Asparagine Availability Is a Critical Limiting Factor for Infectious Spleen and Kidney Necrosis Virus Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viral Strains
2.2. Reagents and Antibodies
2.3. Cell Viability Assay
2.4. Glutamine Depletion and Rescue
2.5. Determination of TCID50 of ISKNV Virus
2.6. The Transcriptional Expression of Genes Was Detected by qPCR
2.7. DNA Extraction and Quantification of ISKNV Genome Copies
2.8. Western Blot
2.9. RNA Interference
2.10. Statistical Analysis
3. Results
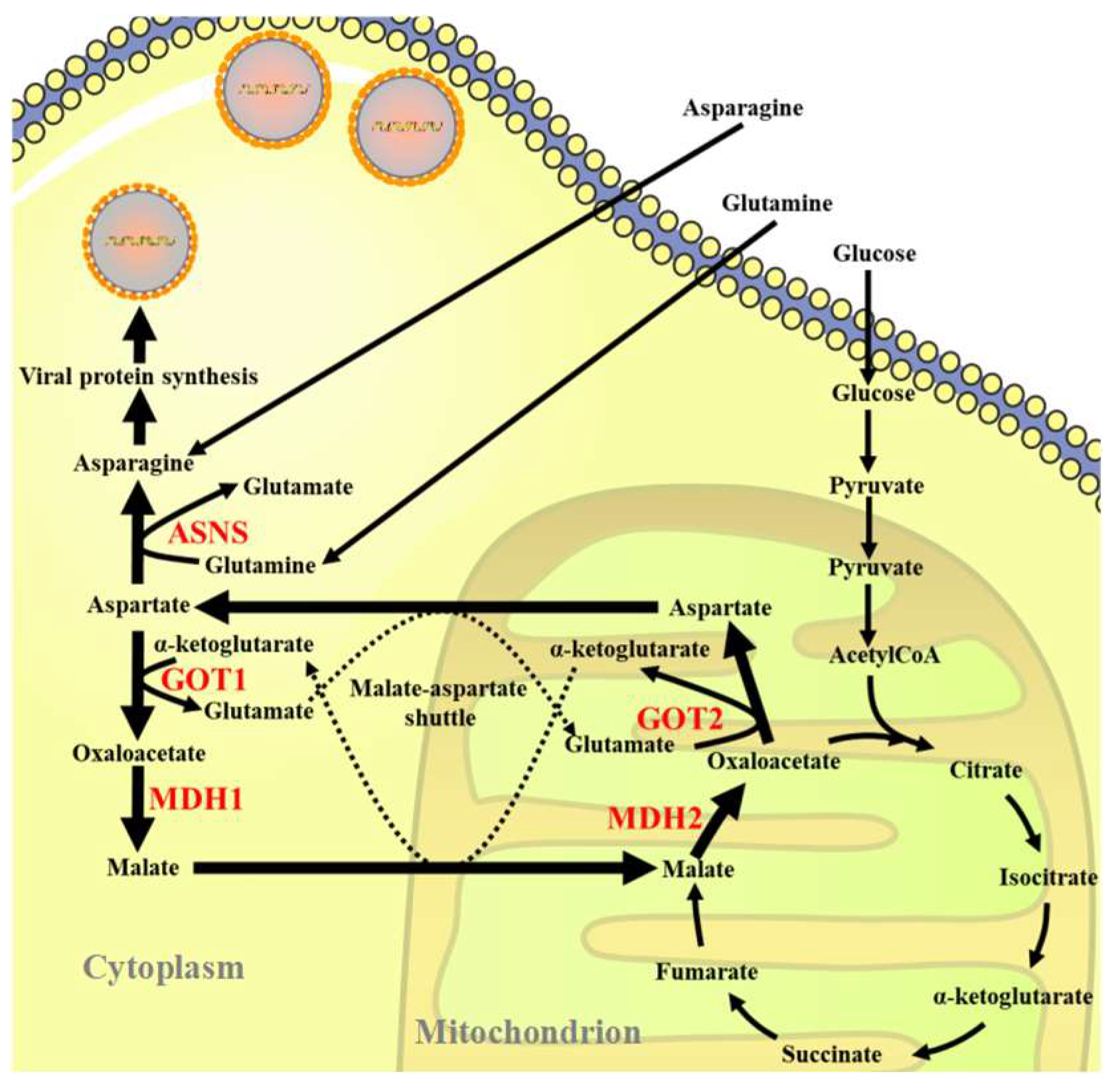
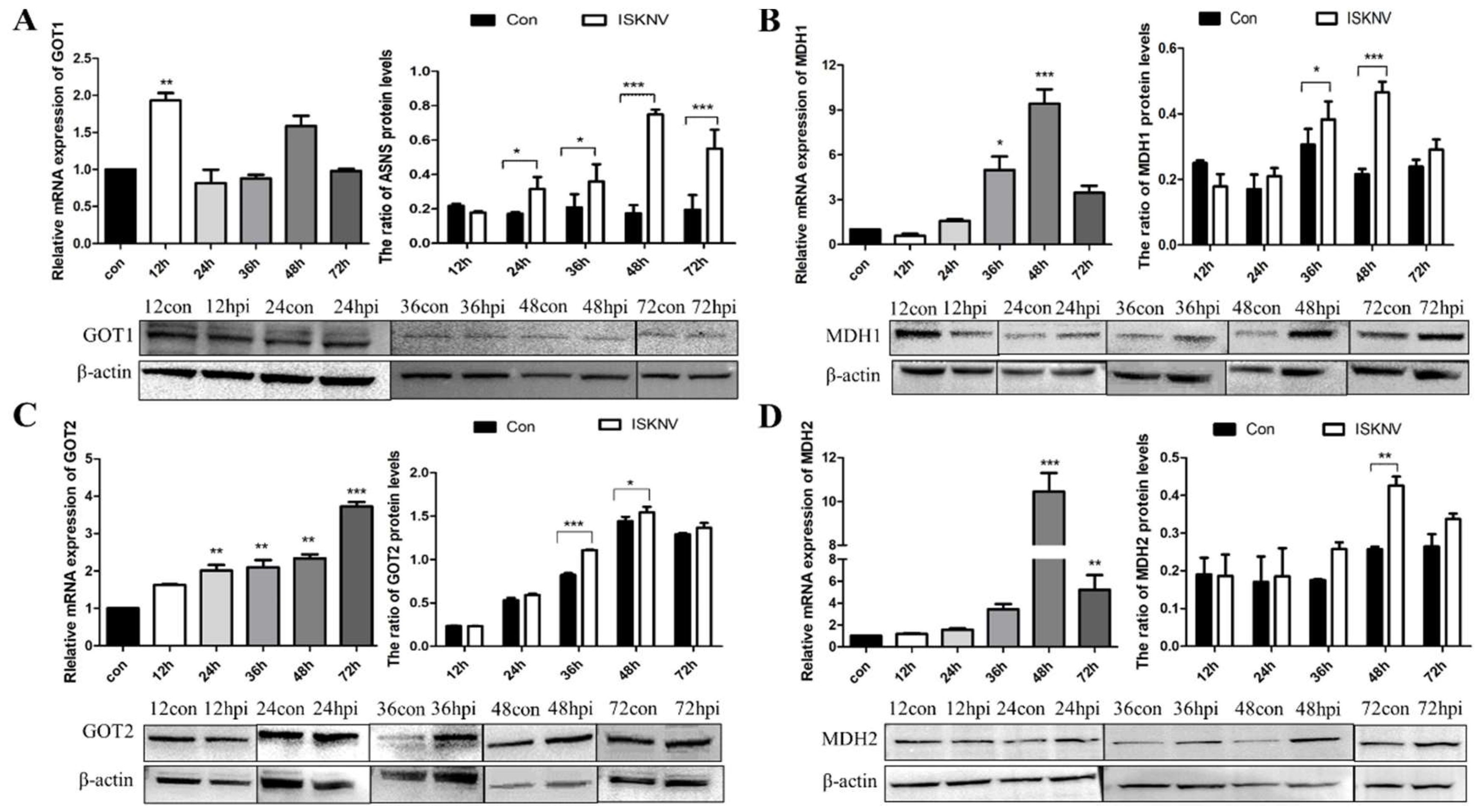
3.1. ISKNV Infection Altered Asparagine Metabolism
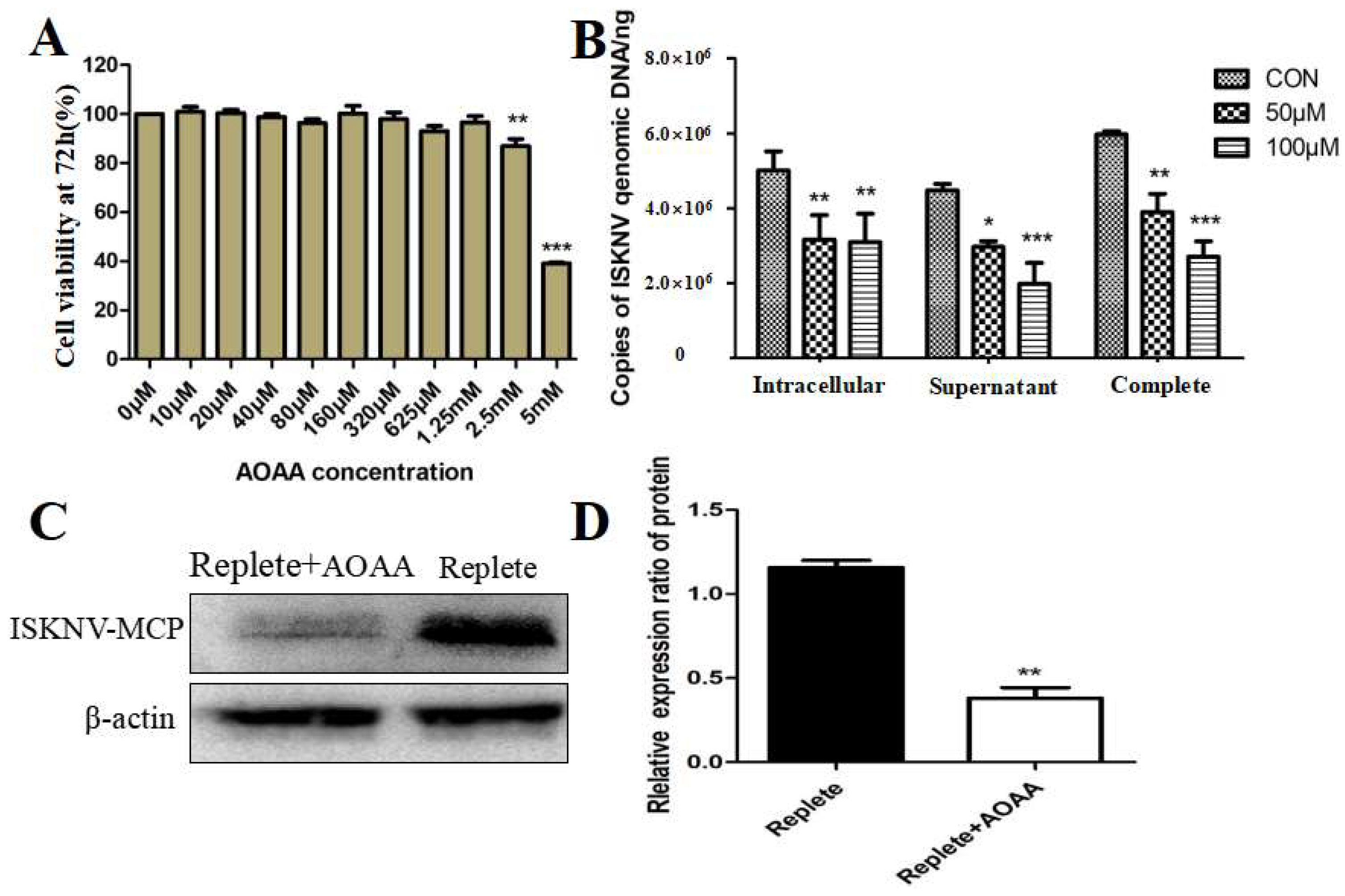
3.2. Inhibition of the MAS-Affected ISKNV Replication
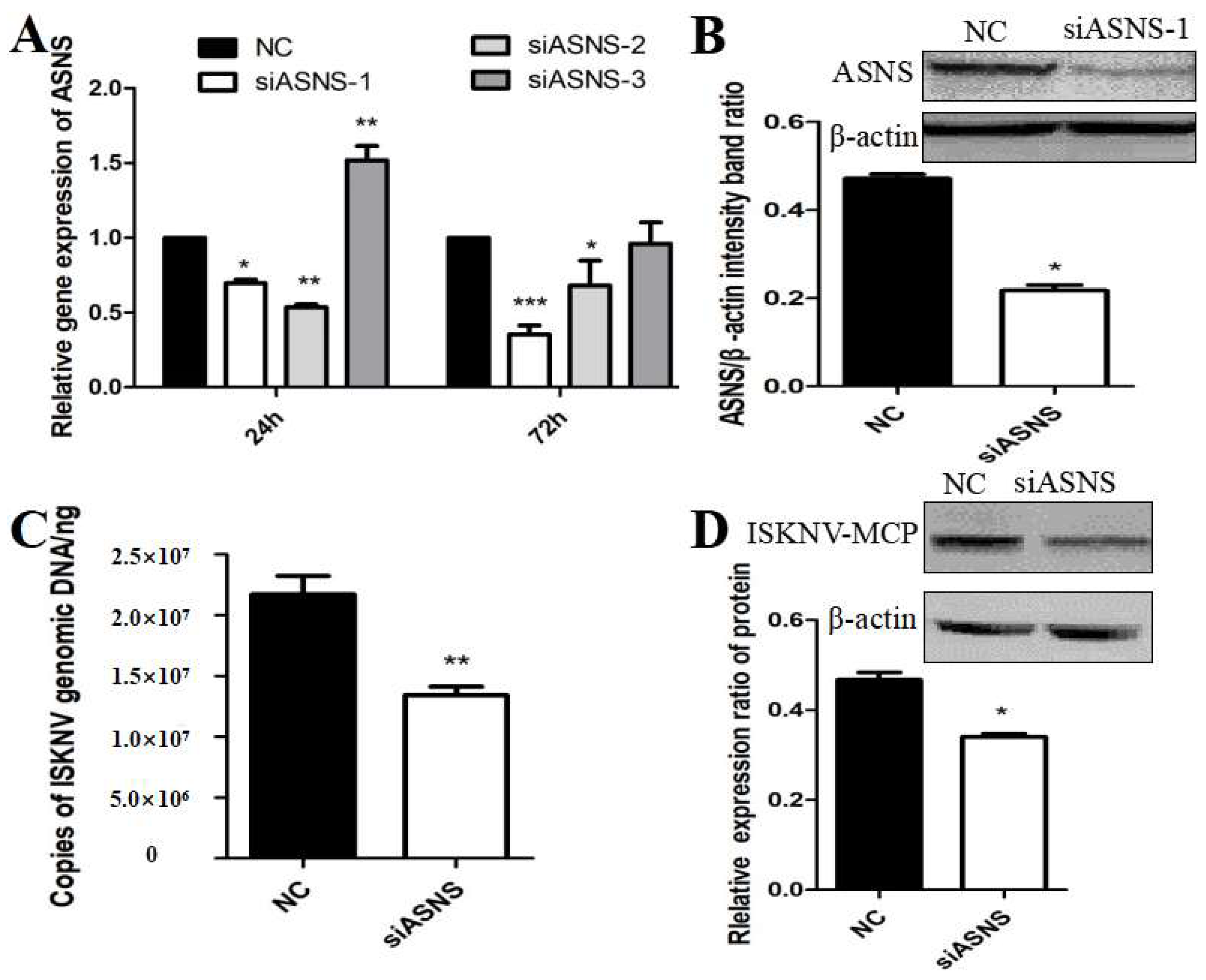
3.3. ASNS Knockdown Reduced ISKNV Replication
3.4. Asparagine Fully Rescued ISKNV Replication in Glutamine Depletion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerddee, P.; Dinh-Hung, N.; Dong, H.T.; Hirono, I.; Soontara, C.; Areechon, N.; Srisapoome, P.; Kayansamruaj, P. Molecular evidence for homologous strains of infectious spleen and kidney necrosis virus (ISKNV) genotype I infecting inland freshwater cultured Asian sea bass (Lates calcarifer) in Thailand. Arch. Virol. 2021, 166, 3061–3074. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Hsueh, T.C.; Wu, J.L.; Hong, J.R. Infectious Spleen and Kidney Necrosis Virus (ISKNV) Triggers Mitochondria-Mediated Dynamic Interaction Signals via an Imbalance of Bax/Bak over Bcl-2/Bcl-xL in Fish Cells. Viruses 2022, 14, 922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lu, L.; Weng, S.P.; Huang, J.N.; Chan, S.M.; He, J.G. Molecular epidemiology and phylogenetic analysis of a marine fish infectious spleen and kidney necrosis virus-like (ISKNV-like) virus. Arch. Virol. 