A Systematic Study of Bovine Viral Diarrhoea Virus Co-Infection with Other Pathogens
Abstract
1. Background
2. Co-Infection of BVDV with Viruses
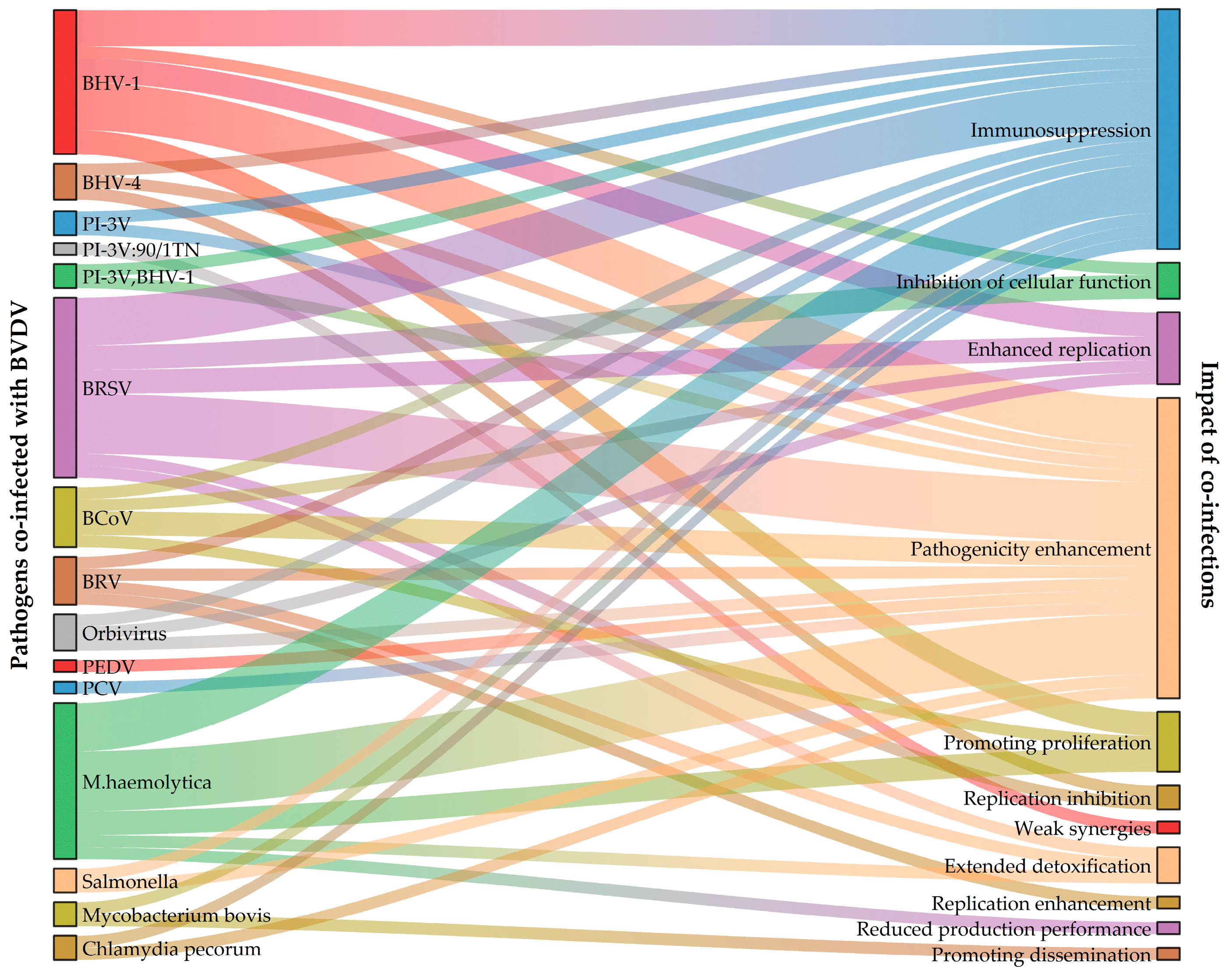
2.1. Co-Infection of BVDV with BHV
2.2. Co-Infection of BVDV with PI-3V
2.3. Co-Infection of BVDV with BCoV
2.4. Co-Infection of BVDV with BRSV
2.5. Co-Infection of BVDV with BRV
2.6. Co-Infection of BVDV with Other Ruminant-Associated Viruses
2.7. Co-Infections Associated with BVDV and Porcine Viruses
3. Co-Infection of BVDV with Bacteria
3.1. Co-Infection of BVDV with Mannheimia haemolytica
3.2. Co-Infection of BVDV with Salmonella
3.3. Co-Infections of BVDV with Other Bacteria
3.4. Co-Infections of BVDV with Atypical Bacteria
3.4.1. Co-Infection of BVDV with Mycoplasma bovis
3.4.2. Co-Infection of BVDV with Chlamydia pecorum
4. Co-Infection of BVDV with Other Pathogens
Co-Infection of BVDV with Neospora caninum
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BVDV | Bovine viral diarrhoea virus |
| BVD-MD | Bovine viral diarrhea/mucocutaneous disease |
| BRDC | Bovine respiratory disease complex |
| BHV | Bovine herpesvirus |
| PI-3V | Parainfluenza virus type 3 |
| BRSV | Bovine respiratory syncytial virus |
| BCoV | Bovine coronavirus |
| BRV | Bovine rotavirus |
| PEDV | Porcine epidemic diarrhea virus |
| PDCOV | Porcine delta coronavirus |
| PKoV | Porcine kobuvirus |
| PASTV | Porcine stellate virus |
| TGEV | Transmissible gastroenteritis virus |
| IBR | Infectious bovine rhinotracheitis |
| BPV | bovine polyomavirus |
| PPRV | Peste des petits ruminants virus |
| PCPV | Pseudocowpox virus |
| BLV | Bovine Leukemia Virus |
| BAV | Bovine adenovirus |
| FMDV | Foot-and-mouth disease virus |
| CSFV | Classical swine fever virus |
| PCV | Porcine circovirus |
| MH | Mannheimia haemolytica |
References
- de Oliveira, P.S.B.; Silva Júnior, J.V.J.; Weiblen, R.; Flores, E.F. Subtyping bovine viral diarrhea virus (BVDV): Which viral gene to choose? Infect. Genet. Evol. 2021, 92, 104891. [Google Scholar] [CrossRef] [PubMed]
- Postler, T.S.; Beer, M.; Blitvich, B.J.; Bukh, J.; de Lamballerie, X.; Drexler, J.F.; Imrie, A.; Kapoor, A.; Karganova, G.G.; Lemey, P.; et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023, 168, 224. [Google Scholar] [CrossRef]
- Collett, M.S.; Larson, R.; Belzer, S.K.; Retzel, E. Proteins encoded by bovine viral diarrhea virus: The genomic organization of a pestivirus. Virology 1988, 165, 200–208. [Google Scholar] [CrossRef]
- Neill, J.D. Molecular biology of bovine viral diarrhea virus. Biologicals 2013, 41, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Callens, N.; Brügger, B.; Bonnafous, P.; Drobecq, H.; Gerl, M.J.; Krey, T.; Roman-Sosa, G.; Rümenapf, T.; Lambert, O.; Dubuisson, J.; et al. Morphology and Molecular Composition of Purified Bovine Viral Diarrhea Virus Envelope. PLoS Pathog. 2016, 12, e1005476. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Krey, T.; Moennig, V.; Thiel, H.J.; Rümenapf, T. CD46 is a cellular receptor for bovine viral diarrhea virus. J. Virol. 2004, 78, 1792–1799. [Google Scholar] [CrossRef]
- Ronecker, S.; Zimmer, G.; Herrler, G.; Greiser-Wilke, I.; Grummer, B. Formation of bovine viral diarrhea virus E1-E2 heterodimers is essential for virus entry and depends on charged residues in the transmembrane domains. J. Gen. Virol. 2008, 89 Pt 9, 2114–2121. [Google Scholar] [CrossRef]
- Passler, T.; Walz, P.H. Bovine viral diarrhea virus infections in heterologous species. Anim. Health Res. Rev. 2010, 11, 191–205. [Google Scholar] [CrossRef]
- Becher, P.; Orlich, M.; Shannon, A.D.; Horner, G.; König, M.; Thiel, H.J. Phylogenetic analysis of pestiviruses from domestic and wild ruminants. J. Gen. Virol. 1997, 78 Pt 6, 1357–1366. [Google Scholar] [CrossRef]
- Ridpath, J.F. Bovine viral diarrhea virus: Global status. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 105–121. [Google Scholar] [CrossRef]
- Bolin, S.R.; McClurkin, A.W.; Coria, M.F. Effects of bovine viral diarrhea virus on the percentages and absolute numbers of circulating B and T lymphocytes in cattle. Am. J. Vet. Res. 1985, 46, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.B.; Bolin, S.R.; Frank, D.E.; Roth, J.A. Defective function of leukocytes from cattle persistently infected with bovine viral diarrhea virus, and the influence of recombinant cytokines. Am. J. Vet. Res. 1991, 52, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Liebler-Tenorio, E.M.; Ridpath, J.F.; Neill, J.D. Lesions and tissue distribution of viral antigen in severe acute versus subclinical acute infection with BVDV2. Biologicals 2003, 31, 119–122. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Falkenberg, S.M.; Bauermann, F.V.; VanderLey, B.L.; Do, Y.; Flores, E.F.; Rodman, D.M.; Neill, J.D. Comparison of acute infection of calves exposed to a high-virulence or low-virulence bovine viral diarrhea virus or a HoBi-like virus. Am. J. Vet. Res. 2013, 74, 438–442. [Google Scholar] [CrossRef]
- Baker, J.C. The clinical manifestations of bovine viral diarrhea infection. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 425–445. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef]
- Van Wyk, B.; Snider, M.; Scruten, E.; van Drunen Littel-van den Hurk, S.; Napper, S. Induction of functional interferon alpha and gamma responses during acute infection of cattle with non-cytopathic bovine viral diarrhea virus. Vet. Microbiol. 2016, 195, 104–114. [Google Scholar] [CrossRef]
