Prevalence of Doravirine Resistance Mutations in a Large-Scale HIV-1 Transmitted Drug Resistance Survey in Buenos Aires, Argentina
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Proceedings
2.3. Inclusion Criteria
- Participants aged 18 years or older, at the time of genotype sampling.
- Documented HIV-1 infection (based on a reactive Enzyme-Linked Immunosorbent Assay plus Western blot or detectable viral load).
- Treatment-naïve, defined as no antiretrovirals (in combination or monotherapy) received before resistance test based on clinical records.
2.4. Exclusion Criteria
- PLWHA without available clinical records to assure ART naïve status.
- PLWHA with genotype assessed outside routine clinical care (e.g., clinical trial).
- PLWHA participating in clinical trials for HIV treatment or prevention.
2.5. Statistical Methods
2.6. Ethical Statement
3. Results
3.1. Study Population Characteristics
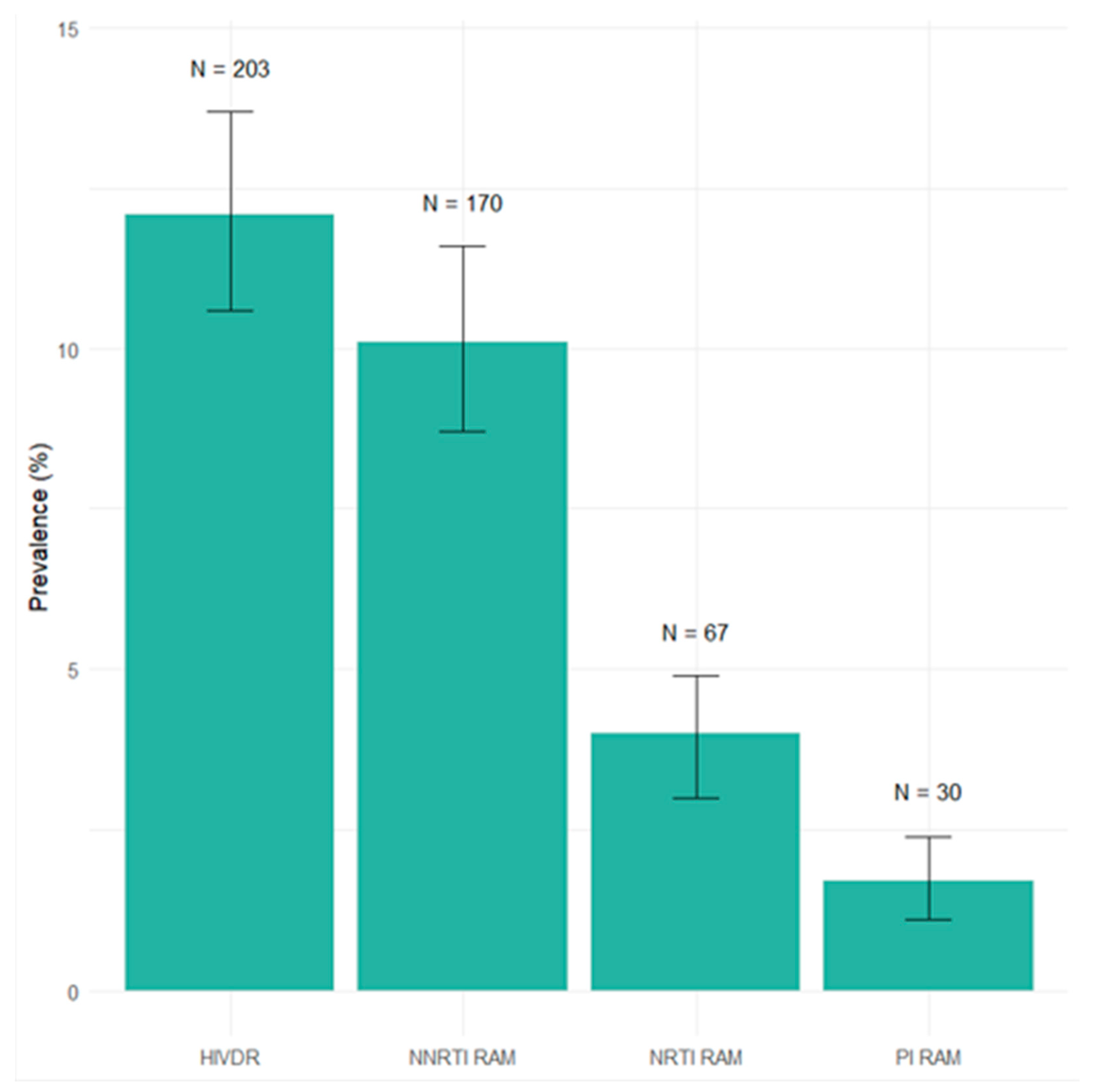
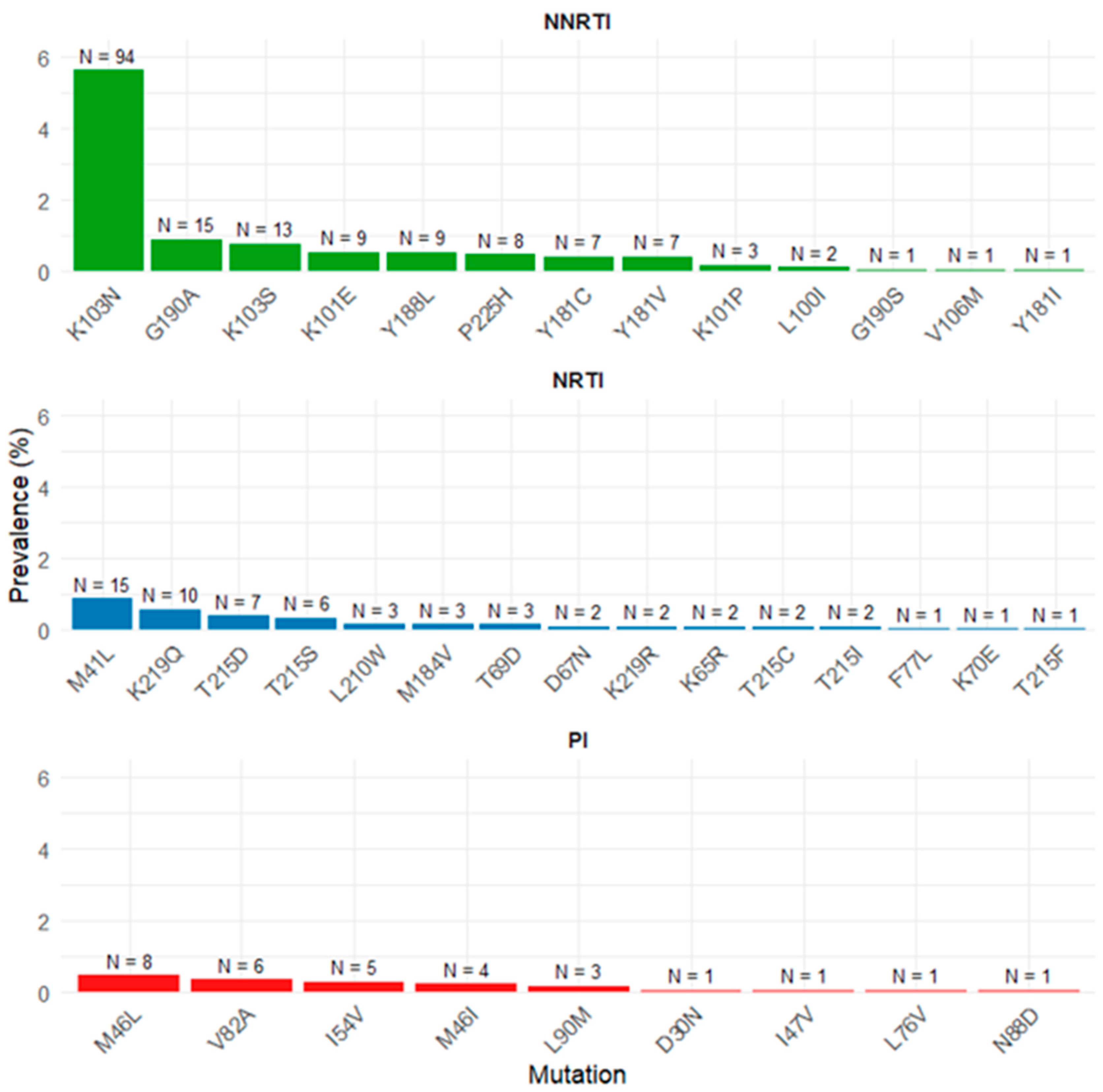
3.2. Transmitted Drug Resistance Mutations According to WHO List
3.3. Transmitted Drug Resistance to Doravirine
3.4. Transmitted Drug Resistance to Rilpivirine
3.5. Predicted Efficacy of NNRTI Drugs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broder, S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 2010, 85, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Bedimo, R.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA 2023, 329, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Arribas, J.R.; Alejos, B.; Bernardino, J.I.; Coiras, M.; Coll, P.; Del Romero, J.; Fuster, M.J.; Górgolas, M.; Gutiérrez, A.; et al. Past and future of HIV infection. A document based on expert opinion. Rev. Esp. Quimioter. 2022, 35, 131–156. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 29 April 2025).