2007, 152, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; He, J.-G.; Weng, S.-P.; Huang, Z.-J.; H, G.-T.; Luo, J.-L.; Huang, W.-B.; Chen, J.-H. Transmission, Host Range, Temperature Sensibility of Infectious Spleen and Kidney Necrosis(ISKN)Virus from Siniperca chuatsi. Virol. Sin. 1999, 14, 353–357. [Google Scholar]
- Mulukutla, B.C.; Yongky, A.; Le, T.; Mashek, D.G.; Hue, W.S. Regulation of Glucose Metabolism—A Perspective from Cell Bioprocessing. Trends Biotechnol. 2016, 34, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fu, X.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Li, N. Research Progress in Virus Infection Altering Cellular Glucose Metabolism. Chin. J. Virol. 2016, 32, 800–809. [Google Scholar]
- Wu, S.; Yu, L.; Fu, X.; Yan, X.; Lin, Q.; Liu, L.; Liang, H.; Li, N. iTRAQ-based proteomic profile analysis of ISKNV-infected CPB cells with emphasizing on glucose metabolism, apoptosis and autophagy pathways. Fish Shellfish Immunol. 2018, 79, 102–111. [Google Scholar] [CrossRef]
- Guo, X.; Wu, S.; Li, N.; Lin, Q.; Liu, L.; Liang, H.; Niu, Y.; Huang, Z.; Fu, X. Accelerated Metabolite Levels of Aerobic Glycolysis and the Pentose Phosphate Pathway Are Required for Efficient Replication of Infectious Spleen and Kidney Necrosis Virus in Chinese Perch Brain Cells. Biomolecules 2019, 9, 440. [Google Scholar] [CrossRef]
- Chambers, J.W.; Maguire, T.G.; Alwine, J.C. Glutamine Metabolism Is Essential for Human Cytomegalovirus Infection. J. Virol. 2010, 84, 1867–1873. [Google Scholar] [CrossRef]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef]
