Transcriptomic Analysis of the Spleen from Asian Seabass (Lates calcarifer) Infected with Infectious Spleen and Kidney Necrosis Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Detection and Isolation
2.2. Viral Plaque Purification
2.3. Experimental Infection
2.4. RNA Isolation and Transcriptome Sequencing
2.5. Analysis of DEGs
2.6. Identification and Visualization of Hub Genes
2.7. Validation of the DEGs by RT-qPCR
2.8. Statistical Analysis
3. Results
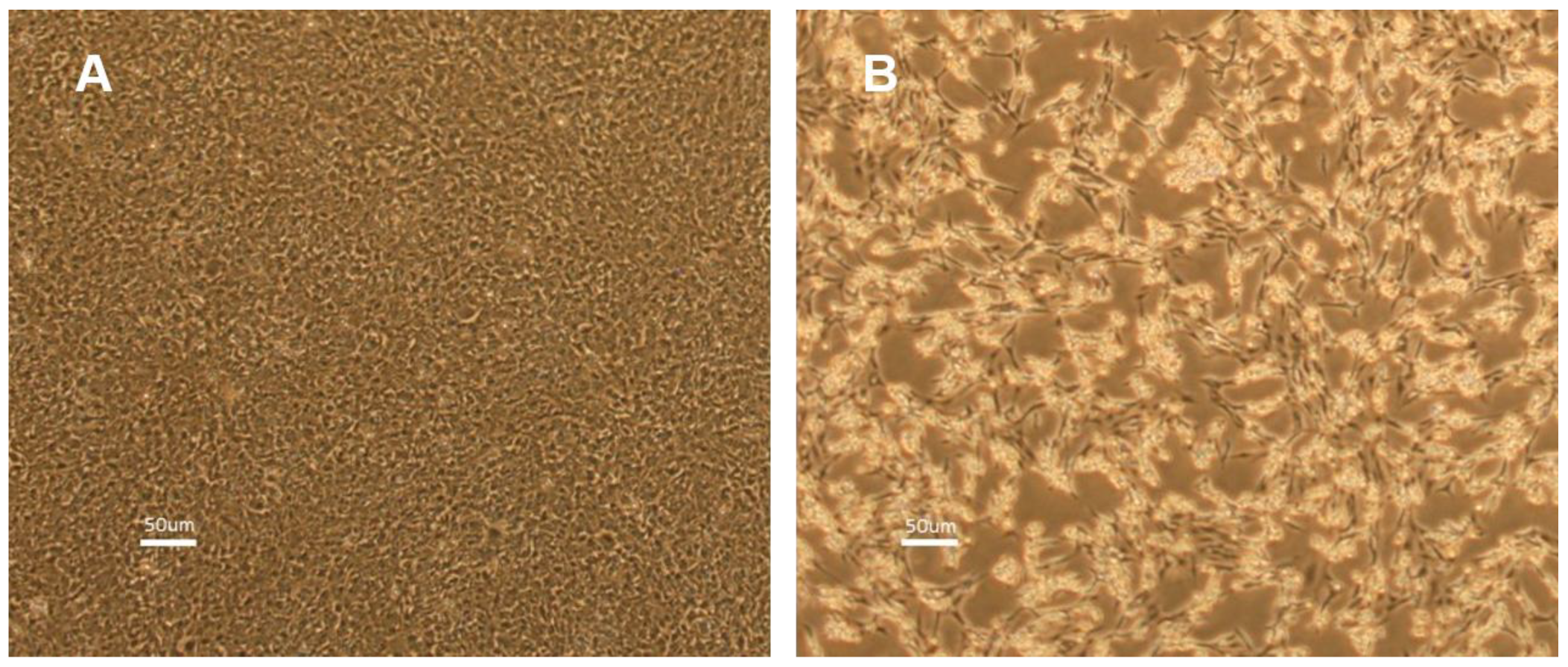
3.1. Isolation and Verification of ISKNV
3.2. DEG Analysis in Spleens of ISKNV-Infected and Healthy Asian Seabass
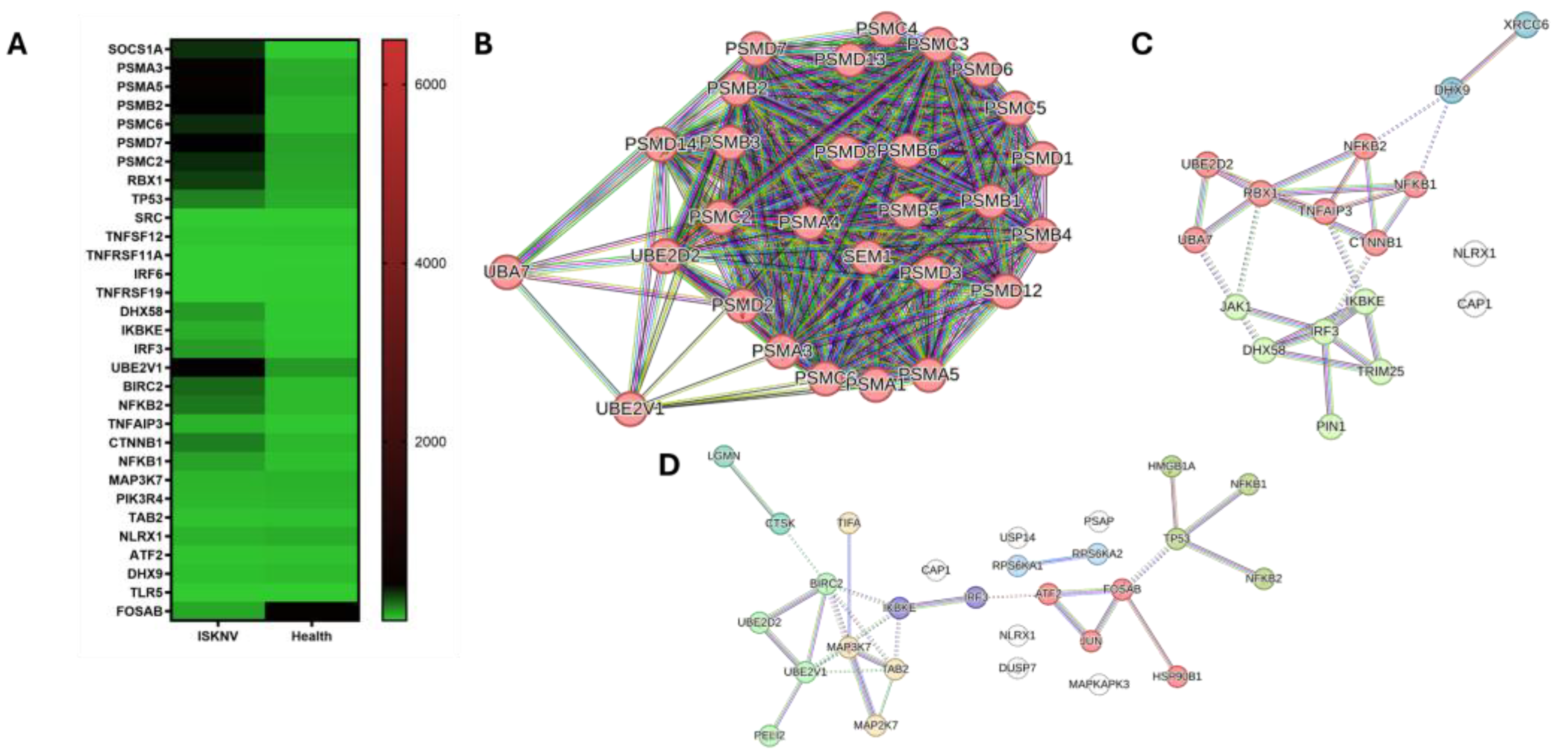
3.3. DEGs Involved in Immune Response
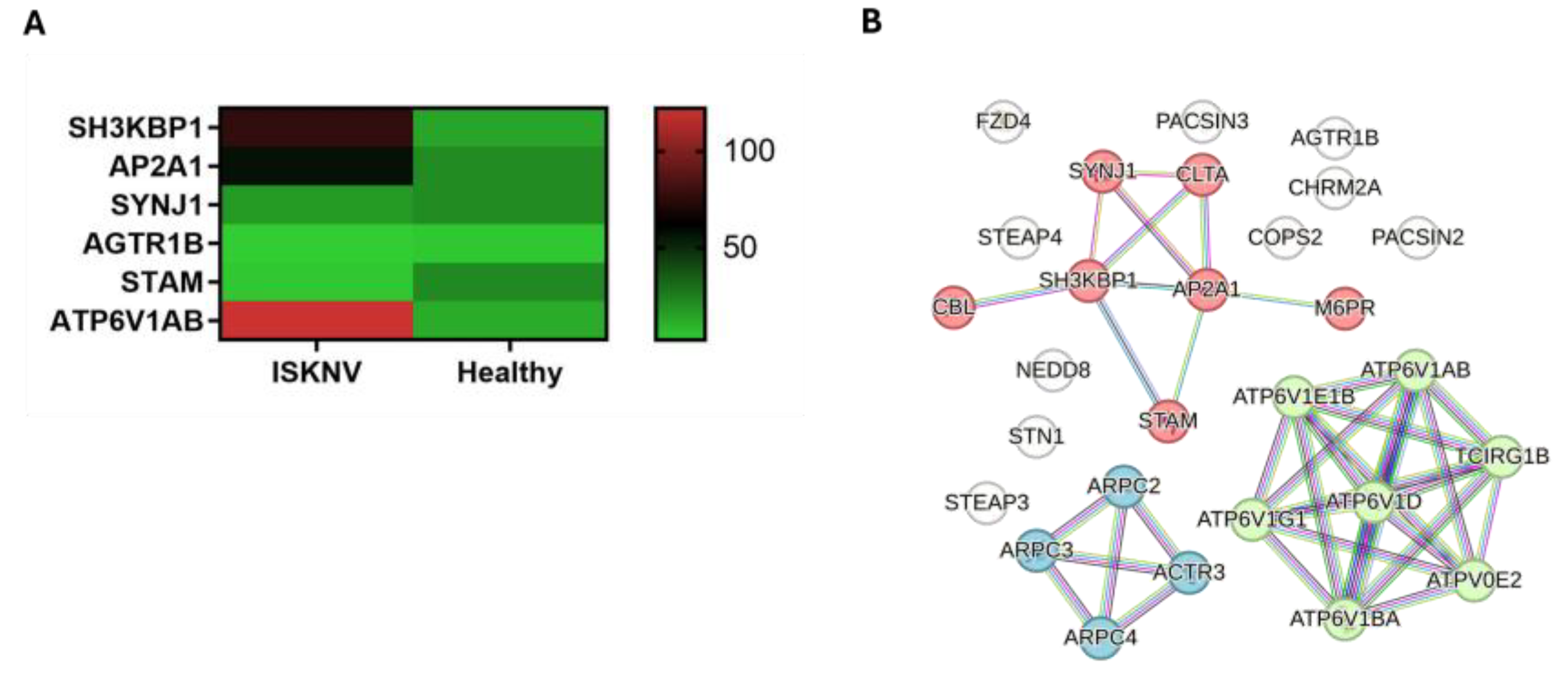
3.4. DEGs Involved in Endocytosis
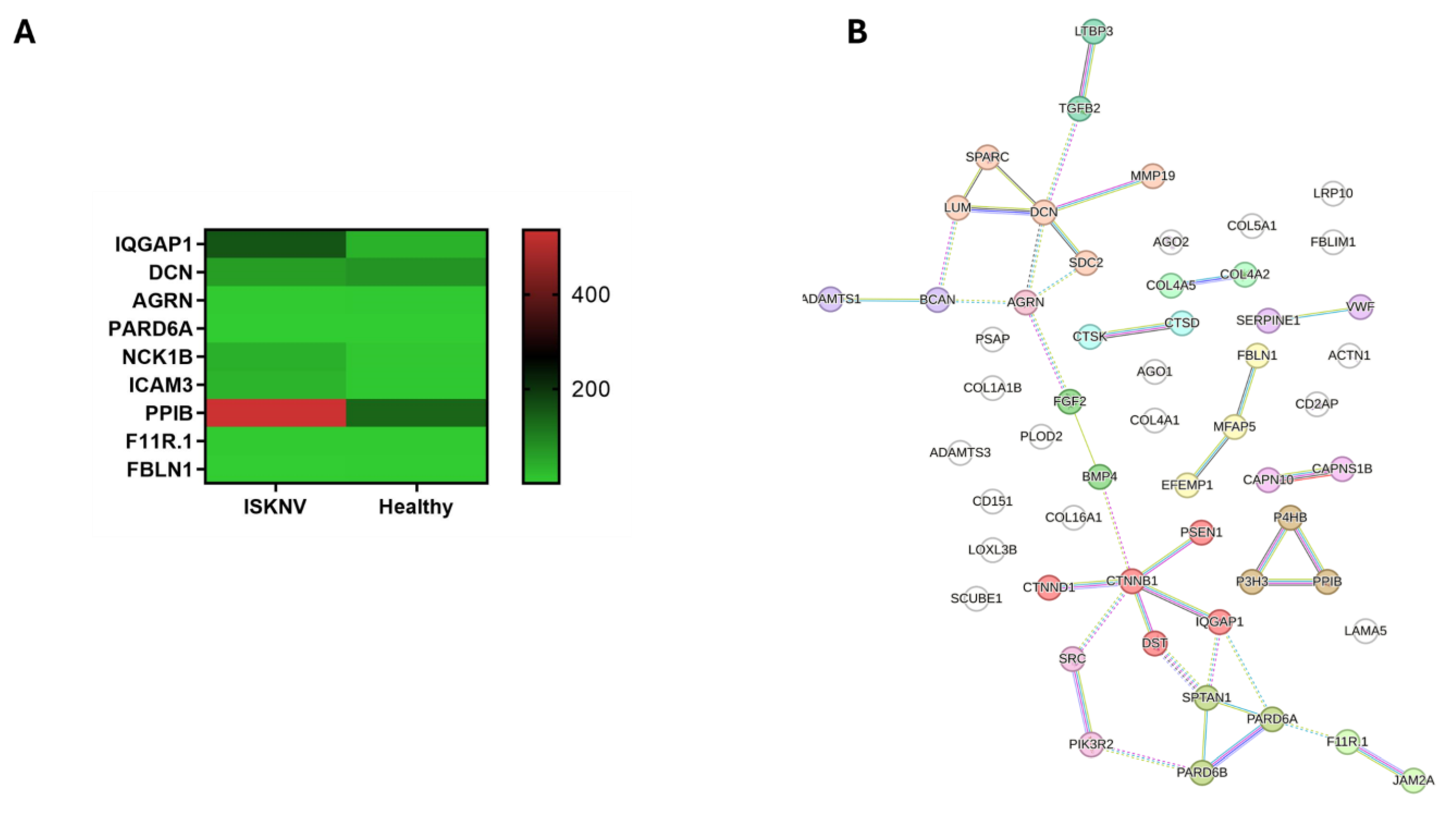
3.5. DEGs Involved in Cell–Cell Communication
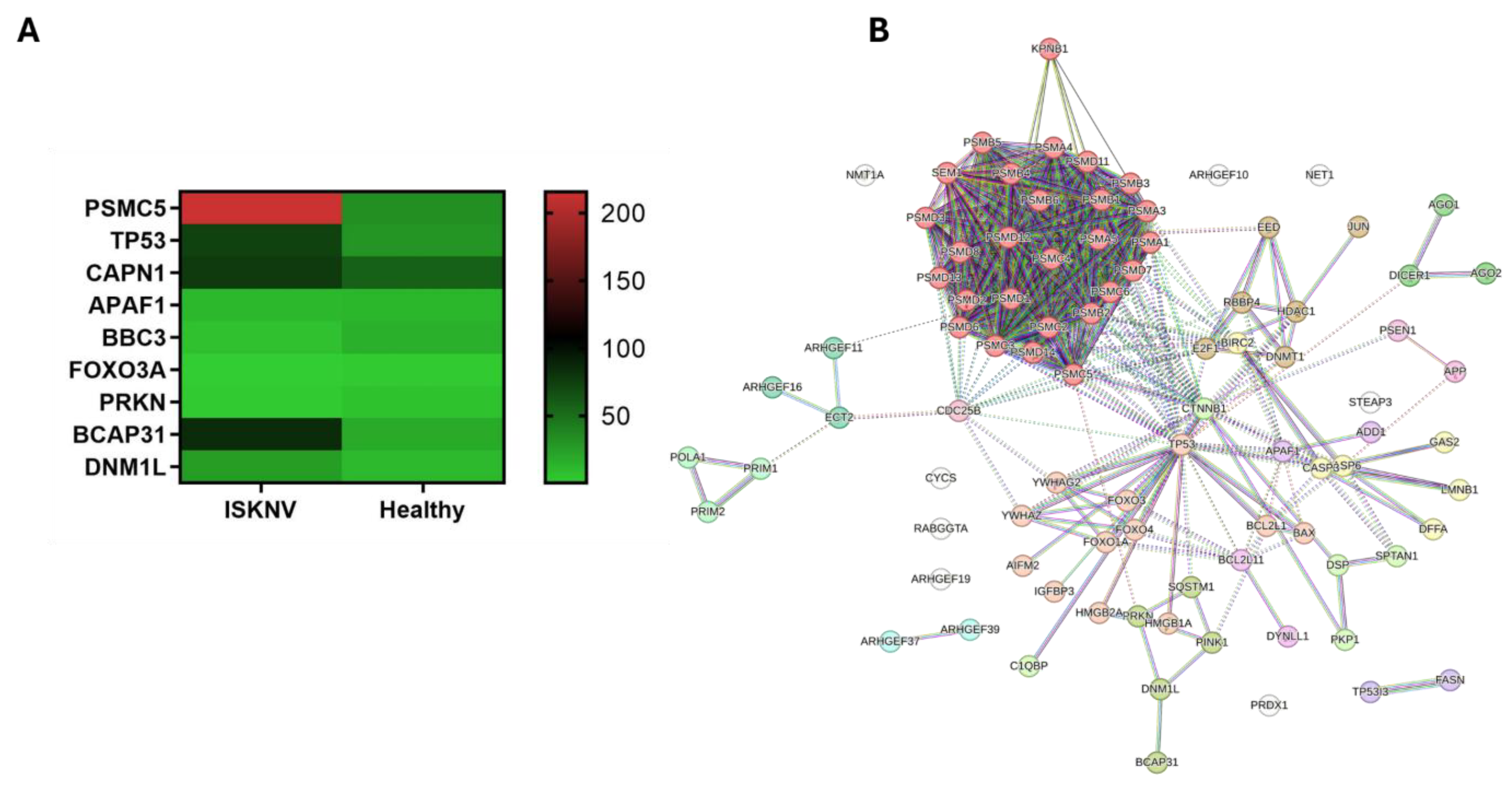
3.6. DEGs Involved in the Programmed Cell Death
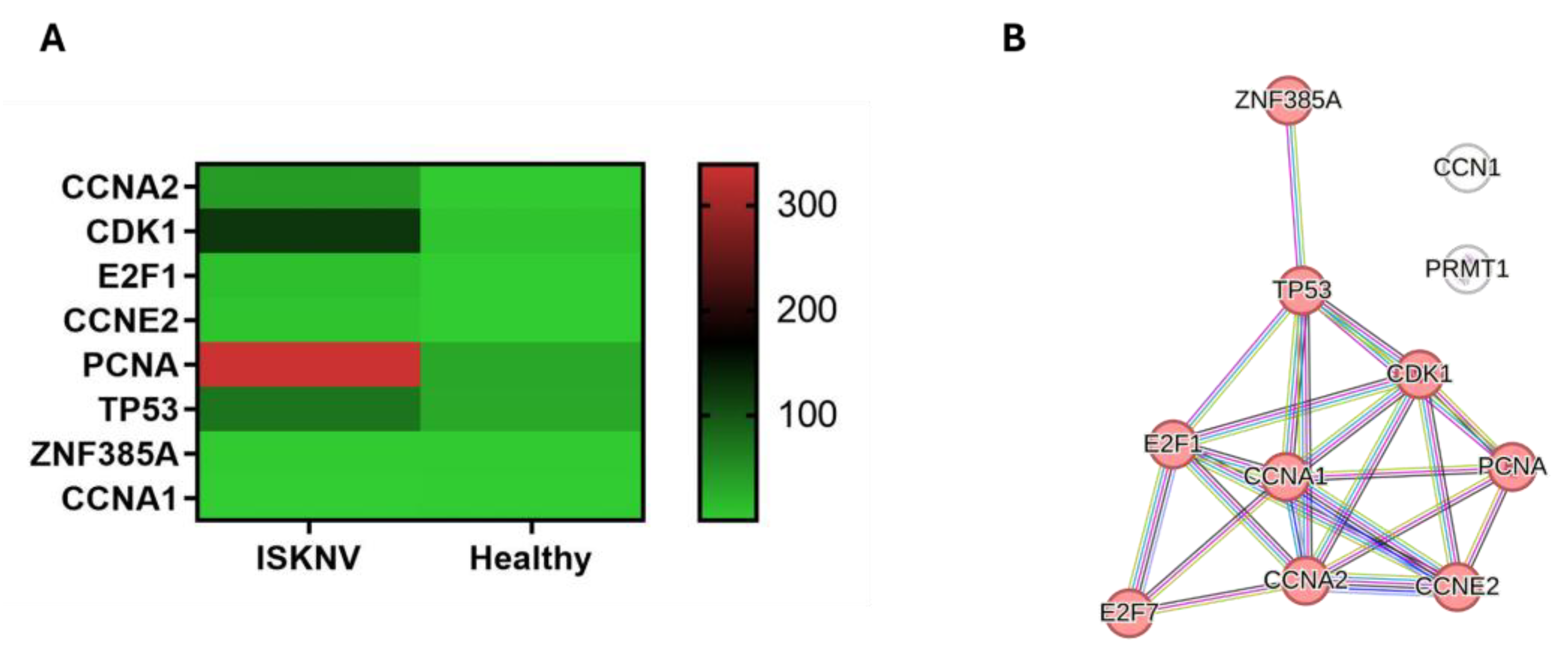
3.7. DEGs Involved in Cell Cycle Arrest
3.8. DEGs Involved in PI3K/AKT Signaling Pathways
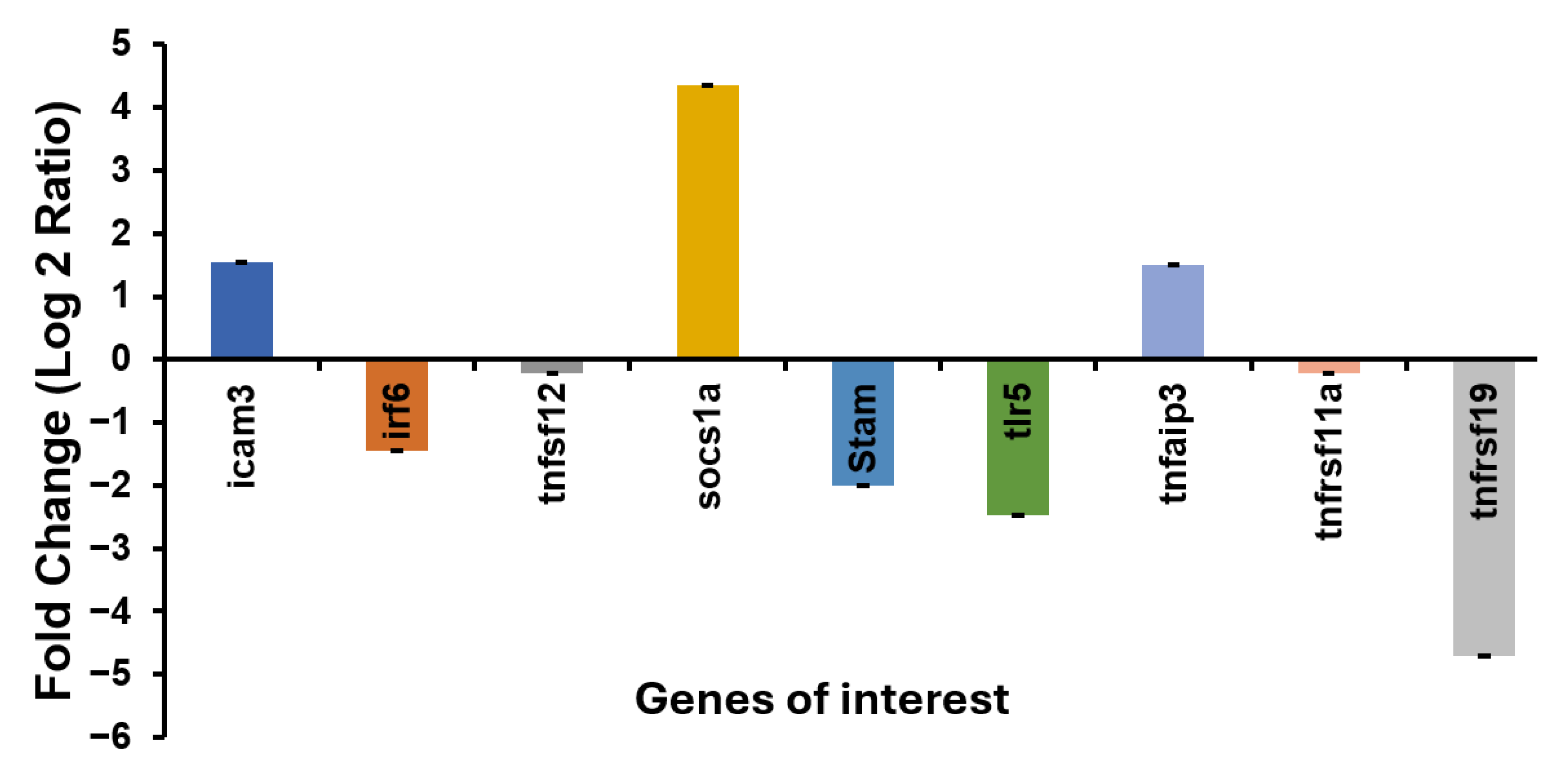