- Pedrera, M.; Gómez-Villamandos, J.C.; Molina, V.; Risalde, M.A.; Rodríguez-Sánchez, B.; Sánchez-Cordón, P.J. Quantification and determination of spread mechanisms of bovine viral diarrhoea virus in blood and tissues from colostrum-deprived calves during an experimental acute infection induced by a non-cytopathic genotype 1 strain. Transbound. Emerg. Dis. 2012, 59, 377–384. [Google Scholar] [CrossRef]
- Brownlie, J.; Clarke, M.C.; Howard, C.J. Experimental production of fatal mucosal disease in cattle. Vet. Rec. 1984, 114, 535–536. [Google Scholar] [CrossRef]
- Bolin, S.R.; McClurkin, A.W.; Cutlip, R.C.; Coria, M.F. Severe clinical disease induced in cattle persistently infected with noncytopathic bovine viral diarrhea virus by superinfection with cytopathic bovine viral diarrhea virus. Am. J. Vet. Res. 1985, 46, 573–576. [Google Scholar] [CrossRef]
- Olafson, P.; MacCallum, A.D.; Fox, F.H. An apparently new transmissible disease of cattle. Cornell Vet. 1946, 36, 205–213. [Google Scholar] [PubMed]
- Childs, T. X Disease of Cattle—Saskatchewan. Can. J. Comp. Med. Vet. Sci. 1946, 10, 316–319. [Google Scholar] [PubMed]
- Liu, L.; Xia, H.; Wahlberg, N.; Belák, S.; Baule, C. Phylogeny, classification and evolutionary insights into pestiviruses. Virology 2009, 385, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, C.; Zhang, L.; Zhu, T.; Li, H.; Wang, Y.; Xue, K.; Qi, M.; Peng, Q.; Chen, Y.; et al. Isolation of BVDV-1a, 1m, and 1v strains from diarrheal calf in china and identification of its genome sequence and cattle virulence. Front. Vet. Sci. 2022, 9, 1008107. [Google Scholar] [CrossRef]
- Malacari, D.A.; Pécora, A.; Pérez Aguirreburualde, M.S.; Cardoso, N.P.; Odeón, A.C.; Capozzo, A.V. In Vitro and In Vivo Characterization of a Typical and a High Pathogenic Bovine Viral Diarrhea Virus Type II Strains. Front. Vet. Sci. 2018, 5, 75. [Google Scholar] [CrossRef]
- Spetter, M.J.; Uriarte, E.L.L.; Altamiranda, E.A.G.; Leunda, M.R.; Pereyra, S.B.; Verna, A.E.; Odeón, A.C. Dual natural infection with bovine viral diarrhea virus-1 and -2 in a stillborn calf: Tissue distribution and molecular characterization. Open Vet. J. 2018, 8, 493–497. [Google Scholar] [CrossRef]
- Grissett, G.P.; White, B.J.; Larson, R.L. Structured literature review of responses of cattle to viral and bacterial pathogens causing bovine respiratory disease complex. J. Vet. Intern. Med. 2015, 29, 770–780. [Google Scholar] [CrossRef]
- Larson, R.L.; Step, D.L. Evidence-based effectiveness of vaccination against Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in feedlot cattle for mitigating the incidence and effect of bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 97–106.e7. [Google Scholar] [CrossRef]
- Theurer, M.E.; Larson, R.L.; White, B.J. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, bovine viral diarrhea virus, bovine respiratory syncytial virus, and parainfluenza type 3 virus for mitigation of bovine respiratory disease complex in cattle. J. Am. Vet. Med. Assoc. 2015, 246, 126–142. [Google Scholar]
- Ridpath, J. The contribution of infections with bovine viral diarrhea viruses to bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 335–348. [Google Scholar] [CrossRef]
- Oliveira, V.H.S.; Dall Agnol, A.M.; Fritzen, J.T.T.; Lorenzetti, E.; Alfieri, A.A.; Alfieri, A.F. Microbial diversity involved in the etiology of a bovine respiratory disease outbreak in a dairy calf rearing unit. Comp. Immunol. Microbiol. Infect. Dis. 2020, 71, 101494. [Google Scholar] [CrossRef] [PubMed]
- Muasya, D.; Leeuwen, J.V.; Gitau, G.; McKenna, S.; Heider, L.; Muraya, J. Evaluation of antibody and antigen cross-reaction in Kenyan dairy cattle naturally infected with two pestiviruses: Bovine viral diarrhea virus and classical swine fever virus. Vet. World 2022, 15, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Liess, B.; Moennig, V. Ruminant pestivirus infection in pigs. Rev. Sci. Tech. 1990, 9, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, B.; Tao, J.; Cheng, J.; Liu, H. The Complex Co-infections of Multiple Porcine Diarrhea Viruses in Local Area Based on the Luminex xTAG Multiplex Detection Method. Front. Vet. Sci. 2021, 8, 602866. [Google Scholar] [CrossRef]
- Woods, R.D.; Kunkle, R.A.; Ridpath, J.E.; Bolin, S.R. Bovine viral diarrhea virus isolated from fetal calf serum enhances pathogenicity of attenuated transmissible gastroenteritis virus in neonatal pigs. J. Vet. Diagn. Investig. 1999, 11, 400–407. [Google Scholar] [CrossRef]
- Richer, L.; Marois, P.; Lamontagne, L. Association of bovine viral diarrhea virus with multiple viral infections in bovine respiratory disease outbreaks. Can. Vet. J. 1988, 29, 713–717. [Google Scholar]
- Potgieter, L.N.; McCracken, M.D.; Hopkins, F.M.; Walker, R.D. Effect of bovine viral diarrhea virus infection on the distribution of infectious bovine rhinotracheitis virus in calves. Am. J. Vet. Res. 1984, 45, 687–690. [Google Scholar] [CrossRef]
- Romero-Palomo, F.; Risalde, M.A.; Molina, V.; Lauzi, S.; Bautista, M.J.; Gómez-Villamandos, J.C. Characterization of thymus atrophy in calves with subclinical BVD challenged with BHV-1. Vet. Microbiol. 2015, 177, 32–42. [Google Scholar] [CrossRef]
- Romero-Palomo, F.; Risalde, M.A.; Gómez-Villamandos, J.C. Immunopathologic Changes in the Thymus of Calves Pre-infected with BVDV and Challenged with BHV-1. Transbound. Emerg. Dis. 2017, 64, 574–584. [Google Scholar] [CrossRef]