- Fu, X.; Li, K.; Niu, Y.; Lin, Q.; Liang, H.; Luo, X.; Liu, L.; Li, N.; Jones, C.J. The mTOR/PGC-1α/SIRT3 Pathway Drives Reductive Glutamine Metabolism to Reduce Oxidative Stress Caused by ISKNV in CPB Cells. Microbiol. Spectr. 2022, 10, e0231021. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hu, X.; Li, N.; Zheng, F.; Dong, X.; Duan, J.; Lin, Q.; Tu, J.; Zhao, L.; Huang, Z.; et al. Glutamine and glutaminolysis are required for efficient replication of infectious spleen and kidney necrosis virus in Chinese perch brain cells. Oncotarget 2017, 8, 2400–2412. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, L.J.; Wice, B.M.; Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979, 254, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Cai, W.F.; Zhang, C.; Wu, Y.Q.; Zhuang, G.; Ye, Z.; Zhang, C.S.; Lin, S.C. Glutaminase GLS1 senses glutamine availability in a non-enzymatic manner triggering mitochondrial fusion. Cell Res. 2018, 28, 865–867. [Google Scholar] [CrossRef]
- Lomelino, C.L.; Andring, J.T.; McKenna, R.; Kilberg, M.S. Asparagine synthetase: Function, structure, and role in disease. J. Biol. Chem. 2017, 292, 19952–19958. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, J.; Venneti, S.; Cross, J.R.; Takagi, T.; Bhinder, B.; Djaballah, H.; Kanai, M.; Cheng, E.H.; Judkins, A.R.; et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol. Cell 2014, 56, 205–218. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, L.; He, J.; Zeng, Q.; Liang, Y.; Shen, Y.; Zu, X. Metabolism of asparagine in the physiological state and cancer. Cell Commun. Signal. 2024, 22, 163. [Google Scholar] [CrossRef]
