Abstract
Human activity in sylvatic environments and resulting contact with wildlife, such as non-human primates (NHPs), can lead to pathogen spillover or spillback. Both NHPs and humans host a variety of herpesviruses. While these viruses typically cause asymptomatic infections in their natural hosts, they can lead to severe disease or even death when they move into novel hosts. In early 2024, deaths of Callithrix penicillata, the black-tufted marmoset, were reported in an urban park in Belo Horizonte, Minas Gerais, Brazil. The epizootic was investigated in collaboration with CETAS/IBAMA and the Zoonoses Department of Belo Horizonte. Nine marmoset carcasses and four sick marmosets were found in the park; the latter exhibited severe neurological symptoms and systemic illness before succumbing within 48 h. Carcasses were tested for rabies virus and were all negative, and necropsy findings revealed widespread organ damage. In addition, the samples were tested for yellow fever virus, with negative results. Finally, molecular testing, viral isolation, and phylogenetic analysis demonstrated human herpesvirus 1 (HHV-1) as the causative agent. The likely source of infection was human-to-marmoset transmission, facilitated by close interactions such as feeding and handling. This study highlights the risks of pathogen spillover between humans and nonhuman primates, emphasizing the need for enhanced surveillance and public awareness to mitigate future epizootics.
1. Introduction
Human activities in sylvatic environments and contact with animals, such as non-human primates (NHPs), increase the risk of bidirectional pathogen transmission between NHPs and humans. There is a long history of infectious diseases spreading from NHPs to humans, but transmission in the opposite direction has also been observed, including Zika virus, SARS-CoV-2, measles, influenza virus, hepatitis virus, herpesviruses, and others [1,2,3,4,5,6]. The breakdown of natural barriers between NHPs and humans poses health risks for a much larger population, including humans living in urban areas [4] and NHPs living in pristine forests. In this context, urban parks serve as key points of interaction between humans and free-living NHPs, facilitating cross-infections [6]. Urban parks are important components of modern human society, enabling outdoor recreation and alleviating urban heat island effects [7]. In many urban parks in the neotropics, marmosets perform essential ecological functions, such as seed dispersal and insect predation, thereby contributing to the regeneration of native plants and to pest control [8]. Additionally, their presence can enhance local biodiversity, promoting ecological balance [8,9].
Between 2016 and 2018, Brazil faced significant yellow fever (YF) outbreaks, with over 2000 human and NHP epizootic cases each, mainly in the State of Minas Gerais (MG) [10,11,12,13,14,15]. Belo Horizonte (BH), the capital of the state, was also affected, with yellow fever virus (YFV, Orthoflavivirus flavi)-positive marmosets’ carcasses being found in different areas of the city, including urban parks [14]. Since the outbreaks, cases of monkeys and humans infected with YFV have been continuously reported outside the Amazon Basin in Brazil, including MG state [10,16] and the metropolitan region of BH [17].
BH is in a region of transition between the Atlantic Forest and the Cerrado (Brazilian savannah) habitats [18] in MG, southeast Brazil. Atlantic Forest and Cerrado biomes are among the most biodiverse in the world. Both biomes face threats to their endemic mammal species, many at risk of extinction, making them critical hotspots for conservation [19,20]. BH has approximately 80 urban parks [21], which play a crucial role in preserving biodiversity in the face of urban growth. Black-tufted marmosets (Callithrix penicillata) live in these parks and other regions of the city, as they are well adapted to urbanized environments. Their ability to exploit diverse habitats, access a variety of food sources, and maintain a versatile diet contributes to their successful adaptation [22,23,24,25].
More recently, between March and June 2024, epizootics took place in an urban park in BH, causing the deaths of several black-tufted marmosets and drastically reducing their population in the affected region. Herein, we report the outcomes of our investigation to identify the pathogen responsible for the outbreak.
2. Materials and Methods
2.1. Study Area and Samples
The urban park Primeiro de Maio is in the northern part of BH, covering an area of 3.3 hectares [26]. It features nine springs, various species of trees, and vertebrate animals including turtles, fish, small mammals, several bird species, and marmosets. The park has different recreational areas and is surrounded by residential areas along its entire perimeter. Park rangers reported that marmosets are attracted to humans, and it is common to observe visitors or neighbors feeding the marmosets.