- Risalde, M.A.; Molina, V.; Sánchez-Cordón, P.J.; Pedrera, M.; Panadero, R.; Romero-Palomo, F.; Gómez-Villamandos, J.C. Response of proinflammatory and anti-inflammatory cytokines in calves with subclinical bovine viral diarrhea challenged with bovine herpesvirus-1. Vet. Immunol. Immunopathol. 2011, 144, 135–143. [Google Scholar] [CrossRef]
- Risalde, M.A.; Molina, V.; Sánchez-Cordón, P.J.; Romero-Palomo, F.; Pedrera, M.; Garfia, B.; Gómez-Villamandos, J.C. Pathogenic mechanisms implicated in the intravascular coagulation in the lungs of BVDV-infected calves challenged with BHV-1. Vet. Res. 2013, 44, 20. [Google Scholar] [CrossRef] [PubMed]
- Risalde, M.A.; Molina, V.; Sónchez-Cordón, P.J.; Pedrera, M.; Romero-Palomo, F.; Bautista, M.J.; Moreno, A.; Gómez-Villamandos, J.C. Comparison of pathological changes and viral antigen distribution in tissues of calves with and without preexisting bovine viral diarrhea virus infection following challenge with bovine herpesvirus-1. Am. J. Vet. Res. 2013, 74, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Risalde, M.A.; Molina, V.; Sánchez-Cordón, P.J.; Romero-Palomo, F.; Pedrera, M.; Gómez-Villamandos, J.C. Effects of Preinfection With Bovine Viral Diarrhea Virus on Immune Cells From the Lungs of Calves Inoculated With Bovine Herpesvirus 1.1. Vet. Pathol. 2015, 52, 644–653. [Google Scholar] [CrossRef]
- Glotov, A.G.; Glotova, T.I.; Sergeev, A.N.; Drozdov, I.G. [Pathogenesis of mixed experimental infection caused by diarrheal viruses--bovine mucosal disease and bovine infectious rhinotracheitis in calves]. Vopr. Virusol. 2007, 52, 40–43. [Google Scholar]
- Aly, N.M.; Shehab, G.G.; Abd el-Rahim, I.H. Bovine viral diarrhoea, bovine herpesvirus and parainfluenza-3 virus infection in three cattle herds in Egypt in 2000. Rev. Sci. Tech. 2003, 22, 879–892. [Google Scholar] [CrossRef]
- Castrucci, G.; Ferrari, M.; Traldi, V.; Tartaglione, E. Effects in calves of mixed infections with bovine viral diarrhea virus and several other bovine viruses. Comp. Immunol. Microbiol. Infect. Dis. 1992, 15, 261–270. [Google Scholar] [CrossRef]
- Romeo, F.; Uriarte, E.L.; Delgado, S.; González-Altamiranda, E.; Pereyra, S.; Morán, P.; Odeón, A.; Pérez, S.; Verna, A. Effect of bovine viral diarrhea virus on subsequent infectivity of bovine gammaherpesvirus 4 in endometrial cells in primary culture: An in vitro model of viral co-infection. J. Virol. Methods 2021, 291, 114097. [Google Scholar] [CrossRef]
- Pollreisz, J.H.; Kelling, C.L.; Brodersen, B.W.; Perino, L.J.; Cooper, V.L.; Doster, A.R. Potentiation of bovine respiratory syncytial virus infection in calves by bovine viral diarrhea virus. Bov. Pr. 1997, 32–38. [Google Scholar] [CrossRef]
- Elvander, M.; Baule, C.; Persson, M.; Egyed, L.; Ballagi-Pordány, A.; Belák, S.; Alenius, S. An experimental study of a concurrent primary infection with bovine respiratory syncytial virus [BRSV] and bovine viral diarrhoea virus [BVDV] in calves. Acta Vet. Scand. 1998, 39, 251–264. [Google Scholar] [CrossRef]
- Brodersen, B.W.; Kelling, C.L. Alteration of leukocyte populations in calves concurrently infected with bovine respiratory syncytial virus and bovine viral diarrhea virus. Viral. Immunol. 1999, 12, 323–334. [Google Scholar] [CrossRef]
- Brodersen, B.W.; Kelling, C.L. Effect of concurrent experimentally induced bovine respiratory syncytial virus and bovine viral diarrhea virus infection on respiratory tract and enteric diseases in calves. Am. J. Vet. Res. 1998, 59, 1423–1430, Erratum in Am. J. Vet. Res. 1999, 60, 13. [Google Scholar] [CrossRef]
- Alkheraif, A.A.; Topliff, C.L.; Reddy, J.; Massilamany, C.; Donis, R.O.; Meyers, G.; Eskridge, K.M.; Kelling, C.L. Type 2 BVDV Npro suppresses IFN-1 pathway signaling in bovine cells and augments BRSV replication. Virology 2017, 507, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lehmkuhl, H.D.; Kaeberle, M.L. Synergistic effects of bovine respiratory syncytial virus and non-cytopathic bovine viral diarrhea virus infection on selected bovine alveolar macrophage functions. Can. J. Vet. Res. 1999, 63, 41–48. [Google Scholar]
- Ridpath, J.F.; Fulton, R.W.; Bauermann, F.V.; Falkenberg, S.M.; Welch, J.; Confer, A.W. Sequential exposure to bovine viral diarrhea virus and bovine coronavirus results in increased respiratory disease lesions: Clinical, immunologic, pathologic, and immunohistochemical findings. J. Vet. Diagn. Investig. 2020, 32, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, R.; Lindberg, A.; Tråvén, M. Failure to spread bovine virus diarrhoea virus infection from primarily infected calves despite concurrent infection with bovine coronavirus. Vet. J. 2002, 163, 251–259. [Google Scholar] [CrossRef]
- Kelling, C.L.; Steffen, D.J.; Cooper, V.L.; Higuchi, D.S.; Eskridge, K.M. Effect of infection with bovine viral diarrhea virus alone, bovine rotavirus alone, or concurrent infection with both on enteric disease in gnotobiotic neonatal calves. Am. J. Vet. Res. 2002, 63, 1179–1186. [Google Scholar] [CrossRef]
- Nakamura, S.; Shimazaki, T.; Sakamoto, K.; Fukusho, A.; Inoue, Y.; Ogawa, N. Enhanced replication of orbiviruses in bovine testicle cells infected with bovine viral diarrhoea virus. J. Vet. Med. Sci. 1995, 57, 677–681. [Google Scholar] [CrossRef][Green Version]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef]
