.B.; Zeng, Y.Y.; Zhao, J.; Mao, X.L. TRIM25 activates AKT/mTOR by inhibiting PTEN via K63-linked polyubiquitination in non-small cell lung cancer. Acta Pharmacol. Sin. 2022, 43, 681–691. [Google Scholar] [CrossRef]
- Yan, H.; Ma, Y.L.; Gui, Y.Z.; Wang, S.M.; Wang, X.B.; Gao, F.; Wang, Y.P. MG132, a proteasome inhibitor, enhances LDL uptake in HepG2 cells in vitro by regulating LDLR and PCSK9 expression. Acta Pharmacol. Sin. 2014, 35, 994–1004. [Google Scholar] [CrossRef]
- Zheng, B.; Qian, F.; Wang, X.; Wang, Y.; Zhou, B.; Fang, L. Neddylation activated TRIM25 desensitizes triple-negative breast cancer to paclitaxel via TFEB-mediated autophagy. J. Exp. Clin. Cancer Res. 2024, 43, 177. [Google Scholar] [CrossRef]
- Nasrullah, U.; Stanke, K.; Recknagel, V.; Bozkurt, S.; Wurzel, P.; Gauer, S.; Imre, G.; Münch, C.; Pfeilschifter, J.; Eberhardt, W. The E3 Ligase TRIM25 Impairs Apoptotic Cell Death in Colon Carcinoma Cells via Destabilization of Caspase-7 mRNA: A Possible Role of hnRNPH1. Cells 2023, 12, 201. [Google Scholar] [CrossRef]
- Nasrullah, U.; Haeussler, K.; Biyanee, A.; Wittig, I.; Pfeilschifter, J.; Eberhardt, W. Identification of TRIM25 as a Negative Regulator of Caspase-2 Expression Reveals a Novel Target for Sensitizing Colon Carcinoma Cells to Intrinsic Apoptosis. Cells 2019, 8, 622. [Google Scholar] [CrossRef]
- Han, Q.; Cheng, P.; Yang, H.; Liang, H.; Lin, F. Altered expression of microRNA-365 is related to the occurrence and development of non-small-cell lung cancer by inhibiting TRIM25 expression. J. Cell Physiol. 2019, 234, 22321–22330. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Eisenacher, K.; Kirchhofer, A.; Brzozka, K.; Lammens, A.; Lammens, K.; Fujita, T.; Conzelmann, K.K.; Krug, A.; Hopfner, K.P. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol. Cell 2008, 29, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aparicio, M.T.; Ayllon, J.; Leo-Macias, A.; Wolff, T.; Garcia-Sastre, A. Subcellular Localizations of RIG-I, TRIM25, and MAVS Complexes. J. Virol. 2017, 91, 10-1128. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Huang, Y.; Sun, M.; Tian, Q.; Zhang, S.; Qin, Y. TRIM25 Promotes TNF-alpha-Induced NF-kappaB Activation through Potentiating the K63-Linked Ubiquitination of TRAF2. J. Immunol. 2020, 204, 1499–1507. [Google Scholar] [CrossRef]
- Khatun, O.; Sharma, M.; Narayan, R.; Tripathi, S. SARS-CoV-2 ORF6 protein targets TRIM25 for proteasomal degradation to diminish K63-linked RIG-I ubiquitination and type-I interferon induction. Cell Mol. Life Sci. 2023, 80, 364. [Google Scholar] [CrossRef]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, Z.; Yu, L.; Shi, D.; Zhu, M.; Yao, H.; Li, L. Interactome Analysis of the Nucleocapsid Protein of SARS-CoV-2 Virus. Pathogens 2021, 10, 1155. [Google Scholar] [CrossRef]
- Gori Savellini, G.; Anichini, G.; Gandolfo, C.; Cusi, M.G. SARS-CoV-2 N Protein Targets TRIM25-Mediated RIG-I Activation to Suppress Innate Immunity. Viruses 2021, 13, 1439. [Google Scholar] [CrossRef]
- Hu, Y.; Li, W.; Gao, T.; Cui, Y.; Jin, Y.; Li, P.; Ma, Q.; Liu, X.; Cao, C. The Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Inhibits Type I Interferon Production by Interfering with TRIM25-Mediated RIG-I Ubiquitination. J. Virol. 2017, 91, e02143-16. [Google Scholar] [CrossRef]
- Zhao, Y.; Sui, L.; Wu, P.; Wang, W.; Wang, Z.; Yu, Y.; Hou, Z.; Tan, G.; Liu, Q.; Wang, G. A dual-role of SARS-CoV-2 nucleocapsid protein in regulating innate immune response. Signal Transduct. Target. Ther. 2021, 6, 331. [Google Scholar] [CrossRef]