In March 2024, the first observations of dead and sick marmosets in Primeiro de Maio park were reported, and the epizootic continued until May 2024. Nine carcasses of marmosets and four sick marmosets (total n = 13) from the same group were found in the park. The four sick marmosets (two adult males, one infant male, and one adult female, named NHP 1342 to 1345) were forwarded to the Wildlife Center of BH (CETAS-BH). They presented fever, apathy, weakness, dehydration, and neurological involvement characterized by multiple seizures, unilateral ptosis, pupil contraction, incoordination, disorientation, and lateral recumbency. Only one individual exhibited ulceration on the lingual mucosa, and all of them died within 48 h. These four carcasses (NHP 1342 to 1345) were sent to LZOON-BH and then to Virus Lab-UFMG for further investigation. At LZOON-BH, all carcasses (n = 13) were tested for rabies, by routine protocols. At Virus Lab-UFMG, necropsy was performed on the four animals that died at CETAS (NHP 1342 to 1345), and liver, kidney, heart, lung, tongue, bladder, spleen, testicle and blood clot samples were collected and preserved at −80 °C and used for the following analyses. In June of 2024, fieldwork and baiting activities were conducted over three days in the park, followed by one day dedicated to trapping and sample collection. During this time, only two marmosets were sighted, with one of them being captured and sampled. Park rangers reported that most marmosets in the park were affected by the epizootic. The outbreak was investigated jointly with the Department and the Laboratory of Zoonoses of BH (LZOON-BH) and the Wildlife Rescue Center (CETAS-BH).
2.2. Virus Investigation
Tissue fragments from four carcasses (NHP 1342 to 1345 that were found sick in the park and died at CETAS-BH) were separately used for total nucleic acid extraction. We performed total viral DNA and RNA extraction using the QIAamp cador Pathogen Mini Kit protocol (QIAGEN, Venlo, The Netherlands) on the liver, blood clot, lung, kidney, testicle, and spleen samples (approximately 30 mg). These samples were then investigated for YFV and human herpesviruses. Further samples (serum, heart, intestine, and bladder, tested only for herpesviruses) had total DNA extracted with a High Pure Viral Nucleic Acid Kit (Roche, Basel, Switzerland). Mechanical tissue disruption was performed using three 2 mm borosilicate beads and a bead beater (Mini-Beadbeater-16, BioSpec Products, Bartlesville, OK, USA). Samples were extracted in batches of up to 14 samples plus a negative extraction control (nuclease-free water).
RT-qPCR was performed using the GoTaq® Probe 1-Step RT-qPCR System (Promega) to assess RNA viability through the endogenous control β-actin gene (Table S1) [27], confirming all samples as suitable for analysis. Total viral RNA/DNA obtained from liver, blood clot, lung, kidney, testicle, and spleen (2.5 µL) along with negative extraction controls were screened in duplicate for YFV RNA, targeting the 5′ untranslated region of the viral genome (Table S1) [28]. In each set of RT-qPCR, a non-template control (nuclease-free water) and a positive control (YFV 17DD RNA) were included. Samples were also tested for human herpesviruses 1 and 3 (HHV-1/2, and HHV-3) by qPCR (GoTaq® qPCR Promega Corporation, Madison, WI, USA), using primers targeting the polymerase gene [29] (Table S1). To distinguish HHV-1 from HHV-2, samples were tested using primers targeting the glycoprotein D (gD) gene of HHV-1 and glycoprotein G (gG) gene of HHV-2 [30] (Table S1). Extracted DNA from HHV-1 and HHV-3 were used as the positive control, as well as a non-template control (nuclease-free water) in each qPCR and PCR reaction.
A fragment of the tongue with a lesion from NHP 1342 was macerated (50 mg of tissue in 200 μL of Eagle’s minimal essential medium (MEM) without fetal bovine serum (FBS) by mechanical disruption as described above. After maceration, the product was centrifuged for 10 min at 10,000× g and used as an inoculum on the Vero cells monolayer. Vero cells cultivated in a 12-well plate (1.8 × 105 Vero cells per well) were inoculated with 180 µL of the 1:2 diluted macerated and maintained in MEM supplemented with 5% FBS at 37 °C in a 5% CO2 incubator. Cells were observed in an inverted microscope and after three days of infection.
