Investigation of HCPro-Mediated Ethylene Synthesis Pathway Through RNA-Seq Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. cDNA Library Construction and RNA Sequencing
2.3. Differential Gene Expression Analysis
2.4. Expression-Based Heatmaps and Principal Component Analysis (PCA)
2.5. GO Enrichment Analysis and KEGG Pathway Enrichment Analysis
2.6. External Application of Hormones
2.7. Staining with Nitro Blue Tetrazolium and 3,3′-Diaminobenzidine
2.8. Assays for Hydrogen Peroxide
2.9. Total RNA Extraction, Reverse-Transcription PCR, and Quantitative PCR Analysis
2.10. Statistical Analyses
3. Results
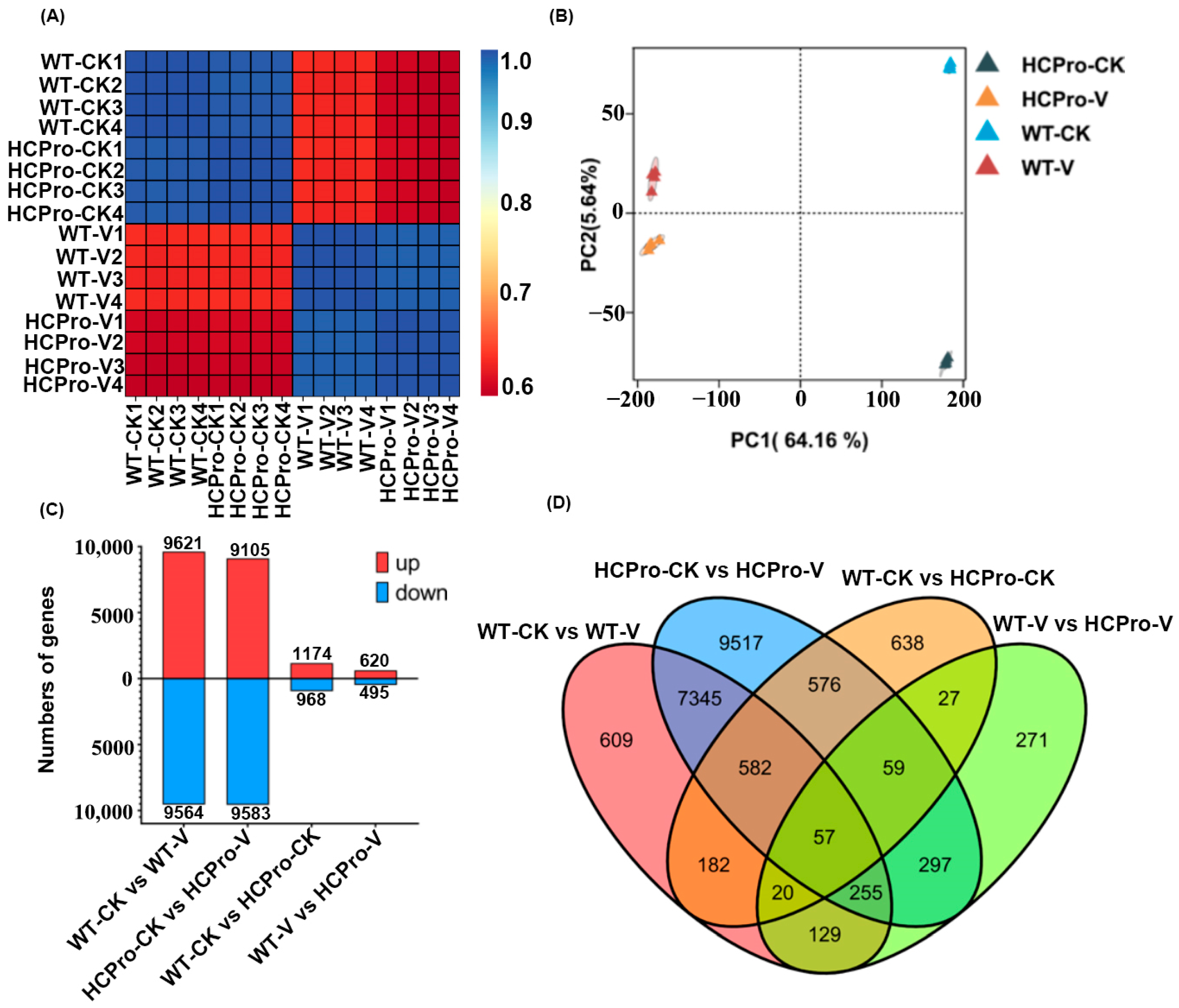
3.1. Transcriptome Sequencing of N. tabacum Leaves Infected with ChiVMV
3.2. The Expression of ChiVMV HCPro Promotes Virus Infection
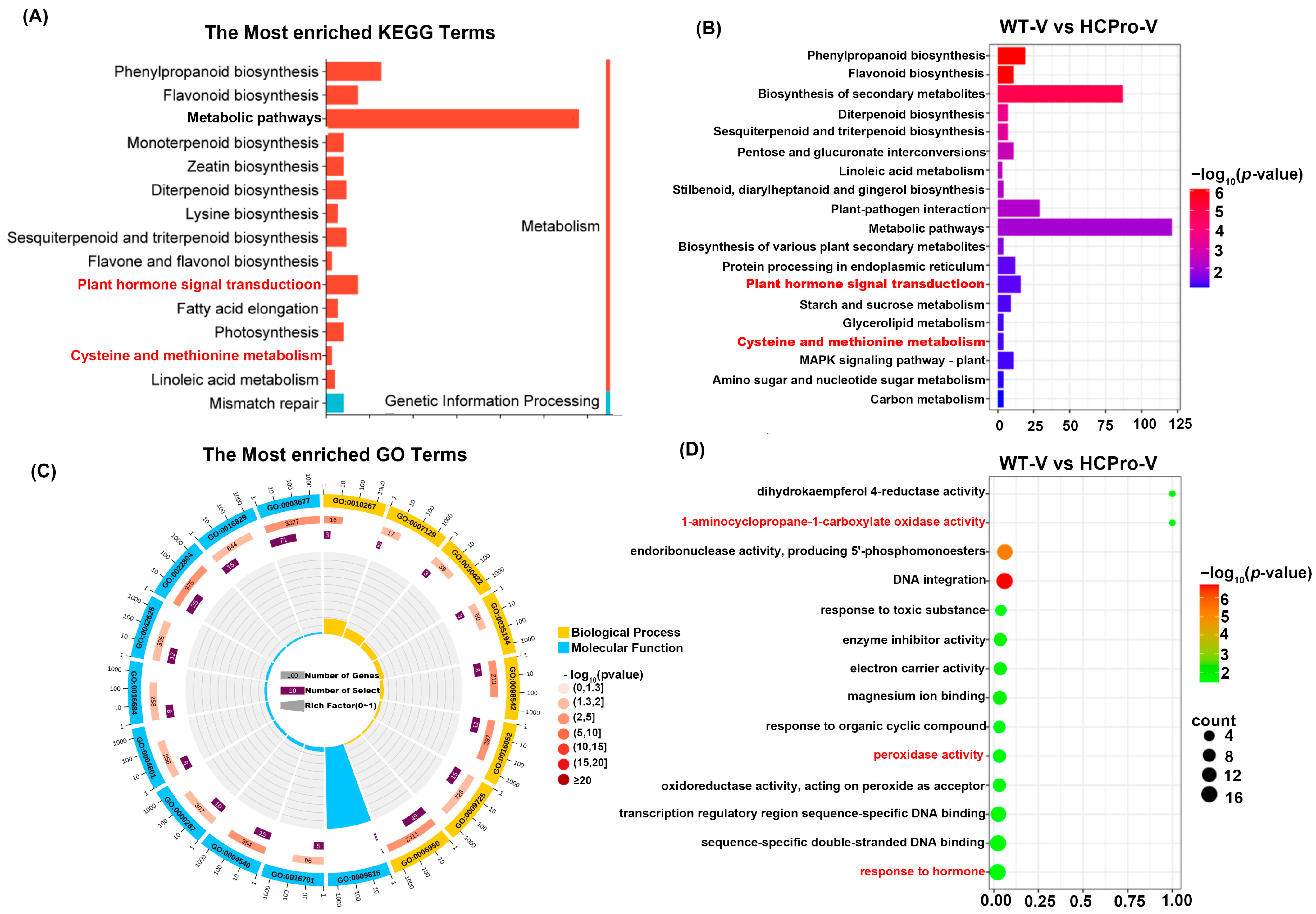