- World Health Organization. Available online: https://www.who.int/data/gho/data/themes/hiv-aids (accessed on 29 April 2025).
- Masters, M.C.; Krueger, K.M.; Williams, J.L.; Morrison, L.; Cohn, S.E. Beyond one pill, once daily: Current challenges of antiretroviral therapy management in the United States. Expert Rev. Clin. Pharmacol. 2019, 12, 1129–1143. [Google Scholar] [CrossRef]
- Apetroaei, M.M.; Velescu, B.Ș.; Nedea, M.I.I.; Dinu-Pîrvu, C.E.; Drăgănescu, D.; Fâcă, A.I.; Udeanu, D.I.; Arsene, A.L. The Phenomenon of Antiretroviral Drug Resistance in the Context of Human Immunodeficiency Virus Treatment: Dynamic and Ever Evolving Subject Matter. Biomedicines 2024, 12, 915. [Google Scholar] [CrossRef]
- Shafer, R.W.; Schapiro, J.M. HIV-1 drug resistance mutations: An updated framework for the second decade of HAART. AIDS Rev. 2008, 10, 67–84. [Google Scholar]
- Wensing, A.M.; Calvez, V.; Günthard, H.F.; Johnson, V.A.; Paredes, R.; Pillay, D.; Shafer, R.W.; Richman, D.D. 2014 Update of the drug resistance mutations in HIV-1. Top. Antivir. Med. 2014, 22, 642–650. [Google Scholar]
- Ge, L.; Luo, Y.; Li, X.; Hu, Y.; Sun, L.; Bu, F.; Shan, D.; Liu, J. Global, regional, and national prevalence of HIV-1 drug resistance in treatment-naive and treatment-experienced children and adolescents: A systematic review and meta-analysis. EClinicalMedicine 2024, 77, 102859. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gregson, J.; Parkin, N.; Haile-Selassie, H.; Tanuri, A.; Andrade Forero, L.; Kaleebu, P.; Watera, C.; Aghokeng, A.; Mutenda, N.; et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: A systematic review and meta-regression analysis. Lancet Infect. Dis. 2018, 18, 346–355. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Zhang, Y.; Liu, X.; Li, A.; Gao, M.; Zhang, T.; Wu, H.; Chen, G.; Huang, X. Transmitted Drug Resistance in Antiretroviral Therapy-Naive Persons with Acute/Early/Primary HIV Infection: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 718763. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Kassaye, S.G.; Barrow, G.; Sundaramurthi, J.C.; Jordan, M.R.; Shafer, R.W. HIV-1 transmitted drug resistance surveillance: Shifting trends in study design and prevalence estimates. J. Int. AIDS Soc. 2020, 23, e25611. [Google Scholar] [CrossRef] [PubMed]
- Mbuagbaw, L.; Garcia, C.; Brenner, B.; Cecchini, D.; Chakroun, M.; Djiadeu, P.; Holguin, A.; Mor, O.; Parkin, N.; Santoro, M.M.; et al. Checklist for studies of HIV drug resistance prevalence or incidence: Rationale and recommended use. Lancet HIV 2023, 10, e684–e689. [Google Scholar] [CrossRef] [PubMed]
- Dirección de Respuesta al VIH, ITS, Hepatitis Virales y Tuberculosis, Ministerio de Salud de la Nación Argentina. Banco de Recursos de Comunicación del Ministerio de Salud de la Nación. Available online: https://bancos.salud.gob.ar/recurso/boletin-ndeg-40-respuesta-al-vih-y-las-its-en-la-argentina (accessed on 29 April 2025).