The supernatant of infected cells was collected and subjected to DNA extraction and PCR targeting gD (HHV-1) and gG (HHV-2) genes [30] and conventional PCR targeting the glycoprotein B (gB) gene, generating a 400 nt amplicon [31] (Table S1). The PCR amplicon was analyzed by electrophoresis in an 8% polyacrylamide gel, stained with SYBR Gold (Invitrogen–Thermo Fisher Scientific, Waltham, MA, USA), and examined under a Blue-Light Transilluminator (Safe Imager™ 2.0 Blue-Light Transilluminator, Invitrogen–Thermo Fisher Scientific, Waltham, MA, USA). The observed amplicon was purified and sequenced by the dideoxy method with the ABI3130 platform (Applied Biosystems, Waltham, MA, USA). The consensus sequence was generated (Chromas software version 2.6.6) and the Basic Local Alignment Search Tool (BLAST) was used to find regions of local similarity between sequences on nucleotide databases. The consensus sequence was aligned with 14 sequences (GenBank accession numbers in Supplementary File) in MEGA12 (https://www.megasoftware.net/, (accessed on 20 January 2025)) using Tamura-Nei (1993) model and the maximum-likelihood method with 1000 bootstrap replicates.
3. Results and Discussion
Considering the persistent circulation of YFV in MG, including the metropolitan region of BH [10,17,32,33,34], we initially investigated YFV infection in carcasses. Samples were negative, indicating no active infection with YFV. Brains were removed in LZOON-BH for rabies investigation and all samples (n = 13) were found to be negative for rabies virus. It was not possible to collect samples from the nine carcasses found in the park for YFV or herpesvirus investigation due to their advanced decomposition state.
The four necropsied carcasses of marmosets, NHP 1342 to 1345, were in an advanced stage of autolysis, but it was possible to note extensive areas of multifocal hepatic necrosis, characterized by pale-colored zones with hemorrhagic centers, indicative of cellular degeneration [35,36]. The lungs showed foci of interstitial pneumonia with consolidation, diffuse edema, and hemorrhage, consistent with severe dyspnea. Brains were removed in LZOON-BH for rabies investigation and were not available for analysis. The regional lymph nodes were enlarged and hyperemic, suggesting an intense systemic inflammatory response (Table S2). The tongue of NHP 1342 exhibited well-defined necrotic ulcers with a hemorrhagic halo, associated with intense sialorrhea and dysphagia (Figure 1A).
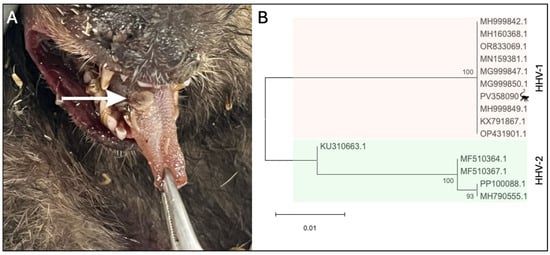
Figure 1.
Human herpesvirus 1 in Callithrix penicillata during an epizootic in Belo Horizonte, Minas Gerais, Brazil. (A) Marmoset tongue (NHP 1342) presenting a herpes lesion (white arrow). (B) Evolutionary analysis of partial sequence obtained from HHV-1 virus isolated from the tongue of animal 1342. Inferences used the maximum likelihood method and Tamura–Nei (1993) model. The percentage of replicate trees in which the associated taxa clustered together (1.000 replicates) is shown next to the branches. The analyses included 15 partial sequences of glycoprotein B coding gene with 351 positions in the final dataset. Samples are identified in the phylogenetic tree by Genbank accession number. The evolutionary analyses were conducted in MEGA12.