- Fu, X.; Li, N.; Lai, Y.; Luo, X.; Wang, Y.; Shi, C.; Huang, Z.; Wu, S.; Su, J. A novel fish cell line derived from the brain of Chinese perch Siniperca chuatsi: Development and characterization. J. Fish Biol. 2015, 86, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Liu, L.; Lin, Q.; Wang, F.; Lai, Y.; Jiang, H.; Pan, H.; Shi, C.; Wu, S. Genotype and host range analysis of infectious spleen and kidney necrosis virus (ISKNV). Virus Genes 2011, 42, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Lin, Q.; Guo, H.; Zhang, D.; Liu, L.; Wu, S. Protective immunity against infectious spleen and kidney necrosis virus induced by immunization with DNA plasmid containing mcp gene in Chinese perch Siniperca chuatsi. Fish Shellfish Immunol. 2014, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fu, X.; Luo, X.; Lin, Q.; Liang, H.; Niu, Y.; Liu, L.; Li, N. Role of asparagine biosynthesis pathway in Siniperca chuatsi rhabdovirus proliferation. Front. Microbiol. 2023, 14, 1165491. [Google Scholar] [CrossRef] [PubMed]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, L.; Stanley, W.C.; Cabrera, M.E.; Saidel, G.M.; Yu, X. Role of the malate-aspartate shuttle on the metabolic response to myocardial ischemia. J. Theor. Biol. 2008, 254, 466–475. [Google Scholar] [CrossRef]
- Pardo, B.; Contreras, L.; Satrústegui, J. De novo Synthesis of Glial Glutamate and Glutamine in Young Mice Requires Aspartate Provided by the Neuronal Mitochondrial Aspartate-Glutamate Carrier Aralar/AGC1. Front. Endocrinol. 2013, 4, 149. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Zhang, J.; Hong, Y.; Ding, X.; Ying, W. Malate-aspartate shuttle mediates the intracellular ATP levels, antioxidation capacity and survival of differentiated PC12 cells. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 109–114. [Google Scholar]