- Jones, C. Bovine Herpesvirus 1 Counteracts Immune Responses and Immune-Surveillance to Enhance Pathogenesis and Virus Transmission. Front. Immunol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Peterhans, E.; Wyler, R. DNA of bovine herpesvirus type 1 in the trigeminal ganglia of latently infected calves. Am. J. Vet. Res. 1982, 43, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Givens, M.D.; Marley, M.S. Infectious causes of embryonic and fetal mortality. Theriogenology 2008, 70, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.; Parr, M.; Fagan, J.; Johnson, A.; Tratalos, J.; Lively, F.; Diskin, M.; Kenny, D. Prevalence of Bovine Viral Diarrhoea Virus (BVDV), Bovine Herpes Virus 1 (BHV 1), Leptospirosis and Neosporosis, and associated risk factors in 161 Irish beef herds. BMC Vet. Res. 2018, 14, 8. [Google Scholar] [CrossRef]
- Barrett, D.; Lane, E.; Lozano, J.M.; O’Keeffe, K.; Byrne, A.W. Bovine Herpes Virus Type 1 (BoHV-1) seroprevalence, risk factor and Bovine Viral Diarrhoea (BVD) co-infection analysis from Ireland. Sci. Rep. 2024, 14, 867. [Google Scholar] [CrossRef]
- Fulton, R.W.; Purdy, C.W.; Confer, A.W.; Saliki, J.T.; Loan, R.W.; Briggs, R.E.; Burge, L.J. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 2000, 64, 151–159. [Google Scholar]
- Fulton, R.W.; Ridpath, J.F.; Saliki, J.T.; Briggs, R.E.; Confer, A.W.; Burge, L.J.; Purdy, C.W.; Loan, R.W.; Duff, G.C.; Payton, M.E. Bovine viral diarrhea virus (BVDV) 1b: Predominant BVDV subtype in calves with respiratory disease. Can. J. Vet. Res. 2002, 66, 181–190. [Google Scholar]
- Mahmoud, M.A.; Allam, A.M. Seroprevalence of bovine viral diarrhea virus (BVDV), bovine herpes virus type 1 (BHV-1), parainfluenza type 3 virus (PI-3V) and bovine respiratory syncytial virus (BRSV) among non vaccinated cattle. Glob. Vet. 2013, 10, 348–353. [Google Scholar]
- Ratta, B.; Yadav, B.S.; Pokhriyal, M.; Saxena, M.; Sharma, B. Microarray chip based identification of a mixed infection of bovine herpesvirus 1 and bovine viral diarrhea 2 from Indian cattle. Curr. Microbiol. 2014, 68, 127–131. [Google Scholar] [CrossRef]
- El-Mohamady, R.S.; Behour, T.S.; Rawash, Z.M. Concurrent detection of bovine viral diarrhoea virus and bovine herpes-virus-1 in bulls’ semen and their effect on semen quality. Int. J. Vet. Sci. Med. 2020, 8, 106–114. [Google Scholar] [CrossRef]
- Sayers, R.G.; Byrne, N.; O’Doherty, E.; Arkins, S. Prevalence of exposure to bovine viral diarrhoea virus (BVDV) and bovine herpesvirus-1 (BoHV-1) in Irish dairy herds. Res. Vet. Sci. 2015, 100, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ibeas, A.M.; Power, C.; McClure, J.; Sayers, R.G. Prevalence of BoHV-1 seropositive and BVD virus positive bulls on Irish dairy farms and associations between bull purchase and herd status. Ir. Vet. J. 2015, 68, 28. [Google Scholar] [CrossRef]
- Roshtkhari, F.; Mohammadi, G.; Mayameei, A. Serological evaluation of relationship between viral pathogens (BHV-1, BVDV, BRSV, PI-3V, and Adeno3) and dairy calf pneumonia by indirect ELISA. Trop. Anim. Health Prod. 2012, 44, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Yoshitani, G.D.; Camilo, S.L.O.; Fritzen, J.T.T.; Oliveira, M.V.; Lorenzetti, E.; Lisbôa, J.A.N.; Alfieri, A.F.; Alfieri, A.A. Serological Profile for Major Respiratory Viruses in Unvaccinated Cows from High-Yielding Dairy Herds. Animals 2024, 14, 1256. [Google Scholar] [CrossRef]
- Molina, V.; Risalde, M.A.; Sánchez-Cordón, P.J.; Romero-Palomo, F.; Pedrera, M.; Garfia, B.; Gómez-Villamandos, J.C. Cell-mediated immune response during experimental acute infection with bovine viral diarrhoea virus: Evaluation of blood parameters. Transbound Emerg Dis. 2014, 61, 44–59. [Google Scholar] [CrossRef]
- Donofrio, G.; Colleoni, S.; Galli, C.; Lazzari, G.; Cavirani, S.; Flammini, C.F. Susceptibility of bovine mesenchymal stem cells to bovine herpesvirus 4. J. Virol. Methods 2005, 127, 168–170. [Google Scholar] [CrossRef]
- Egyed, L. Bovine herpesvirus type 4: A special herpesvirus [review article]. Acta Vet. Hung. 2000, 48, 501–513. [Google Scholar] [CrossRef]
- Egyed, L.; Ballagi-Pordány, A.; Bartha, A.; Belák, S. Studies of in vivo distribution of bovine herpesvirus type 4 in the natural host. J. Clin. Microbiol. 1996, 34, 1091–1095. [Google Scholar] [CrossRef]
- Izumi, Y.; Tsuduku, S.; Murakami, K.; Tsuboi, T.; Konishi, M.; Haritani, M.; Kamiyoshi, T.; Kimura, K.; Sentsui, H. Charac-terization of Bovine herpesvirus type 4 isolated from cattle with mastitis and subclinical infection by the virus among cattle. J. Vet. Med. Sci. 2006, 68, 189–193. [Google Scholar] [CrossRef]
- Czaplicki, G.; Thiry, E. An association exists between bovine herpesvirus-4 seropositivity and abortion in cows. Prev. Vet. Med. 1998, 33, 235–240. [Google Scholar] [CrossRef]
- Dağalp, S.B.; Babaoglu, A.R.; Doğan, F.; Farzani, T.A.; Alkan, F. An assessment of bovine herpes virus 4 as a causative agent in abortions and neonatal death. Onderstepoort J. Vet. Res. 2020, 87, e1–e5. [Google Scholar]
- Falcone, E.; Cordioli, P.; Tarantino, M.; Muscillo, M.; Sala, G.; La Rosa, G.; Archetti, I.L.; Marianelli, C.; Lombardi, G.; Tollis, M. Experimental infection of calves with bovine viral diarrhoea virus type-2 (BVDV-2) isolated from a contaminated vaccine. Vet. Res. Commun. 2003, 27, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Yeşilbağ, K.; Güngör, B. Antibody prevalence against respiratory viruses in sheep and goats in North-Western Turkey. Trop. Anim. Health Prod. 2009, 41, 421–425. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses [ICTV]. Available online: https://ictv.global/taxonomy (accessed on 21 February 2024).