Next, we investigated the marmosets for human herpesvirus infection. Liver, lung, kidney, testicle, heart, intestine, or bladder samples were simultaneously tested for HHV-1/2 and HHV-3 [29] While samples from the 4 marmosets were negative for HHV-3, they were positive for HHV-1/2 by qPCR (Table 1 and Table S3). Liver and lung samples were also tested for HHV-1 [30], being positive (Table S3). HHV-1/2 infections in non-human primates are known to cause generalized and systemic disease. As a result, viral DNA can be detected in various tissues from an infected individual [35]. Viral isolation was conducted using a fragment of the lesion on the tongue (NHP 1342). After three days of infection, cell rounding, detachment, and lysis across most of the cell monolayer were observed in infected cells compared to the control cells. The supernatant was collected and subjected to DNA extraction, diluted 1:2, and tested positive for HHV-1 (Cq = 12.5). A partial sequence of the gB gene obtained from the virus isolated from NHP 1342 (Genbank accession number PV358090) was aligned with other herpesviruses sequences (Figure S1) and used for phylogenetic analysis, confirming the infection by HHV-1 (Figure 1B). Serum from the free-living marmoset captured in June/2024 (CT-24-203) in the park was tested by qPCR and negative for HHV-1 (Table 1).

Table 1.
Molecular investigation of yellow fever virus and human herpesviruses in Callithrix penicilatta.
Both NHPs and humans host a variety of specific herpesviruses. While these viruses typically cause asymptomatic infections in their natural hosts, they can lead to severe disease when transmitted to different species [36]. For example, herpesvirus B (Simplexvirus macacinealpha1) is rarely responsible for disease in its natural host, the macaque, but infection of humans results in a disseminated viral infection characterized by ascending paralysis and a high case fatality rate. The opposite situation can also occur when HHV-1/2 and HHV-3 infect NHPs [35,36]. Although HHV-1 infection in old-world monkeys usually result in mild, self-limiting oral vesicular lesions, New World monkeys usually develop lethal disseminated disease. In NHPs, HHV-1 is commonly responsible for oral lesions and encephalitis in adults, whereas HHV-2 is usually a sexually transmitted disease, causing a genital infection in adults and a disseminated infection in infants [35,37].
The observed lesion pattern and clinical signs [1,2,3,4,35], and molecular testing, confirmed systemic infection by HHV-1. NHPs are not naturally infected by HHV-1 in the wild; thus, infection occurs through contact with infected humans or contaminated fomites [2,3,35]. Once established in a population of New World NHPs, HHV-1 spreads rapidly, potentially leading to high mortality and morbidity within the population [3], as observed in the epizootic studied here. It is well established that Callithrix species live in close-knit family groups, which likely facilitated pathogen transmission. Although we could not analyze samples from the nine carcasses initially collected at the beginning of the epizootics, they belonged to the same group affected by HHV-1, supporting the inference that their deaths were related to the same occurrence. Unfortunately, we were not able to track the exact source of HHV-1 infection in the marmosets in our study. However, given that close contact between NHPs and humans is a key factor in pathogen transmission [3,24,38,39] and frequent interactions—such as physical contact and feeding between park visitors and neighbors—are reported by park rangers, probably humans were the source of HHV-1 infection in NHPs.
Direct and indirect interactions between humans and NHPs increase the risk of interspecific disease transmission, posing challenges for both species conservation and public health [40,41]. Beyond these risks, excessive proximity to black-tufted marmosets can lead to significant behavioral changes, making them dependent on humans for food, which has negative consequences for their health [24,42] This dependency can weaken their ecological roles in pest control and seed dispersal, reduce their natural antipredator responses, and lower their aversion to human presence [43,44,45]. As a result, human–marmoset interactions may escalate into conflicts and, in extreme cases, acts of aggression toward people [24,38].
Monitoring NHP infections is crucial for understanding transmission dynamics, identifying pathogen persistence, and detecting potential spillover or spillback events within disease cycles. Addressing this complex issue requires an integrated approach that combines surveillance efforts with targeted educational initiatives. Promoting responsible human behavior toward wildlife is essential for mitigating the risks of viral spillback and preventing future epizootics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v17050660/s1, Table S1: Primers and probes used for molecular investigation of yellow fever virus and human herpesviruses; Table S2: Necropsies findings in Callithrix penicilatta carcasses; Table S3: Results of yellow fever virus (RTqPCR) and Human Herpesviruses (qPCR) molecular investigation in Callithrix penicilatta. Figure S1: Nucleotide alignment of partial gB coding gene of human herpesvirus 1, used for phylogenetic analyses.