- Wang, C.; Chen, H.; Zhang, M.; Zhang, J.; Wei, X.; Ying, W. Malate-aspartate shuttle inhibitor aminooxyacetic acid leads to decreased intracellular ATP levels and altered cell cycle of C6 glioma cells by inhibiting glycolysis. Cancer Lett. 2016, 378, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Demagny, H.; Faure, A.; Pontanari, F.; Jalil, A.; Bresciani, N.; Yildiz, E.; Korbelius, M.; Perino, A.; Schoonjans, K. Asparagine protects pericentral hepatocytes during acute liver injury. J. Clin. Investig. 2023, 133, e163508. [Google Scholar] [CrossRef]
- Goodwin, C.M.; Xu, S.; Munger, J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends Microbiol. 2015, 23, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Clippinger, A.J.; Alwine, J.C. Viral effects on metabolism: Changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011, 19, 360–367. [Google Scholar] [CrossRef]
- Broeks, M.H.; Meijer, N.W.F.; Westland, D.; Bosma, M.; Gerrits, J.; German, H.M.; Ciapaite, J.; van Karnebeek, C.D.M.; Wanders, R.J.A.; Zwartkruis, F.J.T.; et al. The malate-aspartate shuttle is important for de novo serine biosynthesis. Cell Rep. 2023, 42, 113043. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Waagepetersen, H.S.; Schousboe, A.; Sonnewald, U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: Current evidence and pharmacological tools. Biochem. Pharmacol. 2006, 71, 399–407. [Google Scholar] [CrossRef]
- Gu, H.; Chen, C.; Hao, X.; Su, N.; Huang, D.; Zou, Y.; Lin, S.H.; Chen, X.; Zheng, D.; Liu, L.; et al. MDH1-mediated malate-aspartate NADH shuttle maintains the activity levels of fetal liver hematopoietic stem cells. Blood 2020, 136, 553–571. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Xue, Q.; Yang, F.; Cao, W.; Xue, Z.; Liu, X.; Zheng, H. Picornavirus infection enhances aspartate by the SLC38A8 transporter to promote viral replication. PLoS Pathog. 2023, 19, e1011126. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Griffiths, S.; Digard, P.; Pham, N.; Auer, M.; Haas, J.; Grey, F. Asparagine Deprivation Causes a Reversible Inhibition of Human Cytomegalovirus Acute Virus Replication. mBio 2019, 10, e0165119. [Google Scholar] [CrossRef]
- Pant, A.; Cao, S.; Yang, Z.; Shisler, J.L. Asparagine Is a Critical Limiting Metabolite for Vaccinia Virus Protein Synthesis during Glutamine Deprivation. J. Virol. 2019, 93, e0183418. [Google Scholar] [CrossRef]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Hui, S.; Ghergurovich, J.M.; Fan, J.; Intlekofer, A.M.; White, R.M.; Rabinowitz, J.D.; Thompson, C.B.; Zhang, J. As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell Metab. 2018, 27, 428–438.e5. [Google Scholar] [CrossRef]


| Primer Name | Gene Name | Sequence (5′-3′) |
|---|---|---|
| ASNS-F | asparagine synthetase | GAAATGTGTGGTATCTGGGCTTTGT |
| ASNS-R | TCAAAGACTTCAAAGTGGCCAGGG | |
| MDH2-F | malate dehydrogenase 2 | ACTGCCCCGAAGCTATGA |
| MDH2-R | CAGCGTGACCTCCAATGAC | |
| GOT2-F | Glutamic-oxaloacetic transaminase | ACCATGGCCCTGCTCAAG |
| GOT2-R | TACTTGGTGACAGCATGGATCGC | |
| GOT1-F | Glutamic-oxaloacetic transaminase 1 | CGGCTTATCAGGGTTTCGC |
| GOT1-R | CAGGGCTGTTGAGGGTCTTG | |
| MDH1-F | malate dehydrogenase 1 | ATGGGTGTTTACTCCTCTGGC |
| MDH1-R | TTTGGGGCGGTCTAAGTG | |
| 18S-F | 18S RNA | CATTCGTATTGTGCCGCTAGA |
| 18S-R | CAAATGCTTTCGCTTTGGTC | |
| ISKNV-F | ISKNV-007 | CGAGGCCACATCCAACATC |
| SKNV-R | CGCCTTTAACGTGGGATATATTG | |
| ISKNV-Probe | CACCAAACTGACCGCGGACTCGT |