3.9. Validation of the Selected DEGs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, M.; Pan, W.; You, Y.; Qin, X.; Su, H.; Zhan, Z.; Weng, S.; Guo, C.; He, J. Hypermethylated Genome of a Fish Vertebrate Iridovirus ISKNV Plays Important Roles in Viral Infection. Commun. Biol. 2024, 7, 1–13. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, S.; Jian, J.; Liu, G.; Xu, L. Co-Infection of Infectious Spleen and Kidney Necrosis Virus and Francisella sp. in Farmed Pearl Gentian Grouper (♀Epinephelus fuscoguttatus × ♂E. lanceolatus) in China—A Case Report. Aquaculture 2020, 526, 735409. [Google Scholar] [CrossRef]
- Asian Sea Bass Market Size, Growth, Trends & Forecast to 2034. Available online: https://www.futuremarketinsights.com/reports/sea-bass-market (accessed on 8 January 2025).
- Carmen, L.C.P.; Chan, J.; Lee, S.Q.; Hong, H.Y.; Chee, P.X.; Song, Y.; Yang, D.; Prabakaran, M. Utilization of a Monoclonal Antibody Targeting the Functional P-Domain of Nervous Necrosis Virus (NNV) Coat Protein: Developing an Epitope-Blocking ELISA to Quantify Viral Neutralizing Antibodies. Aquaculture 2025, 598, 742058. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef] [PubMed]
- Biggins, J.E.; Biesinger, T.; Yu Kimata, M.T.; Arora, R.; Kimata, J.T. ICAM-3 Influences Human Immunodeficiency Virus Type 1 Replication in CD4+ T Cells Independent of DC-SIGN-Mediated Transmission. Virology 2007, 364, 383–394. [Google Scholar] [CrossRef][Green Version]