- Oh, S.J.; Shin, O.S. SARS-CoV-2 Nucleocapsid Protein Targets RIG-I-Like Receptor Pathways to Inhibit the Induction of Interferon Response. Cells 2021, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, J.; Han, L.; Zhuang, M.W.; Xu, Y.; Zhang, J.; Nan, M.L.; Xiao, Y.; Zhan, P.; Liu, X.; et al. SARS-CoV-2 NSP5 and N protein counteract the RIG-I signaling pathway by suppressing the formation of stress granules. Signal Transduct. Target. Ther. 2022, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.; Li, J.; Zheng, H.; Li, H.; Lai, Q.; Chen, Y.; Qin, L.; Zuo, Y.; Guo, L.; et al. Engagement of the G3BP2-TRIM25 Interaction by Nucleocapsid Protein Suppresses the Type I Interferon Response in SARS-CoV-2-Infected Cells. Vaccines 2022, 10, 2042. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, S.; Wang, J.; Zhou, L.; Zhang, X.; Billadeau, D.D.; Yang, P.; Zhang, L.; Zhou, F.; Bai, P.; et al. TRIM25 predominately associates with anti-viral stress granules. Nat. Commun. 2024, 15, 4127. [Google Scholar] [CrossRef]
- Choudhury, N.R.; Trus, I.; Heikel, G.; Wolczyk, M.; Szymanski, J.; Bolembach, A.; Dos Santos Pinto, R.M.; Smith, N.; Trubitsyna, M.; Gaunt, E.; et al. TRIM25 inhibits influenza A virus infection, destabilizes viral mRNA, but is redundant for activating the RIG-I pathway. Nucleic Acids Res. 2022, 50, 7097–7114. [Google Scholar] [CrossRef]
- Gack, M.U.; Albrecht, R.A.; Urano, T.; Inn, K.S.; Huang, I.C.; Carnero, E.; Farzan, M.; Inoue, S.; Jung, J.U.; Garcia-Sastre, A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 2009, 5, 439–449. [Google Scholar] [CrossRef]
- Koliopoulos, M.G.; Lethier, M.; van der Veen, A.G.; Haubrich, K.; Hennig, J.; Kowalinski, E.; Stevens, R.V.; Martin, S.R.; Reis e Sousa, C.; Cusack, S.; et al. Molecular mechanism of influenza A NS1-mediated TRIM25 recognition and inhibition. Nat. Commun. 2018, 9, 104502. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Fan, W.; Zheng, W.; Yu, M.; Chen, C.; Sun, L.; Bi, Y.; Ding, C.; Gao, G.F.; et al. Robust Lys63-Linked Ubiquitination of RIG-I Promotes Cytokine Eruption in Early Influenza B Virus Infection. J. Virol. 2016, 90, 6263–6275. [Google Scholar] [CrossRef]
- Galão, R.P.; Wilson, H.; Schierhorn, K.L.; Debeljak, F.; Bodmer, B.S.; Goldhill, D.; Hoenen, T.; Wilson, S.J.; Swanson, C.M.; Neil, S.J.D. TRIM25 and ZAP target the Ebola virus ribonucleoprotein complex to mediate interferon-induced restriction. PLoS Pathog. 2022, 18, e1010530. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chiweshe, S.; McCormick, D.; Raper, A.; Wickenhagen, A.; DeFillipis, V.; Gaunt, E.; Simmonds, P.; Wilson, S.J.; Grey, F. Human cytomegalovirus evades ZAP detection by suppressing CpG dinucleotides in the major immediate early 1 gene. PLoS Pathog. 2020, 16, e1008844. [Google Scholar] [CrossRef]
- Manokaran, G.; Finol, E.; Wang, C.; Gunaratne, J.; Bahl, J.; Ong, E.Z.; Tan, H.C.; Sessions, O.M.; Ward, A.M.; Gubler, D.J.; et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 2015, 350, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Lau, Z.; Cheung, P.; Aguilar, E.G.; Schneider, W.M.; Bozzacco, L.; Molina, H.; Buehler, E.; Takaoka, A.; Rice, C.M.; et al. TRIM25 Enhances the Antiviral Action of Zinc-Finger Antiviral Protein (ZAP). PLoS Pathog. 2017, 13, e1006145. [Google Scholar] [CrossRef] [PubMed]
- Gack, M.U.; Shin, Y.C.; Joo, C.H.; Urano, T.; Liang, C.; Sun, L.; Takeuchi, O.; Akira, S.; Chen, Z.; Inoue, S.; et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 2007, 446, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, R.; Rahimi, P.; Fateh, A.; Hamidi-Fard, M.; Eaybpoosh, S.; Bahramali, G.; Sadeghi, S.A.; Doroud, D.; Aghasadeghi, M. Exploring the impression of TRIM25 gene expression on COVID-19 severity and SARS-CoV-2 viral replication. J. Infect. Public Health 2024, 17, 102489. [Google Scholar] [CrossRef]
- Mibayashi, M.; Martinez-Sobrido, L.; Loo, Y.M.; Cardenas, W.B.; Gale, M., Jr.; Garcia-Sastre, A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 2007, 81, 514–524. [Google Scholar] [CrossRef]
- Goertz, G.P.; Pijlman, G.P. Dengue Non-coding RNA: TRIMmed for Transmission. Cell Host Microbe 2015, 18, 133–134. [Google Scholar] [CrossRef]