| Name | Sequence (5′-3′) | Anti-Sequence (5′-3′) |
|---|---|---|
| siRNA-ASNS (siASNS-1) | GGCUUUGUUUGGCAGUGAUTT | AUCACUGCCAAACAAAGCCTT |
| siRNA-ASNS (siASNS-2) | GCAAGGUUGCAGCUCACAUTT | AUGUGAGCUGCAACCUUGCTT |
| iRNA-ASNS (siASNS-3) | GGUAUGUACUUGGUUUCAATT | UUGAAACCAAGUACAUACCTT |
| Negative control (NC) | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| Sample | Intracellular (TCID50/0.1 mL) | Supernatant (TCID50/0.1 mL) | Complete (TCID50/0.1 mL) | |
|---|---|---|---|---|
| AOOA Concentration | ||||
| control | 10−4.23 | 10−4.16 | 10−4.71 | |
| 50 μM | 10−3.78 | 10−3.71 | 10−4.33 | |
| 100 μM | 10−3.29 | 10−3.13 | 10−3.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Li, F.; Fu, X.; Luo, X.; Lin, Q.; Liang, H.; Niu, Y.; Li, N. Asparagine Availability Is a Critical Limiting Factor for Infectious Spleen and Kidney Necrosis Virus Replication. Viruses 2024, 16, 1540. https://doi.org/10.3390/v16101540
Ma B, Li F, Fu X, Luo X, Lin Q, Liang H, Niu Y, Li N. Asparagine Availability Is a Critical Limiting Factor for Infectious Spleen and Kidney Necrosis Virus Replication. Viruses. 2024; 16(10):1540. https://doi.org/10.3390/v16101540
Chicago/Turabian StyleMa, Baofu, Fangying Li, Xiaozhe Fu, Xia Luo, Qiang Lin, Hongru Liang, Yinjie Niu, and Ningqiu Li. 2024. "Asparagine Availability Is a Critical Limiting Factor for Infectious Spleen and Kidney Necrosis Virus Replication" Viruses 16, no. 10: 1540. https://doi.org/10.3390/v16101540
APA StyleMa, B., Li, F., Fu, X., Luo, X., Lin, Q., Liang, H., Niu, Y., & Li, N. (2024). Asparagine Availability Is a Critical Limiting Factor for Infectious Spleen and Kidney Necrosis Virus Replication. Viruses, 16(10), 1540. https://doi.org/10.3390/v16101540