- Fu, C.; Cao, N.; Liu, W.; Zhang, Z.; Yang, Z.; Zhu, W.; Fan, S. Crosstalk between Mitophagy and Innate Immunity in Viral Infection. Front. Microbiol. 2022, 13, 1064045. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Shuai, L.; Ma, X.; Zhang, H.; Liu, R.; Chen, W.; Wang, X.; Ge, J.; Wen, Z.; et al. The Serine/Threonine Kinase AP2-Associated Kinase 1 Plays an Important Role in Rabies Virus Entry. Viruses 2019, 12, 45. [Google Scholar] [CrossRef]
- Mutso, M.; Morro, A.M.; Smedberg, C.; Kasvandik, S.; Aquilimeba, M.; Teppor, M.; Tarve, L.; Lulla, A.; Lulla, V.; Saul, S.; et al. Mutation of CD2AP and SH3KBP1 Binding Motif in Alphavirus nsP3 Hypervariable Domain Results in Attenuated Virus. Viruses 2018, 10, 226. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Sun, P.; Wang, J.; Li, G.; Cui, Z.; Li, D.; Yuan, H.; Wang, T.; Li, K.; et al. Porcine Reproductive and Respiratory Syndrome Virus Nonstructural Protein 2 Promotes the Autophagic Degradation of Adaptor Protein SH3KBP1 to Antagonize Host Innate Immune Responses by Enhancing K63-Linked Polyubiquitination of RIG-I. PLOS Pathog. 2024, 20, e1012670. [Google Scholar] [CrossRef]