Author Contributions
Conceptualization, B.P.D.; Methodology, B.P.D., G.F.G.-O. and M.F.B.; Formal Analysis, G.F.G.-O., M.F.B. and B.P.D.; Investigation, G.F.G.-O., M.F.B., D.J., M.A.O. and A.M.d.O.P.; Resources, M.F.B., D.J., M.A.O., A.M.d.O.P., P.A.A., C.B., D.A.d.R.V., É.P.T.T., T.L.S., T.M.M., M.C.S., M.G.A., I.V.S., É.M.M. and F.E.N.G.; Writing—Original Draft Preparation, G.F.G.-O., M.F.B., A.M.d.O.P. and B.P.D.; Writing—Review and Editing, G.F.G.-O., M.F.B., D.J., M.A.O., A.M.d.O.P., P.A.A., C.B., D.A.d.R.V., É.P.T.T., T.L.S., T.M.M., M.C.S., M.G.A., I.V.S., É.M.M., F.E.N.G., K.A.H., N.V. and B.P.D.; Visualization, G.F.G.-O. and B.P.D.; Supervision, B.P.D.; Project Administration, K.A.H., N.V. and B.P.D.; Funding Acquisition, K.A.H., N.V. and B.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Institute of Allergy and Infectious Diseases (NIAID) through the Centers for Research in Emerging Infectious Diseases (CREID) “The Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics (CREATE-NEO)” grant U01 AI151807 (to NV/KAH). BPD research is also supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico do Ministério da Ciência e Tecnologia e Inovação (CNPq). GFGO is a student from the Graduation Program in Microbiology of Universidade Federal de Minas Gerais (UFMG), which is supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) (001, and 88882.348380/2010-1).
Institutional Review Board Statement
Ethical approval for the study was obtained from the Committee on Ethics in the Use of Animals at the Universidade Federal de Minas Gerais (protocol CEUA 290/2022), and research in federal conservation units was authorized by the Chico Mendes Institute for Biodiversity Conservation (protocol SISBIO 77400). The project is registered at SISGEN-Brazil under protocol A8091C3.
Informed Consent Statement
Not applicable.
Data Availability Statement
All research data are shared within the manuscript and Supplementary Tables S1–S3 and in the ZENODO repository under https://doi.org/10.5281/zenodo.15262741 (accessed on 20 March 2025).
Acknowledgments
We would like to thank Secretaria Municipal de Saúde de Belo Horizonte, Laboratório de Zoonoses, and Centro de Controle de Zoonoses da Prefeitura de Belo Horizonte, Department of Zoonoses of Belo Horizonte, Secretaria de Estado de Saúde de Minas Gerais, Centro de Triagem de Animais Silvestres of Belo Horizonte, colleagues from Laboratório de Vírus/UFMG, Pró-Reitorias de Graduação, Pós-graduação, and Pesquisa from Universidade Federal de Minas Gerais/Brazil.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, collection, analyses, or interpretation of data, writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NHP | Non-human primate |
| IBAMA | Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis |
| CETAS | Centro de Triagem de Animais Silvestres |
| BH | Linear dichroism |
| MG | Minas Gerais |
| YFV | Yellow fever virus |
| HHV | Human herpes virus |
| LZOON | Laboratório de Zoonoses |
| UFMG | Universidade Federal de Minas Gerais |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| PCR | Polymerase chain reaction |
| RT-qPCR | Reverse Transcriptase Real-Time Polymerase Chain Reaction |
| gD | Glycoprotein D |
| gG | Glycoprotein G |
| gB | Glycoprotein B |
| MEM | Eagle’s minimal essential medium |
| FBS | Fetal bovine serum |
| nt | Nucleotide |
References