- Laufer, N.L.; Bouzas, M.B.; Fernández Giuliano, S.; Zapiola, I.; Mammana, L.; Salomon, H.; Monzani, C.; Castro, G.; Suarez Ornani, M.L.; Rojas Machado, P.; et al. Pretreatment HIV-1 Resistance in Argentina: Results from the Second Surveillance Study Following World Health Organization Guidelines (2019). AIDS Res. Hum. Retroviruses 2024, 40, 464–470. [Google Scholar] [CrossRef]
- Colombier, M.A.; Molina, J.M. Doravirine: A review. Curr. Opin. HIV AIDS 2018, 13, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Sachs, N.A.; Xu, M.; Grobler, J.; Blair, W.; Hazuda, D.J.; Miller, M.D.; Lai, M.T. Doravirine Suppresses Common Nonnucleoside Reverse Transcriptase Inhibitor-Associated Mutants at Clinically Relevant Concentrations. Antimicrob. Agents Chemother. 2016, 60, 2241–2247. [Google Scholar] [CrossRef]
- Hwang, C.; Lai, M.T.; Hazuda, D. Rational Design of Doravirine: From Bench to Patients. ACS Infect. Dis. 2020, 6, 64–73. [Google Scholar] [CrossRef]
- Martin, E.A.; Lai, M.T.; Ngo, W.; Feng, M.; Graham, D.; Hazuda, D.J. Review of Doravirine Resistance Patterns Identified in Participants During Clinical Development. J. Acquir. Immune Defic. Syndr. 2020, 85, 635–642. [Google Scholar] [CrossRef]
- Pham, H.T.; Xiao, M.A.; Principe, M.A.; Wong, A.; Mesplède, T. Pharmaceutical, clinical, and resistance information on doravirine, a novel non-nucleoside reverse transcriptase inhibitor for the treatment of HIV-1 infection. Drugs Context 2020, 9, 2019-11-4. [Google Scholar] [CrossRef]
- Sociedad Argentina de Infectología. Consenso Argentino de Terapia Antirretroviral 2023. Available online: https://www.sadi.org.ar/publicaciones/item/1733-consenso-argentino-de-terapia-antirretroviral (accessed on 29 April 2025).
- Stanford HIV Drug Resistance Database. Available online: https://hivdb.stanford.edu/dr-summary/resistance-notes/NNRTI/ (accessed on 29 April 2025).
- ANRS. HIV-1 Genotypic Drug Resistance Interpretation’s Algorithms. Non-Nucleoside Reverse Transcriptase Inhibitors. Available online: https://hivfrenchresistance.org/hiv-french-resitance-non-nucleoside-reverse-transcriptase-inhibitors/ (accessed on 29 April 2025).
