Emergence of Bictegravir Resistance in a Treatment-Experienced PWH on Functional Monotherapy and Rapid Replacement by an Ancient Wild-Type Strain Following Transient Treatment Interruption
Abstract
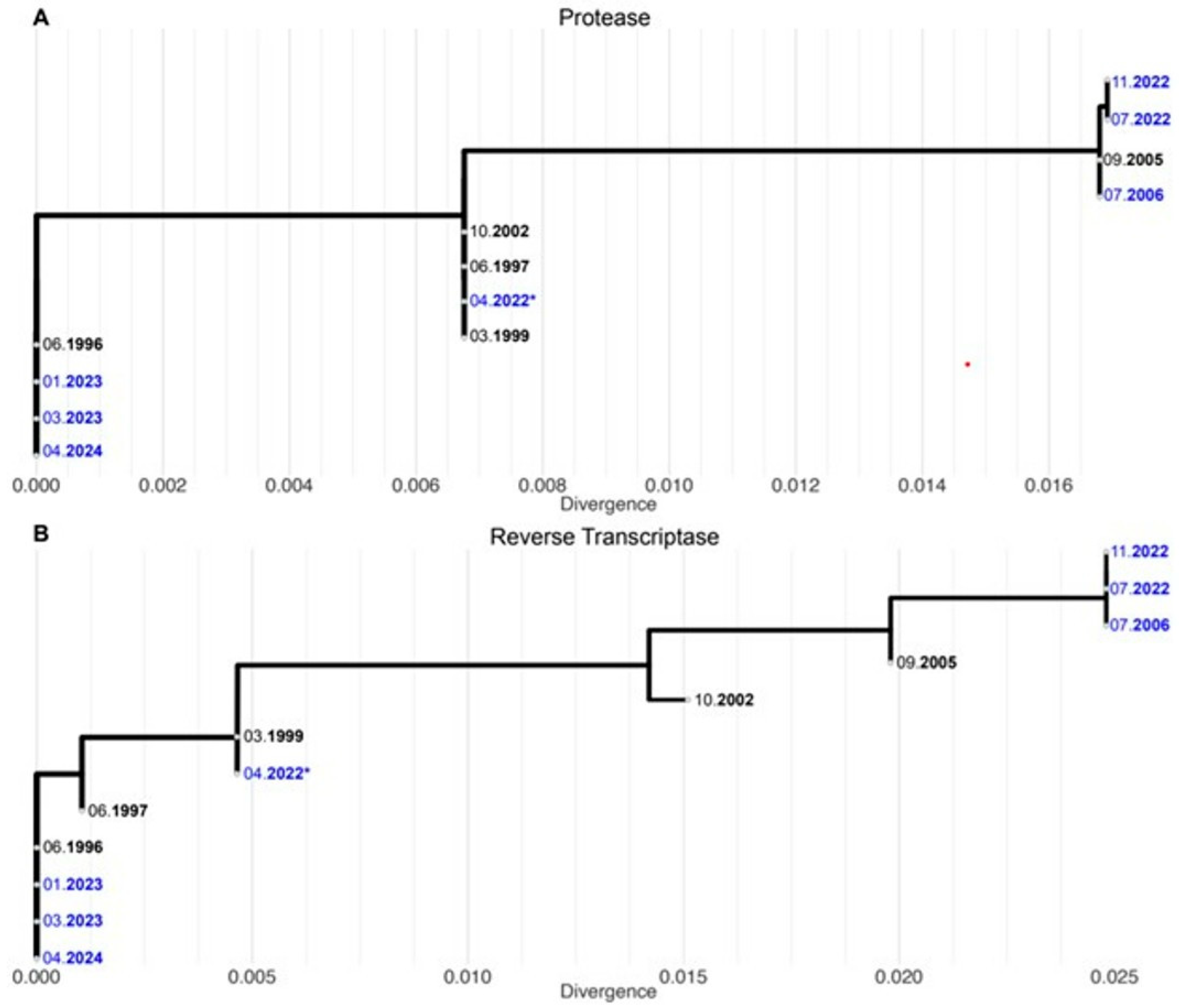
1. Case Report
2. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherrer, A.U.; Traytel, A.; Braun, D.L.; Calmy, A.; Battegay, M.; Cavassini, M.; Furrer, H.; Schmid, P.; Bernasconi, E.; Stoeckle, M.; et al. Cohort Profile Update: The Swiss HIV Cohort Study (SHCS). Leuk. Res. 2022, 51, 33–34j. [Google Scholar] [CrossRef] [PubMed]
- Glass, T.R.; Sterne, J.A.; Schneider, M.-P.; De Geest, S.; Nicca, D.; Furrer, H.; Günthard, H.F.; Bernasconi, E.; Calmy, A.; Rickenbach, M.; et al. Self-reported nonadherence to antiretroviral therapy as a predictor of viral failure and mortality. AIDS 2015, 29, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Metzner, K.J.; Geissberger, F.D.; Shah, C.; Leemann, C.; Klimkait, T.; Böni, J.; Trkola, A.; Zagordi, O. MinVar: A rapid and versatile tool for HIV-1 drug resistance genotyping by deep sequencing. J. Virol. Methods 2017, 240, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rozewicki, J.; Li, S.; Amada, K.M.; Standley, D.M.; Katoh, K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 2019, 47, W5–W10. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [PubMed]
- Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2021.