- Hancock, M.H.; Mitchell, J.; Goodrum, F.D.; Nelson, J.A. Human Cytomegalovirus miR-US5-2 Downregulation of GAB1 Regulates Cellular Proliferation and UL138 Expression through Modulation of Epidermal Growth Factor Receptor Signaling Pathways. mSphere 2020, 5, e00582-20. [Google Scholar] [CrossRef]
- Guo, Q.; Wei, X.; Qi, J.; Li, C.; Xie, F. FGFR3 Upregulates Interferon-Stimulated Genes Via the JAK1-STAT1 Signaling Pathway in HPV2 E2 Stable Expressing Keratinocytes. J. Med. Virol. 2025, 97, e70147. [Google Scholar] [CrossRef] [PubMed]
- Campos-Obando, N.; Zillikens, M.C.; Macaya, R.F. Klotho Deficiency in Severe COVID-19: A Unifying Hypothesis. COVID 2024, 4, 1833–1850. [Google Scholar] [CrossRef]
- Fattahi, S.; Khalifehzadeh-Esfahani, Z.; Mohammad-Rezaei, M.; Mafi, S.; Jafarinia, M. PI3K/Akt/mTOR Pathway: A Potential Target for Anti-SARS-CoV-2 Therapy. Immunol. Res. 2022, 70, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Maecker, H.; Varfolomeev, E.; Kischkel, F.; Lawrence, D.; LeBlanc, H.; Lee, W.; Hurst, S.; Danilenko, D.; Li, J.; Filvaroff, E.; et al. TWEAK Attenuates the Transition from Innate to Adaptive Immunity. Cell 2005, 123, 931–944. [Google Scholar] [CrossRef]