- Bennett, D.E.; Camacho, R.J.; Otelea, D.; Kuritzkes, D.R.; Fleury, H.; Kiuchi, M.; Heneine, W.; Kantor, R.; Jordan, M.R.; Schapiro, J.M.; et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE 2009, 4, e4724. [Google Scholar] [CrossRef]
- PAHO HIV Report. Available online: https://www.paho.org/sites/default/files/PAHO-HIVDR%20Report.pdf (accessed on 29 April 2025).
- Bissio, E.; Barbás, M.G.; Bouzas, M.B.; Cudolá, A.; Salomón, H.; Espínola, L.; Ornani, M.S.; Ravasi, G. Pretreatment HIV-1 drug resistance in Argentina: Results from a surveillance study performed according to WHO-proposed new methodology in 2014–2015. J. Antimicrob. Chemother. 2017, 72, 504–510. [Google Scholar] [CrossRef]
- Ferreira, A.C.G.; Coelho, L.E.; Grinsztejn, E.; Jesus, C.S.; Guimarães, M.L.; Veloso, V.G.; Grinsztejn, B.; Cardoso, S.W. Transmitted drug resistance in patients with acute/recent HIV infection in Brazil. Braz. J. Infect. Dis. 2017, 21, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Aulicino, P.C.; Zapiola, I.; Kademian, S.; Valle, M.M.; Fernandez Giuliano, S.; Toro, R.; Barbas, G.; Canizal, A.M.; Mayon, P.; Golemba, M.D.; et al. Pre-treatment drug resistance and HIV-1 subtypes in infants from Argentina with and without exposure to antiretroviral drugs for prevention of mother-to-child transmission. J. Antimicrob. Chemother. 2019, 74, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rios, S.; Sued, O.; Rhee, S.Y.; Shafer, R.W.; Reyes-Teran, G.; Ravasi, G. Surveillance of HIV Transmitted Drug Resistance in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158560. [Google Scholar] [CrossRef]
- Melikian, G.L.; Rhee, S.Y.; Varghese, V.; Porter, D.; White, K.; Taylor, J.; Towner, W.; Troia, P.; Burack, J.; DeJesus, E.; et al. Non-nucleoside reverse transcriptase inhibitor (NNRTI) cross-resistance: Implications for preclinical evaluation of novel NNRTIs and clinical genotypic resistance testing. J. Antimicrob. Chemother. 2014, 69, 12–20. [Google Scholar] [CrossRef] [PubMed]
- EACS Guidelines. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed on 29 April 2025).
- Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. What to Start: Non-Nucleoside Reverse Transcriptase Inhibitor Regimens. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-non-nucleoside-reverse?view=full (accessed on 29 April 2025).
- Miranda, M.N.S.; Pingarilho, M.; Pimentel, V.; Martins, M.D.R.O.; Kaiser, R.; Seguin-Devaux, C.; Paredes, R.; Zazzi, M.; Incardona, F.; Abecasis, A.B. Trends of Transmitted and Acquired Drug Resistance in Europe from 1981 to 2019: A Comparison Between the Populations of Late Presenters and Non-late Presenters. Front. Microbiol. 2022, 13, 846943. [Google Scholar] [CrossRef]
- Kagan, R.M.; Dunn, K.J.; Snell, G.P.; Nettles, R.E.; Kaufman, H.W. Trends in HIV-1 Drug Resistance Mutations from a U.S. Reference Laboratory from 2006 to 2017. AIDS Res. Hum. Retroviruses 2019, 35, 698–709. [Google Scholar] [CrossRef]