- European AIDS Clinical Society. European AIDS Clinical Society Guidelines. Version 12.0. 2023. Available online: https://www.eacsociety.org/guidelines/ (accessed on 8 May 2025).
- Andreatta, K.; D’Antoni, M.L.; Chang, S.; Parvangada, A.; Martin, R.; Blair, C.; Hagins, D.; Kumar, P.; Hindman, J.T.; Martin, H.; et al. High efficacy of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in Black adults in the United States, including those with pre-existing HIV resistance and suboptimal adherence. J. Med. Virol. 2024, 96, e29899. [Google Scholar] [CrossRef] [PubMed]
- de Salazar, A.; Viñuela, L.; Fuentes, A.; Teyssou, E.; Charpentier, C.; Lambert-Niclot, S.; Serrano-Conde, E.; Pingarilho, M.; Fabeni, L.; De Monte, A.; et al. Transmitted Drug Resistance to Integrase-Based First-Line Human Immunodeficiency Virus Antiretroviral Regimens in Mediterranean Europe. Clin. Infect. Dis. 2023, 76, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.; Mena, L.; Brock, J.B. Case Report: Emergent Resistance in a Treatment-Naive Person With Human Immunodeficiency Virus Under Bictegravir-Based Therapy. Open Forum Infect. Dis. 2021, 8, ofab297. [Google Scholar] [CrossRef] [PubMed]
- Blanco-ArãValo, J.L.; Garcãa-Deltoro, M.; Torralba, M.; VãLez-Dãaz-PallarãS, M.; Castro, A.; Rubio-Rodrãguez, D.; Rubio-TerrãS, C. HIV-1 resistance and virological failure to treatment with the integrase inhibitors bictegravir, cabotegravir, and dolutegravir: A systematic literature review. Aids Rev. 2024, 26, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, A.-G.; Soulie, C.; Wirden, M.; Barriere, G.; Durand, F.; Charpentier, C.; Descamps, D.; Calvez, V. Emergent resistance-associated mutations at first- or second-line HIV-1 virologic failure with second-generation InSTIs in two- and three-drug regimens: The Virostar-1 study. J. Antimicrob. Chemother. 2025, 80, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Walter, H.; Beerenwinkel, N.; Keulen, W.; Kaiser, R.; Hoffmann, D.; Lengauer, T.; Selbig, J.; Vandamme, A.-M.; Korn, K.; et al. Tenofovir Resistance and Resensitization. Antimicrob. Agents Chemother. 2003, 47, 3478–3484. [Google Scholar] [CrossRef] [PubMed]
- Sterman, F.L.; Lalezari, J.P.; Kowalczyk, U.M.; Main, D.W.; Grant, E.M.; Caro, L.; Manning, C.M.; Burke, R.L. Bictegravir/emtricitabine/tenofovir alafenamide plus doravirine in highly treatment-experienced men with multidrug-resistant HIV. AIDS 2023, 37, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Sterrantino, G.; Borghi, V.; Callegaro, A.P.; Bruzzone, B.; Saladini, F.; Maggiolo, F.; Maffongelli, G.; Andreoni, M.; De Gennaro, M.; Gianotti, N.; et al. Prevalence of predicted resistance to doravirine in HIV-1-positive patients after exposure to non-nucleoside reverse transcriptase inhibitors. Int. J. Antimicrob. Agents 2019, 53, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lan, Y.; Liang, S.; Wang, J.; Ni, M.; Zhang, X.; Yu, F.; Chen, M.; Zhang, H.; Yan, L.; et al. Prevalence of doravirine cross-resistance in HIV-infected adults who failed first-line ART in China, 2014–2018. J. Antimicrob. Chemother. 2022, 77, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.L.; Scheier, T.; Ledermann, U.; Flepp, M.; Metzner, K.J.; Böni, J.; Günthard, H.F. Emergence of Resistance to Integrase Strand Transfer Inhibitors during Dolutegravir Containing Triple-Therapy in a Treatment-Experienced Patient with Pre-Existing M184V/I Mutation. Viruses 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Loosli, T.; Hossmann, S.; Ingle, S.M.; Okhai, H.; Kusejko, K.; Mouton, J.; Bellecave, P.; van Sighem, A.; Stecher, M.; Monforte, A.D.; et al. HIV-1 drug resistance in people on dolutegravir-based antiretroviral therapy: A collaborative cohort analysis. Lancet HIV 2023, 10, e733–e741. [Google Scholar] [CrossRef]