- Zhang, H.; Sheng, X.; Tang, X.; Xing, J.; Chi, H.; Zhan, W. Transcriptome Analysis Reveals Molecular Mechanisms of Lymphocystis Formation Caused by Lymphocystis Disease Virus Infection in Flounder (Paralichthys Olivaceus). Front. Immunol. 2023, 14, 1268851. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, L.-F.; Wang, Z.-X.; Chen, D.-D.; Zhang, Y.-A. Fish IRF6 Is a Positive Regulator of IFN Expression and Involved in Both of the MyD88 and TBK1 Pathways. Fish Shellfish Immunol. 2016, 57, 262–268. [Google Scholar] [CrossRef]
- Shi, Y.; Muenzner, P.; Schanz-Jurinka, S.; Hauck, C.R. The Phosphatidylinositol-5′ Phosphatase Synaptojanin1 Limits Integrin-Mediated Invasion of Staphylococcus aureus. Microbiol. Spectr. 2024, 12, e02006-23. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gutierrez-Jensen, A.D.; Glenn, H.L.; Abrantes, M.; Moussatche, N.; McFadden, G. RNA Helicase A/DHX9 Forms Unique Cytoplasmic Antiviral Granules That Restrict Oncolytic Myxoma Virus Replication in Human Cancer Cells. J. Virol. 2021, 95, e00151-21. [Google Scholar] [CrossRef]
- Feng, H.; Lenarcic, E.M.; Yamane, D.; Wauthier, E.; Mo, J.; Guo, H.; McGivern, D.R.; González-López, O.; Misumi, I.; Reid, L.M.; et al. NLRX1 Promotes Immediate IRF1-Directed Antiviral Responses by Limiting dsRNA-Activated PKR Translational Inhibition. Nat. Immunol. 2017, 18, 1299–1309. [Google Scholar] [CrossRef]