- Mätz-Rensing, K.; Jentsch, K.D.; Rensing, S.; Langenhuyzen, S.; Verschoor, E.; Niphuis, H.; Kaup, F.-J. Fatal Herpes simplex Infection in a Group of Common Marmosets (Callithrix jacchus). Vet. Pathol. 2003, 40, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Longa, C.S.; Bruno, S.F.; Pires, A.R.; Romijn, P.C.; Kimura, L.S.; Costa, C.H.C. Human Herpesvirus 1 in Wild Marmosets, Brazil, 2008. Emerg. Infect. Dis. 2011, 17, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.A.; Luppi, M.M.; de Campos Cordeiro Malta, M.; Luiz, A.P.M.F.; de Araujo, M.R.; Coelho, F.M.; da Fonseca, F.G.; Ecco, R.; Resende, M. Outbreak of Human Herpesvirus Type 1 Infection in Nonhuman Primates (Callithrix penincillata). J. Wildl. Dis. 2011, 47, 690–693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, T.M.; Ritter, J.M.; Martines, R.B.; Bullock, H.A.; Fair, P.; Radford, K.W.; Macêdo, I.L.; Sousa, D.E.; Gonçalves, A.A.; Romano, A.P.; et al. Fatal Human Alphaherpesvirus 1 Infection in Free-Ranging Black-Tufted Marmosets in Anthropized Environments, Brazil, 2012–2019. Emerg. Infect. Dis. 2022, 28, 802–811. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Zini, N.; Sacchetto, L.; Rocha, R.F.; Parra, M.C.P.; Del Sarto, J.L.; Dias, A.C.F.; Coutinho, F.; Rayra, J.; da Silva, R.A.; et al. Evidence of natural Zika virus infection in neotropical non-human primates in Brazil. Sci. Rep. 2018, 8, 16034. [Google Scholar] [CrossRef]
- Fagre, A.C.; Cohen, L.E.; Eskew, E.A.; Farrell, M.; Glennon, E.; Joseph, M.B.; Frank, H.K.; Ryan, S.J.; Carlson, C.J.; Albery, G.F. Assessing the risk of human-to-wildlife pathogen transmission for conservation and public health. Ecol. Lett. 2022, 25, 1534–1549. [Google Scholar] [CrossRef]
- Sadeghian, M.M.; Vardanyan, Z. The Benefits of Urban Parks, a Review of Urban Research. Available online: www.jnasci.org (accessed on 10 January 2025).
- Auricchio, P. Primatas do Brasil; Terra Brasilis Editora: São Paulo, Brazil, 1995. [Google Scholar]
- Chiarello, A.G. Primates in Fragments—Ecology and Conservation. In Primates in Fragments; Laura, K.M., Ed.; Springer: Boston, MA, USA, 2003; Available online: https://link.springer.com/chapter/10.1007/978-1-4757-3770-7_8 (accessed on 21 March 2025).
- Silva, N.I.O.; Albery, G.F.; Arruda, M.S.; Oliveira, G.F.G.; Costa, T.A.; de Mello, É.M.; Moreira, G.D.; Reis, E.V.; da Silva, S.A.; Silva, M.C.; et al. Ecological drivers of sustained enzootic yellow fever virus transmission in Brazil, 2017–2021. PLoS Negl. Trop. Dis. 2023, 17, e0011407. [Google Scholar] [CrossRef]
- Mares-Guia, M.A.M.D.M.; Horta, M.A.; Romano, A.; Rodrigues, C.D.; Mendonça, M.C.; Dos Santos, C.C.; Torres, M.C.; Araujo, E.S.; Fabri, A.; de Souza, E.R.; et al. Yellow fever epizootics in non-human primates, Southeast and Northeast Brazil (2017 and 2018). Parasit Vectors 2020, 13, 90. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Secretaria de Vigilância Epidemiológica. Monitoramento do Período Sazonal da Febre Amarela—Informe 26. 2018. Available online: https://zenodo.org/records/15306394 (accessed on 29 April 2025).
- Ministério da Saúde. Emergência epidemiológica de febre amarela no Brasil, no período de dezembro de 2016 a julho de 2017. Bol. Epidemiológico 2017, 48, 1–22. [Google Scholar]
- Sacchetto, L.; Silva, N.I.O.; de Rezende, I.M.; Arruda, M.S.; Costa, T.A.; de Mello, E.M.; Oliveira, G.F.G.; Alves, P.A.; de Mendonca, V.E.; Stumpp, R.G.A.V.; et al. Neighbor danger: Yellow fever virus epizootics in urban and urban-rural transition areas of minas gerais state, during 2017–2018 yellow fever outbreaks in Brazil. PLoS Negl. Trop. Dis. 2020, 14, e0008658. [Google Scholar] [CrossRef]
- Rezende, I.M.; Sacchetto, L.; Munhoz de Mello, É.; Alves, P.A.; Iani, F.C.M.; Adelino, T.É.R.; Duarte, M.M.; Cury, A.L.F.; Bernardes, A.F.L.; Santos, T.A.; et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl. Trop. Dis. 2018, 12, e0006538. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Nota Técnica Conjunta Nº 27/2025-DEDT/DPNI/SVSA. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/notas-tecnicas/2025/nota-tecnica-conjunta-no-27-2025-dedt-dpni-svsa.pdf (accessed on 20 March 2025).