- Karron, R.A.; Collins, P.L. Parainfluenza viruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Wil-liams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1, pp. 1497–1526. [Google Scholar]
- Ellis, J.A. Bovine parainfluenza-3 virus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 575–593. [Google Scholar] [CrossRef]
- Hoerlein, A.B.; Mansfield, M.E.; Abinanti, F.R.; Huebner, R.J. Studies of shipping fever of cattle. I. Para-influenza 3 virus an-tibodies in feeder calves. J. Am. Vet. Med. Assoc. 1959, 135, 153–160. [Google Scholar]
- Murray, G.M.; More, S.J.; Sammin, D.; Casey, M.J.; McElroy, M.C.; O’Neill, R.G.; Byrne, W.J.; Earley, B.; Clegg, T.A.; Ball, H.; et al. Pathogens, patterns of pneumonia, and epidemiologic risk factors associated with respiratory disease in recently weaned cattle in Ireland. J. Vet. Diagn. Investig. 2017, 29, 20–34. [Google Scholar] [CrossRef]
- Betancur, C.; Orrego, A.; González, M. Seroepidemiological study of parainfluenza 3 virus in cattle of the municipality of Monteria (Colombia) with reproductive disorders. J. Vet. Med. 2010, 20, 63–70. [Google Scholar]
- León, J.C.P.; Diaz, W.; Vasquez, M.C.; Tobón, J.C.; Sánchez, A.; Ortiz, D. Seroprevalence and risk factor associated with respiratory viral pathogens in dual-purpose cattle of Aguachica, Rio de Oro, and La Gloria municipalities in Cesar department, Colombia. Vet. World 2019, 12, 951–958. [Google Scholar] [CrossRef]
- Martin, S.W.; Bateman, K.G.; Shewen, P.E.; Rosendal, S.; Bohac, J.G.; Thorburn, M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can. J. Vet. Res. 1990, 54, 337–342. [Google Scholar]
- Hashemi, M.; Bakhshesh, M.; Khezri, M.; Gharagouzlouian, M.M.; Tavakoli, G. A Two-Year Serological Study of Bovine Viral Diarrhea Virus, Bovine Alphaherpesvirus 1 and Bovine Parainfluenza Virus Type 3 in Qazvin Dairy Cattle Farms, Northwestern of Iran. Vet. Arh. 2022, 92, 1–10. [Google Scholar] [CrossRef]
- Booker, C.W.; Abutarbush, S.M.; Morley, P.S.; Jim, G.K.; Pittman, T.J.; Schunicht, O.C.; Perrett, T.; Wildman, B.K.; Fenton, R.K.; Guichon, P.T.; et al. Microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in Western Canada. Can. Vet. J. 2008, 49, 473–481. [Google Scholar] [PubMed]
- McClurkin, A.W.; Littledike, E.T.; Cutlip, R.C.; Frank, G.H.; Coria, M.F.; Bolin, S.R. Production of cattle immunotolerant to bovine viral diarrhea virus. Can. J. Comp. Med. 1984, 48, 156–161. [Google Scholar]
- Edwards, T.A. Control methods for bovine respiratory disease for feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M.C.; Holmes, K.V. Coronaviridae: The viruses and their replication. In Fields Virology; Knipe, D.M., Howley, P.M., Griffin, D.E., et al., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; Volume 1, pp. 1163–1185. [Google Scholar]
- De Groot, R.J.; Baker, S.C.; Baric, R.; Enjuanes, L.; Gorbalenya, A.E.; Holmes, K.V.; Perlman, S.; Poon, L.; Rottier, P.J.M.; Talbot, P.J.; et al. Family coronaviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M., Carstens, E., Lefkowitz, E., Eds.; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2012. [Google Scholar]
- Saif, L.J.; Wang, Q.; Vlasova, A.N.; Jung, K.; Xiao, S. Coronaviruses. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2019; pp. 488–529. [Google Scholar]
- Clark, M.A. Bovine coronavirus. Br. Vet. J. 1993, 149, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J.; Jung, K. Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans. J. Clin. Microbiol. 2020, 58, e01355-20. [Google Scholar] [CrossRef]
- Pratelli, A.; Lucente, M.S.; Cordisco, M.; Ciccarelli, S.; Di Fonte, R.; Sposato, A.; Mari, V.; Capozza, P.; Pellegrini, F.; Carelli, G.; et al. Natural Bovine Coronavirus Infection in a Calf Persistently Infected with Bovine Viral Diarrhea Virus: Viral Shedding, Immunological Features and S Gene Variations. Animals 2021, 11, 3350. [Google Scholar] [CrossRef]
- Hägglund, S.; Svensson, C.; Emanuelson, U.; Valarcher, J.F.; Alenius, S. Dynamics of virus infections involved in the bovine respiratory disease complex in Swedish dairy herds. Vet. J. 2006, 172, 320–328. [Google Scholar] [CrossRef]
- Paccaud, M.F.; Jacquier, C.I. A respiratory syncytial virus of bovine origin. Arch. Gesammte Virusforsch 1970, 30, 327–342. [Google Scholar] [CrossRef]
- Brodersen, B.W. Bovine respiratory syncytial virus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 323–333. [Google Scholar] [CrossRef]
- Valarcher, J.F.; Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 2007, 38, 153–180. [Google Scholar] [CrossRef]
- Larsen, L.E. Bovine respiratory syncytial virus (BRSV): A review. Acta Vet. Scand. 2000, 41, 1–24. [Google Scholar]
- Tuncer, P.; Yeşilbağ, K. Serological detection of infection dynamics for respiratory viruses among dairy calves. Vet. Microbiol. 2015, 180, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Swiatek, D.L.; Palombo, E.A.; Lee, A.; Coventry, M.J.; Britz, M.L.; Kirkwood, C.D. Characterisation of G8 human rotaviruses in Australian children with gastroenteritis. Virus Res. 2010, 148, 1–7. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Bertoni, E.; Aduriz, M.; Bok, M.; Vega, C.; Saif, L.; Aguirre, D.; Cimino, R.O.; Miño, S.; Parreño, V. First report of group A rotavirus and bovine coronavirus associated with neonatal calf diarrhea in the northwest of Argentina. Trop. Anim. Health Prod. 2020, 52, 2761–2768. [Google Scholar] [CrossRef]
- Bai, X.Q.; Wei, S.C. Establishment of bovine rotavirus animal model and development of corresponding vaccine. J. North Minzu Univ. 2020, 41, 32–37. [Google Scholar]
- Cao, C.; Yu, D.B.; Xu, X.J.; Yu, L.; Chang, J.T. Isolation, identification and complete gene sequence analysis of a bovine ro-tavirus G6P [5] strain. Chin. J. Prev. Vet. Med. 2021, 43, 939–945. [Google Scholar]