- Wang, R.; Simoneau, C.R.; Kulsuptrakul, J.; Bouhaddou, M.; Travisano, K.A.; Hayashi, J.M.; Carlson-Stevermer, J.; Zengel, J.R.; Richards, C.M.; Fozouni, P.; et al. Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell 2021, 184, 106–119.e14. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Lin, Q.; Liu, H.; Ye, G.; Liu, X.; Jiao, S.; Li, J.; Tang, Y.; Shi, D.; et al. Pseudorabies Virus Infection Activates the TLR-NF-κB Axis and AIM2 Inflammasome To Enhance Inflammatory Responses in Mice. J. Virol. 2023, 97, e00003-23. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Zhang, J.; Wang, L.; Wang, J.; Wen, Z.; Wang, Z.; Shuai, L.; Wang, X.; Ge, J.; et al. The ATPase ATP6V1A Facilitates Rabies Virus Replication by Promoting Virion Uncoating and Interacting with the Viral Matrix Protein. J. Biol. Chem. 2020, 296, 100096. [Google Scholar] [CrossRef] [PubMed]
- Kouhpayeh, H.R.; Tabasi, F.; Dehvari, M.; Naderi, M.; Bahari, G.; Khalili, T.; Clark, C.; Ghavami, S.; Taheri, M. Association between Angiotensinogen (AGT), Angiotensin-Converting Enzyme (ACE) and Angiotensin-II Receptor 1 (AGTR1) Polymorphisms and COVID-19 Infection in the Southeast of Iran: A Preliminary Case-Control Study. Transl. Med. Commun. 