- Garcia-Oliveira, G.F.; Guimarães, A.C.D.S.; Moreira, G.D.; Costa, T.A.; Arruda, M.S.; de Mello, É.M.; Silva, M.C.; de Almeida, M.G.; Hanley, K.A.; Vasilakis, N.; et al. YELLOW ALERT: Persistent Yellow Fever Virus Circulation among Non-Human Primates in Urban Areas of Minas Gerais State, Brazil (2021–2023). Viruses 2024, 16, 1527. [Google Scholar] [CrossRef] [PubMed]
- Milan, E.; Moro, R.S. O conceito biogeográfico de ecótono. Terra Plur. 2016, 10, 75–88. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Gil, P.R.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited, 1st ed.; CEMEX: Mexico City, Mexico, 2004. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Fundação de Parques e Zoobotânica de belo Horizonte. Prefeitura de Belo Horizonte. 2023. Available online: https://prefeitura.pbh.gov.br/fundacao-de-parques-e-zoobotanica (accessed on 15 February 2025).
- De Miranda, G.H.B.; de Faria, D.S. Ecological aspects of black-pincelled marmoset (Callithrix penicillata) in the cerradão and dense cerrado of the Brazilian Central Plateau. Braz. J. Biol. 2001, 61, 397–404. [Google Scholar] [CrossRef]
- Vilela, S.L.; Faria, D.S.d. Seasonality of the activity pattern of Callithrix penicillata (Primates, Callitrichidae) in the cerrado (scrub savanna vegetation). Braz. J. Biol. 2004, 64, 363–370. [Google Scholar] [CrossRef]
- Leite, G.C.; Duarte, M.H.; Young, R.J. Human-marmoset interactions in a city park. Appl. Anim. Behav. Sci. 2011, 132, 187–192. [Google Scholar] [CrossRef]
- Bernardes Dias, A.C.; Hannibal, W.; Martinez, R.A.; Machado, P.R.R. Distribution, activity patterns and diet of Callithrix penicillata (Primates: Callithrichidae) in an urban area in central Brazil. Res. Soc. Dev. 2022, 11, e14811830717. [Google Scholar] [CrossRef]
- Fundação De Parques E Zoobotânica De Belo Horizonte. Prefeitura de Belo Horizonte. 2025. Available online: https://prefeitura.pbh.gov.br/fundacao-de-parques-e-zoobotanica/informacoes/parques/parque-primeiro-de-maio (accessed on 15 February 2025).