- Wang, M.; Yan, Y.; Wang, R.; Wang, L.; Zhou, H.; Li, Y.; Tang, L.; Xu, Y.; Jiang, Y.; Cui, W.; et al. Simultaneous Detection of Bovine Rotavirus, Bovine Parvovirus, and Bovine Viral Diarrhea Virus Using a Gold Nanoparticle-Assisted PCR Assay with a Dual-Priming Oligonucleotide System. Front. Microbiol. 2019, 10, 2884. [Google Scholar] [CrossRef]
- Xie, M.; Chen, K.; Liu, P.; Wang, X.; Chen, Y.; Shang, H.; Hao, Y.; Gao, P.; He, X.; Xu, X. Seroprevalence of five diarrhea-related pathogens in bovine herds of scattered households in Inner Mongolia, China between 2019 and 2022. PeerJ 2023, 11, e16013. [Google Scholar] [CrossRef]
- Roy, P. Orbivirus structure and assembly. Virology 1996, 216, 1–11. [Google Scholar] [CrossRef]
- Shiokawa, M.; Omatsu, T.; Katayama, Y.; Nishine, K.; Fujimoto, Y.; Uchiyama, S.; Kameyama, K.I.; Nagai, M.; Mizutani, T.; Sakoda, Y.; et al. END-phenomenon negative bovine viral diarrhea virus that induces the host’s innate immune response supports propagation of BVDVs with different immunological properties. Virology 2019, 538, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Kapusinszky, B.; Yugo, D.M.; Phan, T.G.; Deng, X.; Kanevsky, I.; Opriessnig, T.; Woolums, A.R.; Hurley, D.J.; Meng, X.J.; et al. Virome of US bovine calf serum. Biologicals 2017, 46, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.M.; Pillatzki, A.; Clement, T.; Bragg, T.; Ridpath, J.; Chase, C.C.L. Persistent infection of American bison [Bison bison] with bovine viral diarrhea virus and bosavirus. Vet. Microbiol. 2021, 252, 108949. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.A.; Figueiredo, P.O.; de Oliveira, C.H.S.; Barbosa, J.D.; Lima, D.H.S.; Bomjardim, H.A.; Silva, N.S.; Campos, K.F.; Oliveira, C.M.C.; Barbosa-Stancioli, E.F.; et al. Occurrence of Pseudocowpox virus associated to Bovine viral diarrhea virus-1, Brazilian Amazon. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 70–75. [Google Scholar] [CrossRef]
- Saeed, I.K.; Ali, Y.H.; AbdulRahman, M.B.; Mohammed, Z.A.; Osman, H.M.; Taha, K.M.; Musa, M.Z.; Khalafalla, A.I. Mixed infection of peste des petits ruminants virus (PPRV) and other respiratory viruses in dromedary camels in Sudan, an abattoir study. Trop. Anim. Health Prod. 2015, 47, 995–998. [Google Scholar] [CrossRef]
- Vanleeuwen, J.A.; Haddad, J.P.; Dohoo, I.R.; Keefe, G.P.; Tiwari, A.; Scott, H.M. Risk factors associated with Neospora caninum seropositivity in randomly sampled Canadian dairy cows and herds. Prev. Vet. Med. 2010, 93, 129–138. [Google Scholar] [CrossRef]
- Fent, G.M.; Fulton, R.W.; Saliki, J.T.; Caseltine, S.L.; Lehmkuhl, H.D.; Confer, A.W.; Purdy, C.W.; Briggs, R.E.; Loan, R.W.; Duff, G.C. Bovine adenovirus serotype 7 infections in postweaning calves. Am. J. Vet. Res. 2002, 63, 976–978. [Google Scholar] [CrossRef]
- Hosny, G.A.; Omran, R.M.A.; Shehab, G.G.; Aly, N.M.; Karim, I.A. Pathological, immunopathological and virological findings in field outbreak of mixed infection by bovine viral diarrhea (BVD) and foot and mouth (FMD) in cattle and buffaloes. Vet. Med. J. 1996, 44, 349–369. [Google Scholar]
- Cheng, J.; Tao, J.; Li, B.; Shi, Y.; Liu, H. Coinfection with PEDV and BVDV induces inflammatory bowel disease pathway highly enriched in PK-15 cells. Virol. J. 2022, 19, 119. [Google Scholar] [CrossRef]
- Langohr, I.M.; Stevenson, G.W.; Nelson, E.A.; Lenz, S.D.; Wei, H.; Pogranichniy, R.M. Experimental co-infection of pigs with Bovine viral diarrhea virus 1 and Porcine circovirus-2. J. Vet. Diagn. Investig. 2012, 24, 51–64. [Google Scholar] [CrossRef]
- Tao, J.; Liao, J.; Wang, Y.; Zhang, X.; Wang, J.; Zhu, G. Bovine viral diarrhea virus (BVDV) infections in pigs. Vet. Microbiol. 2013, 165, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Darbyshire, J.H. A serological relationship between swine fever and mucosal disease of cattle. Vet. Rec. 1960, 72, 331. [Google Scholar]
- Wieringa-Jelsma, T.; Quak, S.; Loeffen, W.L. Limited BVDV transmission and full protection against CSFV transmission in pigs experimentally infected with BVDV type 1b. Vet. Microbiol. 2006, 118, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Carbrey, E.A.; Stewart, W.C.; Kresse, J.I.; Snyder, M.L. Natural infection of pigs with bovine viral diarrhea virus and its differential diagnosis from hog cholera. J. Am. Vet. Med. Assoc. 1976, 169, 1217–1219. [Google Scholar] [CrossRef]
- Castrucci, G.; Torlone, V.; Cilli, V.; Titoli, F.; Valente, C. Rapporti immunologici fra il virus delladiarrea virale del bovino (BVD) e il virus della peste suina (HC). Influenza del ceppo di virus HC in prove d’immunita crociata eseguite nel suino [Immu-nological relationship between bovine viral diarrhea (BVD) virus and hog cholera (HC) virus. Influence of the strain of HC virus in cross immunity tests in swine]. Boll Ist Sieroter Milan 1973, 52, 309–314. [Google Scholar]
- Potgieter, L.N.; McCracken, M.D.; Hopkins, F.M.; Walker, R.D.; Guy, J.S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am. J. Vet. Res. 1984, 45, 1582–1585. [Google Scholar] [CrossRef]
- Gånheim, C.; Hultén, C.; Carlsson, U.; Kindahl, H.; Niskanen, R.; Waller, K.P. The acute phase response in calves experi-mentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 183–190. [Google Scholar] [CrossRef]
- Potgieter, L.N.; McCracken, M.D.; Hopkins, F.M.; Guy, J.S. Comparison of the pneumopathogenicity of two strains of bovine viral diarrhea virus. Am. J. Vet. Res. 1985, 46, 151–153. [Google Scholar] [CrossRef]
- Burciaga-Robles, L.O.; Step, D.L.; Krehbiel, C.R.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Mannheima haemolytica on clinical signs and immune variables: Model for bovine respiratory disease via viral and bacterial interaction. J. Anim. Sci. 2010, 88, 2166–2178. [Google Scholar]