- Brisse, M.; Ly, H. Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front. Immunol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- Min, J.; Li, Y.; Li, X.; Wang, M.; Li, H.; Bi, Y.; Xu, P.; Liu, W.; Ye, X.; Li, J. The circRNA circVAMP3 restricts influenza A virus replication by interfering with NP and NS1 proteins. PLoS Pathog. 2023, 19, e1011577. [Google Scholar] [CrossRef]
- Diaz-Beneitez, E.; Cubas-Gaona, L.L.; Candelas-Rivera, O.; Benito-Zafra, A.; Sanchez-Aparicio, M.T.; Miorin, L.; Rodriguez, J.F.; Garcia-Sastre, A.; Rodriguez, D. Interaction between chicken TRIM25 and MDA5 and their role in mediated antiviral activity against IBDV infection. Front. Microbiol. 2022, 13, 1068328. [Google Scholar] [CrossRef]
- Jiang, X.; Kinch, L.N.; Brautigam, C.A.; Chen, X.; Du, F.; Grishin, N.V.; Chen, Z.J. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity 2012, 36, 959–973. [Google Scholar] [CrossRef]
- Okamoto, M.; Kouwaki, T.; Fukushima, Y.; Oshiumi, H. Regulation of RIG-I Activation by K63-Linked Polyubiquitination. Front. Immunol. 2018, 8, 1942. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Miyashita, M.; Matsumoto, M.; Seya, T. A distinct role of Riplet-mediated K63-Linked polyubiquitination of the RIG-I repressor domain in human antiviral innate immune responses. PLoS Pathog. 2013, 9, e1003533. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-R.; Kim, H.-I.; Choi, M.-S.; Yi, C.-M.; Inn, K.-S. Regulation of MDA5-MAVS Antiviral Signaling Axis by TRIM25 through TRAF6-Mediated NF-κB Activation. Mol. Cells 2015, 38, 759–764. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, R.; Liang, D.; Wang, W.; Min, K.; Luo, T.; Li, X. Uncovering the Interaction between TRAF1 and MAVS in the RIG-I Pathway to Enhance the Upregulation of IRF1/ISG15 during Classical Swine Fever Virus Infection. Cells 2024, 13, 1165. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, Y.; Fu, X.; Bai, G.; Li, X.; Mao, J.; Yan, Y.; Hu, L. Multiple functions of stress granules in viral infection at a glance. Front. Microbiol. 2023, 14, 1138864. [Google Scholar] [CrossRef]
- McVean, G.; Kerns, J.A.; Emerman, M.; Malik, H.S. Positive Selection and Increased Antiviral Activity Associated with the PARP-Containing Isoform of Human Zinc-Finger Antiviral Protein. PLoS Genet. 2008, 4, e21. [Google Scholar] [CrossRef]
- Ficarelli, M.; Neil, S.J.D.; Swanson, C.M. Targeted Restriction of Viral Gene Expression and Replication by the ZAP Antiviral System. Annu. Rev. Virol. 2021, 8, 265–283. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Tu, F.; Wang, Q.; Fan, Z.; Gao, G.; Diamond, M.S. TRIM25 Is Required for the Antiviral Activity of Zinc Finger Antiviral Protein. J. Virol. 2017, 91, e00088-17. [Google Scholar] [CrossRef]
- Yang, E.; Nguyen, L.P.; Wisherop, C.A.; Kan, R.L.; Li, M.M.H. The Role of ZAP and TRIM25 RNA Binding in Restricting Viral Translation. Front. Cell Infect. Microbiol. 2022, 12, 886929. [Google Scholar] [CrossRef]
- Nchioua, R.; Kmiec, D.; Müller, J.A.; Conzelmann, C.; Groß, R.; Swanson, C.M.; Neil, S.J.D.; Stenger, S.; Sauter, D.; Münch, J.; et al. SARS-CoV-2 Is Restricted by Zinc Finger Antiviral Protein despite Preadaptation to the Low-CpG Environment in Humans. mBio 2020, 11. [Google Scholar] [CrossRef]
- Meyerson, N.R.; Zhou, L.; Guo, Y.R.; Zhao, C.; Tao, Y.J.; Krug, R.M.; Sawyer, S.L. Nuclear TRIM25 Specifically Targets Influenza Virus Ribonucleoproteins to Block the Onset of RNA Chain Elongation. Cell Host Microbe 2017, 22, 627–638.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Pang, X.; Liu, Z.; Li, Q.; Yi, D.; Zhang, Y.; Fang, X.; Zhang, T.; Zhou, R.; et al. An anti-influenza A virus microbial metabolite acts by degrading viral endonuclease PA. Nat. Commun. 2022, 13, 2079. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).