2021, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Watashi, K.; Ishii, N.; Hijikata, M.; Inoue, D.; Murata, T.; Miyanari, Y.; Shimotohno, K. Cyclophilin B Is a Functional Regulator of Hepatitis C Virus RNA Polymerase. Mol. Cell 2005, 19, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Fan, X.; Hong, E.; Chen, J.J. Interaction of Oncogenic Papillomavirus E6 Proteins with Fibulin-1. Biochem. Biophys. Res. Commun. 2002, 296, 962–969. [Google Scholar] [CrossRef]
- Torres-Flores, J.M.; Silva-Ayala, D.; Espinoza, M.A.; López, S.; Arias, C.F. The Tight Junction Protein JAM-A Functions as Coreceptor for Rotavirus Entry into MA104 Cells. Virology 2015, 475, 172–178. [Google Scholar] [CrossRef]




| Primer | Oligonucleotide Sequence (5′–3′) |
|---|---|
| IRF6-F | TTGGTTAAGCAGGCCGAGAG |
| IRF6-R | ATCCACACCAGACCCGGATA |
| ICAM3-F | CTGCATCCGTGTCTCCAACT |
| ICAM3-R | TTGATACCACCGCACAACGA |
| TNFRSF19-F | GGTTATGGAGAGGATGCCCG |
| TNFRSF19-R | CCCTTCTGGAAGCGGTTGAT |
| TLR5-F | CAGGATGAAACAAATCCCCAGC |
| TLR5-R | TGTTCAGGTCTGTCTGGAGC |
| SOCS1A-F | GCCAAAAGTTCTCACTGGCG |
| SOCS1A-R | GTATGGGGTGCTGAGGCTTT |
| STAM-F | ATGACGCAGACGCCAAACTA |
| STAM-R | AGGACTGGTGCATCTGTGTG |
| TNFAIP3-F | CCTTTGCCCAGAGTGCCATA |
| TNFAIP3-R | TCTTGGTTGGCGTAGTGGTC |
| TNFRSF11A-F | AATACCAGCCCAGCTTGACC |