- Burciaga-Robles, L.O.; Krehbiel, C.R.; Step, D.L.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and Mannheimia haemolytica challenge on animal performance, nitrogen balance, and visceral organ mass in beef steers. J. Anim. Sci. 2010, 88, 2179–2188. [Google Scholar] [CrossRef]
- Gånheim, C.; Johannisson, A.; Ohagen, P.; Persson Waller, K. Changes in peripheral blood leucocyte counts and subpopu-lations after experimental infection with BVDV and/or Mannheimia haemolytica. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.; Roeder, P.L. Effect of bovine virus diarrhoea-mucosal disease virus infection on salmonella infection in calves. Res. Vet. Sci. 1987, 42, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.R.; Gravenor, M.B.; Charleston, B.; Hope, J.C.; Martin, M.; Howard, C.J. Mycobacterium bovis shedding patterns from experimentally infected calves and the effect of concurrent infection with bovine viral diarrhoea virus. J. R. Soc. Interface 2007, 4, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Gaussen, J.; Trott, D.J.; Spiers, Z.; Jenkins, C.; Griffiths, H. Sporadic bovine encephalopathy caused by Chlamydia pecorum secondary to bovine viral diarrhoea virus infection in calves in South Australia. Aust. Vet. J. 2024, 102, 80–86. [Google Scholar] [CrossRef]
- Highlander, S.K. Molecular genetic analysis of virulence in Mannheimia [pasteurella] haemolytica. Front Biosci. 2001, 6, D1128–D1150. [Google Scholar]
- Rice, J.A.; Carrasco-Medina, L.; Hodgins, D.C.; Shewen, P.E. Mannheimia haemolytica and bovine respiratory disease. Anim. Health Res. Rev. 2007, 8, 117–128. [Google Scholar] [CrossRef]
- Boyce, J.D.; Lo, R.Y.C.; Wilkie, I.; Adler, B. Pasteurella and Mannheimia. In Pathogenesis of Bacterial Infections in Animals; Gyles, C.L., Prescott, J.F., Songer, J.G., Thoen, C.O., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Singh, K.; Ritchey, J.W.; Confer, A.W. Mannheimia haemolytica: Bacterial–Host Interactions in Bovine Pneumonia. Vet. Erinary Pathol. 2011, 48, 338–348. [Google Scholar] [CrossRef]
- LaRock, D.L.; Chaudhary, A.; Miller, S.I. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 2015, 13, 191–205. [Google Scholar] [CrossRef]
- Santos, R.L.; Zhang, S.; Tsolis, R.M.; Kingsley, R.A.; Adams, L.G.; Bäumler, A.J. Animal models of Salmonella infections: Enteritis versus typhoid fever. Microbes Infect. 2001, 3, 1335–1344. [Google Scholar] [CrossRef]
- Penny, C.D.; Low, J.C.; Nettleton, P.F.; Scott, P.R.; Sargison, N.D.; Strachan, W.D.; Honeyman, P.C. Concurrent bovine viral diarrhoea virus and Salmonella typhimurium DT104 infection in a group of pregnant dairy heifers. Vet. Rec. 1996, 138, 485–489. [Google Scholar] [CrossRef]
- Monies, R.J.; Head, J.C. Bovine tuberculosis in housed calves. Vet. Rec. 1999, 145, 743. [Google Scholar] [PubMed]
- Byrne, A.W.; Graham, J.; Brown, C.; Donaghy, A.; Guelbenzu-Gonzalo, M.; McNair, J.; Skuce, R.; Allen, A.; McDowell, S. Bovine tuberculosis visible lesions in cattle culled during herd breakdowns: The effects of individual characteristics, trade movement and co-infection. BMC Vet. Res. 2017, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.M.; Moline, K.M.; Sargent, R.A.; Campbell, J.R.; Myers, D.J.; Doig, P.A. Immunohistochemical study of Hemophilus somnus, Mycoplasma bovis, Mannheimia hemolytica, and bovine viral diarrhea virus in death losses due to myocarditis in feedlot cattle. Can. Vet. J. 2004, 45, 231–234. [Google Scholar] [PubMed]
- Razin, S. The mycoplasmas. Microbiol. Rev. 1978, 42, 414–470. [Google Scholar] [CrossRef]
- Hale, H.H.; Helmboldt, C.F.; Plastridge, W.N.; Stula, E.F. Bovine mastitis caused by a Mycoplasma species. Cornell Vet. 1962, 52, 582–591. [Google Scholar]
- Nicholas, R.A.; Ayling, R.D. Mycoplasma bovis: Disease, diagnosis, and control. Res. Vet. Sci. 2003, 74, 105–112. [Google Scholar] [CrossRef]
- Maunsell, F.P.; Woolums, A.R.; Francoz, D.; Rosenbusch, R.F.; Step, D.L.; Wilson, D.J.; Janzen, E.D. Mycoplasma bovis infec-tions in cattle. J. Vet. Intern. Med. 2011, 25, 772–783. [Google Scholar] [CrossRef]
- Bürki, S.; Frey, J.; Pilo, P. Virulence, persistence and dissemination of Mycoplasma bovis. Vet. Microbiol. 2015, 179, 15–22. [Google Scholar] [CrossRef]
- Casas, E.; Falkenberg, S.M.; Dassanayake, R.P.; Register, K.B.; Neill, J.D. MicroRNA profiles for different tissues from calves challenged with Mycoplasma bovis or challenged with Mycoplasma bovis and bovine viral diarrhea virus. PLoS ONE 2022, 17, e0271581. [Google Scholar] [CrossRef]
- Bürgi, N.; Josi, C.; Bürki, S.; Schweizer, M.; Pilo, P. Mycoplasma bovis co-infection with bovine viral diarrhea virus in bovine macrophages. Vet. Res. 2018, 49, 2. [Google Scholar] [CrossRef]
- Prysliak, T.; van der Merwe, J.; Lawman, Z.; Wilson, D.; Townsend, H.; van Drunen Littel-van den Hurk, S.; Perez-Casal, J. Respiratory disease caused by Mycoplasma bovis is enhanced by exposure to bovine herpes virus 1 (BHV-1) but not to bovine viral diarrhea virus (BVDV)type 2. Can. Vet. J. 2011, 52, 1195–1202. [Google Scholar] [PubMed]
- Haines, D.M.; Martin, K.M.; Clark, E.G.; Jim, G.K.; Janzen, E.D. The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. Can. Vet. J. 2001, 42, 857–860. [Google Scholar] [PubMed]
- Gagea, M.I.; Bateman, K.G.; Shanahan, R.A.; van Dreumel, T.; McEwen, B.J.; Carman, S.; Archambault, M.; Caswell, J.L. Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J. Vet. Diagn. Investig. 2006, 18, 29–40. [Google Scholar] [CrossRef]
- Shahriar, F.M.; Clark, E.G.; Janzen, E.; West, K.; Wobeser, G. Coinfection with bovine viral diarrhea virus and Mycoplasma bovis in feedlot cattle with chronic pneumonia. Can. Vet. J. 2002, 43, 863–868. [Google Scholar]
- Borel, N.; Polkinghorne, A.; Pospischil, A. A Review on Chlamydial Diseases in Animals: Still a Challenge for Pathologists? Vet. Pathol. 2018, 55, 374–390. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G.; Ortega-Mora, L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007, 20, 323–367. [Google Scholar] [CrossRef]