- Ross, L.L.; Shortino, D.; Shaefer, M.S. Changes from 2000 to 2009 in the Prevalence of HIV-1 Containing Drug Resistance-Associated Mutations from Antiretroviral Therapy-Naive, HIV-1-Infected Patients in the United States. AIDS Res. Hum. Retroviruses 2018, 34, 672–679. [Google Scholar] [CrossRef]
- Cecchini, D.; Castillo, S.; Vecchio, C.; Sandoval, C.; Cabral, L.; Rodríguez Iantorno, P.; Cassetti, I. Primary HIV resistance in Buenos Aires metropolitan area. Medicina 2015, 75, 163–168. [Google Scholar]
- Zapiola, I.; Cecchini, D.; Fernández Giuliano, S.; Martínez, M.; Rodríguez, C.; Bouzas, M.B. HIV-1 resistance to antiretroviral drugs in pregnant women from Buenos Aires metropolitan area. Medicina 2016, 76, 349–354. [Google Scholar]
- Cecchini, D.; Sfalcin, J.; Zapiola, I.; Gómez, A.; Fernández Giuliano, S.; Mammana, L.; Seravalle, A.; Rodríguez, C.; Fay, F.; Bouzas, M.B. Reverse transcriptase and protease inhibitors mutational viral load in HIV infected pregnant women with transmitted drug resistance in Argentina. Rev. Esp. Quimioter. 2021, 34, 371–375. [Google Scholar] [CrossRef]
- Rozenszajn, M.; Arazi-Caillaud, S.; Taicz, M.; Bologna, R.; Mangano, A.; Aulicino, P.C. HIV-1 pretreatment drug resistance in vertically infected children is associated with poor virological response to protease inhibitor (PI)-based first-line antiretroviral therapy (ART): Results from a cohort study in Argentina. J. Antimicrob. Chemother. 2022, 77, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Bissio, E.; Barbás, M.G.; Kademián, S.; Bouzas, M.B.; Salomón, H.; Cudolá, A.; Giuliano, S.F.; Falistocco, C. Prevalence of rilpivirine resistance in people starting antiretroviral treatment in Argentina. Antivir. Ther. 2017, 24, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Luz, P.M.; Morris, B.L.; Grinsztejn, B.; Freedberg, K.A.; Veloso, V.G.; Walensky, R.P.; Losina, E.; Nakamura, Y.M.; Girouard, M.P.; Sax, P.E.; et al. Cost-effectiveness of genotype testing for primary resistance in Brazil. J. Acquir. Immune Defic. Syndr. 2015, 68, 152–161. [Google Scholar] [CrossRef]
- Sax, P.E.; Islam, R.; Walensky, R.P.; Losina, E.; Weinstein, M.C.; Goldie, S.J.; Sadownik, S.N.; Freedberg, K.A. Should resistance testing be performed for treatment-naive HIV-infected patients? A cost-effectiveness analysis. Clin. Infect. Dis. 2005, 41, 1316–1323. [Google Scholar] [CrossRef]
- Soulie, C.; Santoro, M.M.; Charpentier, C.; Storto, A.; Paraskevis, D.; Di Carlo, D.; Gennari, W.; Sterrantino, G.; Zazzi, M.; Perno, C.F.; et al. Rare occurrence of doravirine resistance-associated mutations in HIV-1-infected treatment-naive patients. J. Antimicrob. Chemother. 2019, 74, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, K.; Urbańska, A.; Jakubowski, P.; Hlebowicz, M.; Bociąga-Jasik, M.; Raczyńska, A.; Szymczak, A.; Szetela, B.; Łojewski, W.; Parczewski, M. Low prevalence of doravirine-associated resistance mutations among polish human immunodeficiency-1 (HIV-1)-infected patients. Antivir. Ther. 2021, 26, 69–78. [Google Scholar] [CrossRef]