| TNFRSF11A-R | ATGGGACAGCATTGGAGGTG |
| TNFSF12-F | GCAGGCGTCTACTTCCTGTT |
| TNFSF12-R | CAAAGTGGAAAGAGTGCGGC |
| 18S-F | AACGAGACTCCGGCATGCTA |
| 18S-F | CCGGACATCTAAGGGCATCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, H.-Y.; Ying, L.X.; Carmen, L.C.P.; Prabakaran, M. Transcriptomic Analysis of the Spleen from Asian Seabass (Lates calcarifer) Infected with Infectious Spleen and Kidney Necrosis Virus. Viruses 2025, 17, 728. https://doi.org/10.3390/v17050728
Xin H-Y, Ying LX, Carmen LCP, Prabakaran M. Transcriptomic Analysis of the Spleen from Asian Seabass (Lates calcarifer) Infected with Infectious Spleen and Kidney Necrosis Virus. Viruses. 2025; 17(5):728. https://doi.org/10.3390/v17050728
Chicago/Turabian StyleXin, Hong-Yi, Lim Xin Ying, Lee Ching Pei Carmen, and Mookkan Prabakaran. 2025. "Transcriptomic Analysis of the Spleen from Asian Seabass (Lates calcarifer) Infected with Infectious Spleen and Kidney Necrosis Virus" Viruses 17, no. 5: 728. https://doi.org/10.3390/v17050728
APA StyleXin, H.-Y., Ying, L. X., Carmen, L. C. P., & Prabakaran, M. (2025). Transcriptomic Analysis of the Spleen from Asian Seabass (Lates calcarifer) Infected with Infectious Spleen and Kidney Necrosis Virus. Viruses, 17(5), 728. https://doi.org/10.3390/v17050728