- Mesplède, T.; Quashie, P.K.; Osman, N.; Han, Y.; Singhroy, D.N.; Lie, Y.; Petropoulos, C.J.; Huang, W.; A Wainberg, M. Viral fitness cost prevents HIV-1 from evading dolutegravir drug pressure. Retrovirology 2013, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Loosli, T.; Han, N.; Hauser, A.; Josi, J.; Ingle, S.M.; van Sighem, A.; Wittkop, L.; Vehreschild, J.; Ceccherini-Silberstein, F.; Maartens, G.; et al. Predicted dolutegravir resistance in people living with HIV in South Africa during 2020–35: A modelling study. Lancet Glob. Health 2025, 13, e698–e706. [Google Scholar] [CrossRef]

| Date | VL Copies/mL | Ongoing ART for Each Time Interval by Class | Resistance Test | Relevant Resistance Associated Mutations by ART Class | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NRTI | PI | NNRTI | INSTI | Other | NRTI | NNRTI | PI | INSTI | |||
| 07.2006 | 14,500 | ddI + ABC + TDF | ATV/r | RAL | RNA | D67N, K70R | V108I, L234I, Y318 F | L10I, K20R, K43T, M46L, I54V, V82M, L90M | not done | ||
| 04.2022 | <20 | TAF + FTC | DOR | BIC | proviral DNA (PBMC) | D67N, K70R | V108I, L234I, Y318 F | L10I, K20R, K43T, M46L, I54V, V82M, L90M | none | ||
| 07.2022 | 134 | TAF + FTC | DOR | BIC | RNA | D67N, K70R | V108I, L234I, Y318 F | L10I, K20R, K43T, M46L, I54V, V82M, L90M | T97A, G140S, Q148H | ||
| 11.2022 | 217 | TAF + FTC | DOR | BIC | RNA | D67N, K70R, M184V | V108I, L234I, Y318 F | L10I, K20R, K43T, M46L, I54V, V82M, L90M | T97A, G140S, Q148H, G149A | ||
| 01.2023 | 106,000 | TAF + FTC | DRV/c | ETR | FTR | RNA | none | none | none | none | |
| 03.2023 | 1590 | TAF + FTC | DRV/c | ETR | FTR | RNA | none | none | none | none | |
| 07.2023 | <20 | TAF + FTC | DRV/c | ETR | FTR | not done | not done | not done | not done | not done | |
| 04.2024 | 3900 | TAF + FTC | DRV/c | ETR | FTR | RNA | none | none | none | none | |
| 07.2024 | <20 | TAF + FTC | DRV/c | ETR | FTR | not done | not done | not done | not done | not done | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faré, P.B.; Ziltener, G.; Bergadà Pijuan, J.; Abela, I.A.; Hirsch, B.L.; Huber, M.; Nemeth, J.; Günthard, H.F. Emergence of Bictegravir Resistance in a Treatment-Experienced PWH on Functional Monotherapy and Rapid Replacement by an Ancient Wild-Type Strain Following Transient Treatment Interruption. Viruses 2025, 17, 699. https://doi.org/10.3390/v17050699
Faré PB, Ziltener G, Bergadà Pijuan J, Abela IA, Hirsch BL, Huber M, Nemeth J, Günthard HF. Emergence of Bictegravir Resistance in a Treatment-Experienced PWH on Functional Monotherapy and Rapid Replacement by an Ancient Wild-Type Strain Following Transient Treatment Interruption. Viruses. 2025; 17(5):699. https://doi.org/10.3390/v17050699
Chicago/Turabian StyleFaré, Pietro B., Gabriela Ziltener, Judith Bergadà Pijuan, Irene A. Abela, Britta L. Hirsch, Michael Huber, Johannes Nemeth, and Huldrych F. Günthard. 2025. "Emergence of Bictegravir Resistance in a Treatment-Experienced PWH on Functional Monotherapy and Rapid Replacement by an Ancient Wild-Type Strain Following Transient Treatment Interruption" Viruses 17, no. 5: 699. https://doi.org/10.3390/v17050699
APA StyleFaré, P. B., Ziltener, G., Bergadà Pijuan, J., Abela, I. A., Hirsch, B. L., Huber, M., Nemeth, J., & Günthard, H. F. (2025). Emergence of Bictegravir Resistance in a Treatment-Experienced PWH on Functional Monotherapy and Rapid Replacement by an Ancient Wild-Type Strain Following Transient Treatment Interruption. Viruses, 17(5), 699. https://doi.org/10.3390/v17050699