- De Rezende, I.M.; Alves, P.A.; Arruda, M.S.; Gonçalves, A.P.; Oliveira, G.F.G.; Pereira, L.S.; Dutra, M.R.T.; Campi-Azevedo, A.C.; Valim, V.; Tourinho, R.; et al. Yellow Fever Virus Genotyping Tool and Investigation of Suspected Adverse Events Following Yellow Fever Vaccination. Vaccines 2019, 7, 206. [Google Scholar] [CrossRef]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef]
- De Oliveira, D.B.; Candiani, T.M.; Franco-Luiz, A.P.M.; Almeida, G.M.; Abrahão, J.S.; Rios, M.; Coimbra, R.S.; Kroon, E.G. Etiological agents of viral meningitis in children from a dengue-endemic area, Southeast region of Brazil. J. Neurol. Sci. 2017, 375, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, M.; Meyer-König, U.; Hufert, F.T. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time PCR. J. Clin. Microbiol. 2003, 41, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Orle, K.A.; Gates, C.A.; Martin, D.H.; Body, B.A.; Weiss, J.B. Simultaneous PCR Detection of Haemophilus ducreyi, Treponema pallidum, and Herpes Simplex Virus Types 1 and 2 from Genital Ulcers. J. Clin. Microbiol. 1996, 34, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.S.; Campos, F.S.; de Oliveira, C.H.; Oliveira, R.S.; Campos, A.A.S.; de Almeida, M.A.B.; Fonseca, V.d.S.; Simonini-Teixeira, D.; Sevá, A.d.P.; Temponi, A.O.D.; et al. Fast Surveillance Response Reveals the Introduction of a New Yellow Fever Virus Sub-Lineage in 2021, in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2022, 117, e220127. [Google Scholar] [CrossRef]
- Secretaria de Estado de Saúde; Subsecretaria de Vigilância em Saúde; Superintendência de Vigilância Epidemiológica; Diretoria de Vigilância de Agravos Transmissíveis; Coordenação Estadual de Vigilância de Arboviroses. Nota Informativa No 2313—Boletim Epidemiológico Especial: Encerramento do Período de Monitoramento da Febre Amarela (Julho/2020 a Junho/2021) e Fortalecimento das Ações de Vigilância e Imunização Mediante a Confirmação de Primata Não Humano em Minas Gerais. Available online: https://zenodo.org/records/15306394 (accessed on 29 April 2025).
- Secretaria de Estado de Saúde; Subsecretaria de Vigilância em Saúde; Superintendência de Vigilância Epidemiológica; Diretoria de Vigilância de Agravos Transmissíveis; Coordenação Estadual de Vigilância de Arboviroses. Atualização Epidemiológica—Febre Amarela Minas Gerais—Setembro 2024. Belo Horizonte. Available online: https://zenodo.org/records/15306394 (accessed on 29 April 2025).
- Wachtman, L.; Mansfield, K. Viral Diseases of Nonhuman Primates. In Nonhuman Primates in Biomedical Research: Diseases, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–104. [Google Scholar]
- Eberle, R.; Jones-Engel, L. Understanding Primate Herpesviruses. J. Emerg. Dis. Virol. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Rao, K.B. Herpes Viruses-An Overview. IOSR J. Pharm. 2014, 4, 39–41. [Google Scholar] [CrossRef]
- De Paula, H.M.G.; Távora, R.S.; de Almeida, M.V.; Pelegrini, L.S.; da Silva, G.V.; Zaganini, R.L.; Lucindo, A. Estudos preliminares da presença de sagüis no município de Bauru, São Paulo, Brasil. Neotrop. Primates 2005, 13, 6–11. [Google Scholar] [CrossRef]
- Sabbatini, G.; Stammati, M.; Tavares, M.C.H.; Giuliani, M.V.; Visalberghi, E. Interactions between humans and capuchin monkeys (Cebus libidinosus) in the Parque Nacional de Brasília, Brazil. Appl. Anim. Behav. Sci. 2006, 97, 272–283. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, Z.; Li, S.; Yin, H. A Review on Zoonotic Pathogens Associated with Non-Human Primates: Understanding the Potential Threats to Humans. Microorganisms 2023, 11, 246. [Google Scholar] [CrossRef]
- Torres-Romero, E.J.; Nijman, V.; Fernández, D.; Eppley, T.M. Human-modified landscapes driving the global primate extinction crisis. Glob. Change Biol. 2023, 29, 5775–5787. [Google Scholar] [CrossRef]
- Ribeiro, C.V.; Do Vale, C.A.; Andriolo, A.; Prezoto, F. Caracterização das interações entre sagüis (Callithrix penicillata) e humanos. Neotrop. Primates 2018, 24, 17–21. [Google Scholar] [CrossRef]
- Sobroza, T.V.; Dunn, J.C.; Gordo, M.; Barnett, A.A. Do pied tamarins increase scent-marking in response to urban noise? Ethol. Ecol. Evol. 2024, 36, 136–149. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C. Urbanization (and Primate Conservation). In The International Encyclopedia of Primatology; Wiley: Hoboken, NJ, USA, 2017; pp. 1–5. [Google Scholar]
- Lima, I.A.; Bicca-Marques, J.C. Opportunistic meat-eating by urban folivorous-frugivorous monkeys. Primates 2023, 65, 25–32. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).