- Dubey, J.P. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003, 41, 1–16. [Google Scholar]
- Björkman, C.; Alenius, S.; Manuelsson, U.; Uggla, A. Neospora caninum and bovine virus diarrhoea virus infections in Swedish dairy cows in relation to abortion. Vet. J. 2000, 159, 201–206. [Google Scholar] [CrossRef]
- Ståhl, K.; Björkman, C.; Emanuelson, U.; Rivera, H.; Zelada, A.; Moreno-López, J. A prospective study of the effect of Neospora caninum and BVDV infections on bovine abortions in a dairy herd in Arequipa, Peru. Prev. Vet. Med. 2006, 75, 177–188. [Google Scholar] [CrossRef]
- Okumu, T.A.; John, N.M.; Wabacha, J.K.; Tsuma, V.; VanLeeuwen, J. Seroprevalence of antibodies for bovine viral diarrhoea virus, Brucella abortus and Neospora caninum, and their roles in the incidence of abortion/foetal loss in dairy cattle herds in Nakuru District, Kenya. BMC Vet. Res. 2019, 15, 95. [Google Scholar] [CrossRef]
- Olum, M.O.; Mungube, E.O.; Njanja, J.; Kidali, J.; Njenga, E.; Maichomo, M.; Tsuma, V.T.; Mugambi, J. Seroprevalence of canine neosporosis and bovine viral diarrhoea in dairy cattle in selected regions of Kenya. Transbound. Emerg. Dis. 2020, 67 (Suppl. S2), 154–158. [Google Scholar] [CrossRef] [PubMed]
| Coinfections | Protocols | Selected Cells or Bovine | Observations | References |
|---|---|---|---|---|
| BVDV BHV | BVDV BHV-1: Colorado natural infection In vivo | Cattle: Calves and Adult cattle |
| [36,37] |
| BVDV-1 BHV-1.1 BVDV then BHV In vivo | Cattle: 8-to 9-month-old calves (without BVDV/BHV-1 antibodies and antigens) |
| [38,39,40,41,42,43] | |
| BVDV BHV-1 BVDV then BHV-1 In vivo | Cattle: 4-to 6-month-old calves |
| [44] | |
| BVDV BHV-1 PI-3V natural infection In vivo | Cattle: 1-week-to 12-month-old calves |
| [45] | |
| BVDV: NY-1; TVM2 BHV-4: 81/16TV BHV-1: 90/180TN BVDV then BHV-4, BHV-1 In vivo | Cattle: 30-to 40-day-old calves (without antibodies to associated viruses) |
| [46] | |
| Simultaneous infection In vivo |
| [46] | ||
| BVDV-1 BHV-4: 10/154 BVDV then BHV-4 In vitro | Cells: Endometrial cells (Natural infection with NCP-type BVDV) |
| [47] | |
| BVDV PI-3V | BVDV PI-3V Natural infection In vivo | Cattle: 1-week-to 12-month-old calves |
| [45] |
| BVDV: NY-1; TVM PI-3V: 90/1TN Simultaneous infection In vivo | Cattle: 30- to 40-day-old calves (without antibodies to associated viruses) |
| [46] | |
| BVDV BRSV | BVDV: NY-1 BRSV: 165 Simultaneous infection In vivo | Cattle: Calves |
| [48] |
| BVDV: 187/92 BRSV: 504/93 Simultaneous infection In vivo | Cattle: 14-to 17-week-old calves (without antibodies to BVDV, IBR and BLV) |
| [49] | |
| BVDV: NY-1 BRSV: 236-652 Simultaneous infection In vivo | Cattle: 9-to 12-month-old calves |
| [50] | |
| BVDV BRSV Simultaneous infection In vivo | Cattle: 9- to 12-month-old calves |
| [51] | |
| BVDV-2-wt BRSV: 236-652 BVDV then BRSV In vitro | Cell: Bovine turbinate cells; MDBK cells |
| [52] | |
| BVDV: ncp BRSV: 375 Simultaneous infection In vitro | Cell: Bovine alveolar macrophage |
| [53] | |
| BVDV BCoV | BVDV-2a: RS886 BCoV: OK 1776 BVDV then BCoV In vivo | Cattle: 2-to 5-week-old calves |
| [54] |
| BCoV then BVDV In vivo |
| [54] | ||
| BVDV-1 BCoV BVDV then BCoV In vivo | Cattle: 59-to 165-day-old calves |
| [55] | |
| BVDV BRV | BVDV: NY-1c BRV: NS-1 BVDV then BRV In vivo | Cattle: 1-day-old calves (GF) |
| [56] |
| Simultaneous infection In vivo |
| [56] | ||
| BVDV Orbivirus | BVDV: ncp (Positive END phenomenon) Orbivirus BVDV then Orbivirus In vitro | Cells: Bovine testicle cells |
| [57] |
| Coinfections | Protocols | Selected Cells or Porcine | Observations | References |
|---|---|---|---|---|
| BVDV PEDV | BVDV-2: SH-28 PEDV: JS-2/2014 Simultaneous infection In vitro | Cells: Porcine kidney (PK15) cells |
| [124] |
| BVDV PCV | BVDV-1 PCV-2 Simultaneous infection In vivo | Porcine: 24-day-old piglets (GF) |
| [125] |
| Coinfections | Protocols | Selected Cells or Bovine | Observations | References |
|---|---|---|---|---|
| BVDV M. haemolytica | BVDV: 72 M. haemolytica-1 BVDV then M. haemolytica In vivo | Cattle: 4- to 6-month-old calves |
| [131] |
| BVDV 1 M. haemolytica: Ab 35 BVDV then M. haemolytica In vivo | Cattle: 9-to 18-week-old calves |
| [132] | |
| BVDV: 2724; 72 M. haemolytica-1 BVDV then M. haemolytica In vivo | Cattle: Calves |
| [133] | |
| BVDV-1b M. haemolytica BVDV then M. haemolytica In vivo | Cattle: Crossbred beef cattle |
| [134] | |
| BVDV-1b M. haemolytica-1 BVDV then M. haemolytica In vivo | Cattle: Angus crossbred steers |
| [135] | |
| BVDV M. haemolytica BVDV then M. haemolytica In vivo | Cattle: 12-week-old calves |
| [136] | |
| BVDV Salmonella | BVDV Salmonella Co-infection In vivo | Cattle: Calves |
| [137] |
| BVDV Mycobacterium bovis | BVDV: 11249 Mycobacterium bovis: AF 2122/97 Mycobacterium bovis then BVDV In vivo | Cattle: Calves |
| [138] |
| BVDV Chlamydia pecorum | BVDV: ncp Chlamydia pecorum Natural infection In vivo | Cattle: 12-to 18-week-old calves |
| [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Wang, J.; Tan, B.; Zhang, S. A Systematic Study of Bovine Viral Diarrhoea Virus Co-Infection with Other Pathogens. Viruses 2025, 17, 700. https://doi.org/10.3390/v17050700
Hou Z, Wang J, Tan B, Zhang S. A Systematic Study of Bovine Viral Diarrhoea Virus Co-Infection with Other Pathogens. Viruses. 2025; 17(5):700. https://doi.org/10.3390/v17050700
Chicago/Turabian StyleHou, Zhiwei, Jiahui Wang, Bin Tan, and Shuqin Zhang. 2025. "A Systematic Study of Bovine Viral Diarrhoea Virus Co-Infection with Other Pathogens" Viruses 17, no. 5: 700. https://doi.org/10.3390/v17050700
APA StyleHou, Z., Wang, J., Tan, B., & Zhang, S. (2025). A Systematic Study of Bovine Viral Diarrhoea Virus Co-Infection with Other Pathogens. Viruses, 17(5), 700. https://doi.org/10.3390/v17050700