- Bareng, O.T.; Seselamarumo, S.; Seatla, K.K.; Choga, W.T.; Bakae, B.; Maruapula, D.; Kelentse, N.; Moraka, N.O.; Mokaleng, B.; Mokgethi, P.T.; et al. Doravirine-associated resistance mutations in antiretroviral therapy naïve and experienced adults with HIV-1 subtype C infection in Botswana. J. Glob. Antimicrob. Resist. 2022, 31, 128–134. [Google Scholar] [CrossRef]
| Variable | N, (%) |
|---|---|
| Age (median, IQR) | 34 (28–43) |
| Gender | |
| Male | 1356 (81.2) |
| Female | 318 (19) |
| Transgender | 3 (0.17) |
| Route of infection (n = 1442) | |
| Sexual | |
| Heterosexual | 681 (47.23) |
| MSM | 759 (52.6) |
| Other | 2 (0.14) |
| Viral load (copies/mL), median (IQR) | 50,550 (15,300–183,500) |
| CD4 T-cell count (cell/uL), median (IQR) | 363 (212–532) |
| Recent infection * | 166 (9.97) |
| Clinical category (n = 1670) | |
| Asymptomatic | 1263 (75.6) |
| Class B symptoms | 139 (8.3) |
| Class C symptoms | 173 (10.3) |
| Acute retroviral syndrome | 95 (5.63) |
| N (%) | |
| K101E | 9 (0.53) |
| Y188L | 9 (0.53) |
| Y181V | 7 (0.41) |
| L100I | 2 (1.17) |
| V106M | 1 (0.05) |
| G190S | 1 (0.05) |
| Y181I | 1 (0.05) |
| N (%) | |
|---|---|
| Y188L | 9 (0.53) |
| K103N + P225H | 6 (0.35) |
| K103N + Y181C | 3 (0.17) |
| L100I + K103N | 2 (0.11) |
| G190S | 1 (0.05) |
| V106M | 1 (0.05) |
| At least 4 of A98G, L100I, K101E, V106I, E138K, | |
| Y181C, Y181V, G190A or H221Y, L100I, K103N, | |
| K103N + Y181C, K103N + P225H, F227C | 1 (0.05) |
| Mutations Associated with Possible Resistance | N (%) |
|---|---|
| E138A | 64 (3.83) |
| E138G | 9 (0.53) |
| Y188L | 8 (0.47) |
| Y181C | 7 (0.41) |
| E138K | 7 (0.41) |
| K101E | 5 (0.29) |
| Y181V | 5 (0.29) |
| K101P | 3 (0.17) |
| E138Q | 1 (0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchini, D.; Cassetti, I.; Scarnato, F.; Fiori, A.; Nuevo, J.; Villaverde, C.; Sucari, A.; Torroija, M.C.; Bissio, E.; Bugarin, G.; et al. Prevalence of Doravirine Resistance Mutations in a Large-Scale HIV-1 Transmitted Drug Resistance Survey in Buenos Aires, Argentina. Viruses 2025, 17, 731. https://doi.org/10.3390/v17050731
Cecchini D, Cassetti I, Scarnato F, Fiori A, Nuevo J, Villaverde C, Sucari A, Torroija MC, Bissio E, Bugarin G, et al. Prevalence of Doravirine Resistance Mutations in a Large-Scale HIV-1 Transmitted Drug Resistance Survey in Buenos Aires, Argentina. Viruses. 2025; 17(5):731. https://doi.org/10.3390/v17050731
Chicago/Turabian StyleCecchini, Diego, Isabel Cassetti, Florencia Scarnato, Agustina Fiori, Jimena Nuevo, Clara Villaverde, Adriana Sucari, María C. Torroija, Emiliano Bissio, Gabriela Bugarin, and et al. 2025. "Prevalence of Doravirine Resistance Mutations in a Large-Scale HIV-1 Transmitted Drug Resistance Survey in Buenos Aires, Argentina" Viruses 17, no. 5: 731. https://doi.org/10.3390/v17050731
APA StyleCecchini, D., Cassetti, I., Scarnato, F., Fiori, A., Nuevo, J., Villaverde, C., Sucari, A., Torroija, M. C., Bissio, E., Bugarin, G., & Lopardo, G. (2025). Prevalence of Doravirine Resistance Mutations in a Large-Scale HIV-1 Transmitted Drug Resistance Survey in Buenos Aires, Argentina. Viruses, 17(5), 731. https://doi.org/10.3390/v17050731