3.3. Functional Annotation of DEGs in N. tabacum Leaves Infected with ChiVMV
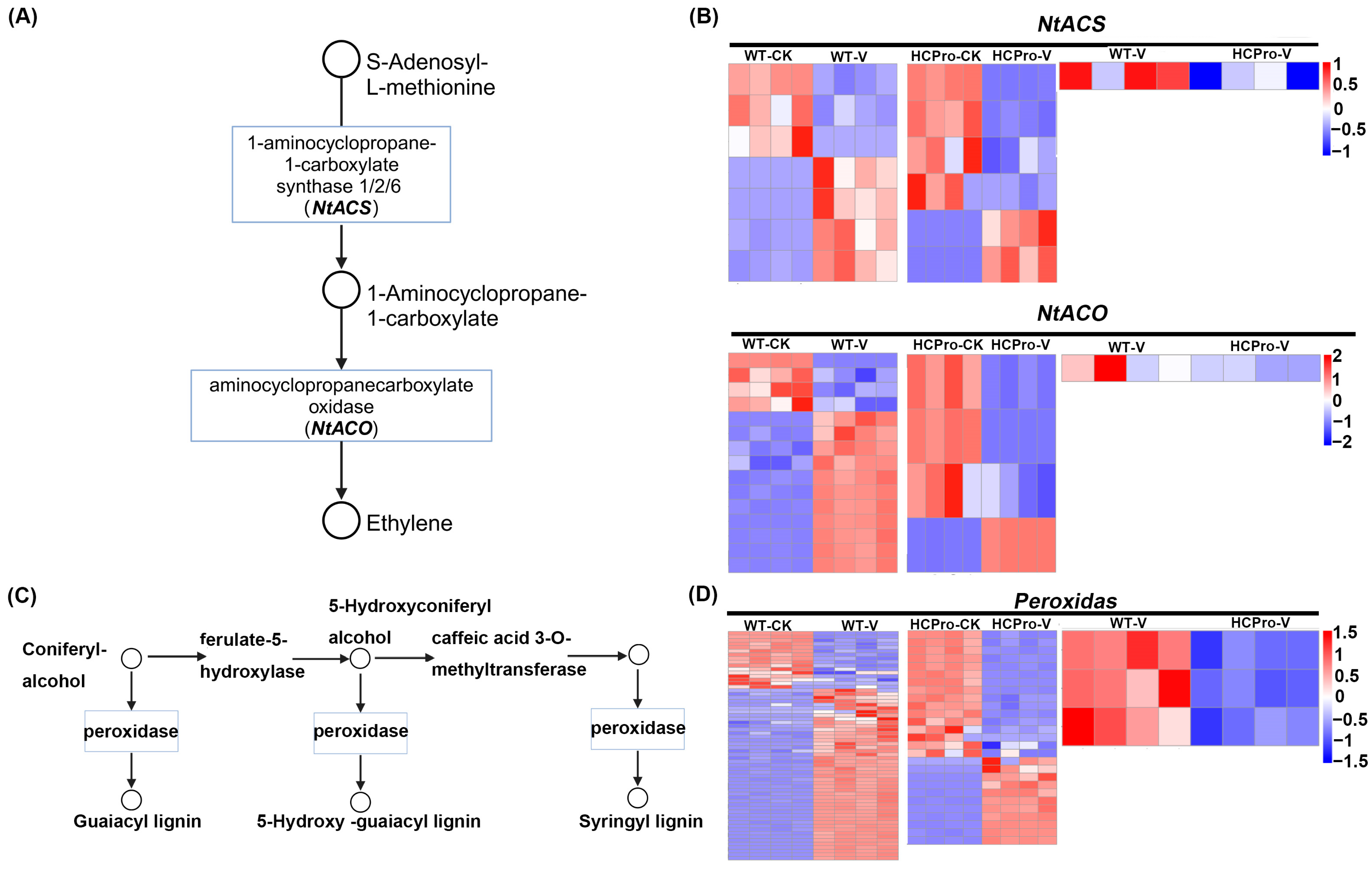
3.4. HCPro Modulates ET Biosynthesis in Plants During ChiVMV Infection
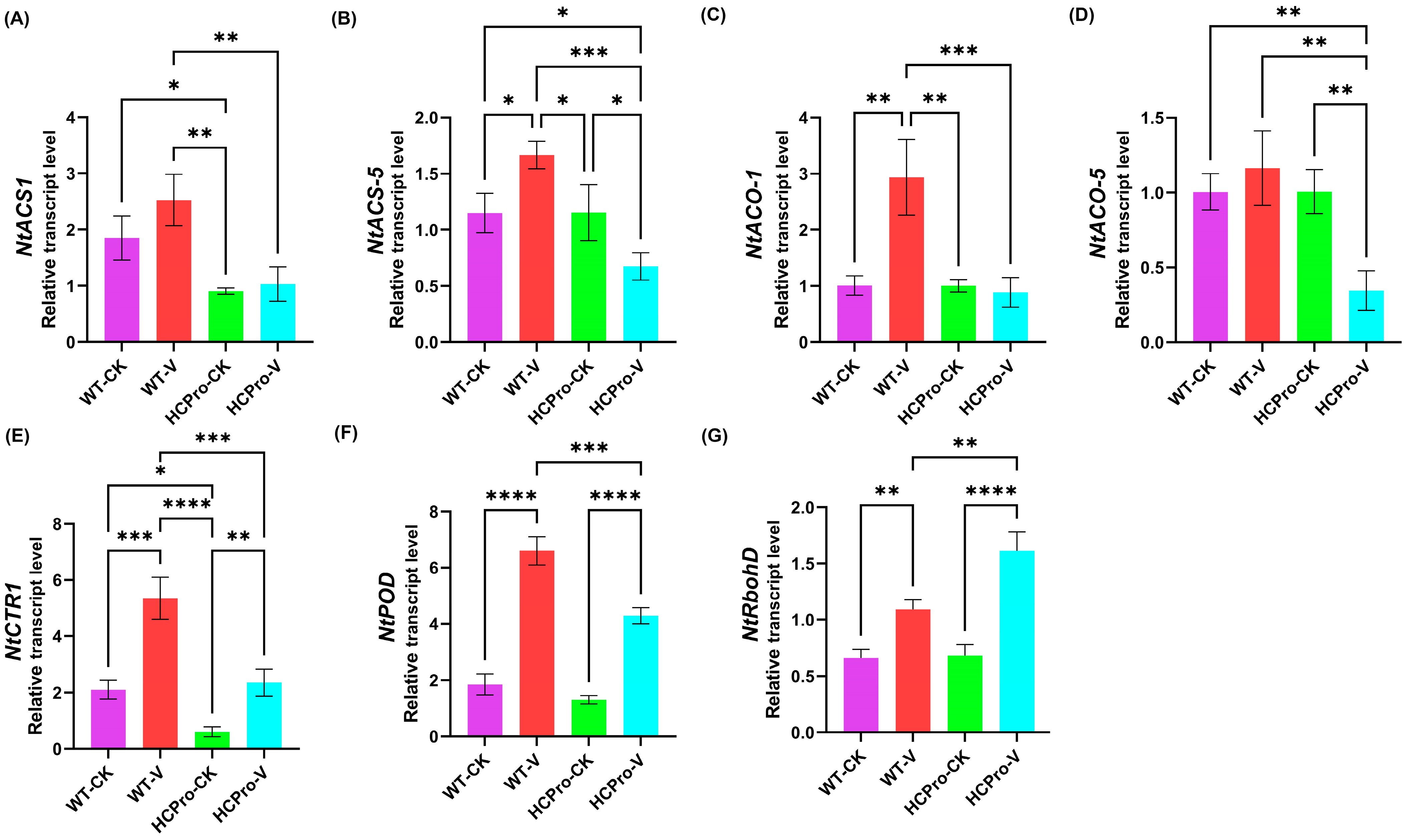
3.5. Validation of Differentially Expressed Genes in HCPro-Regulated Pathways
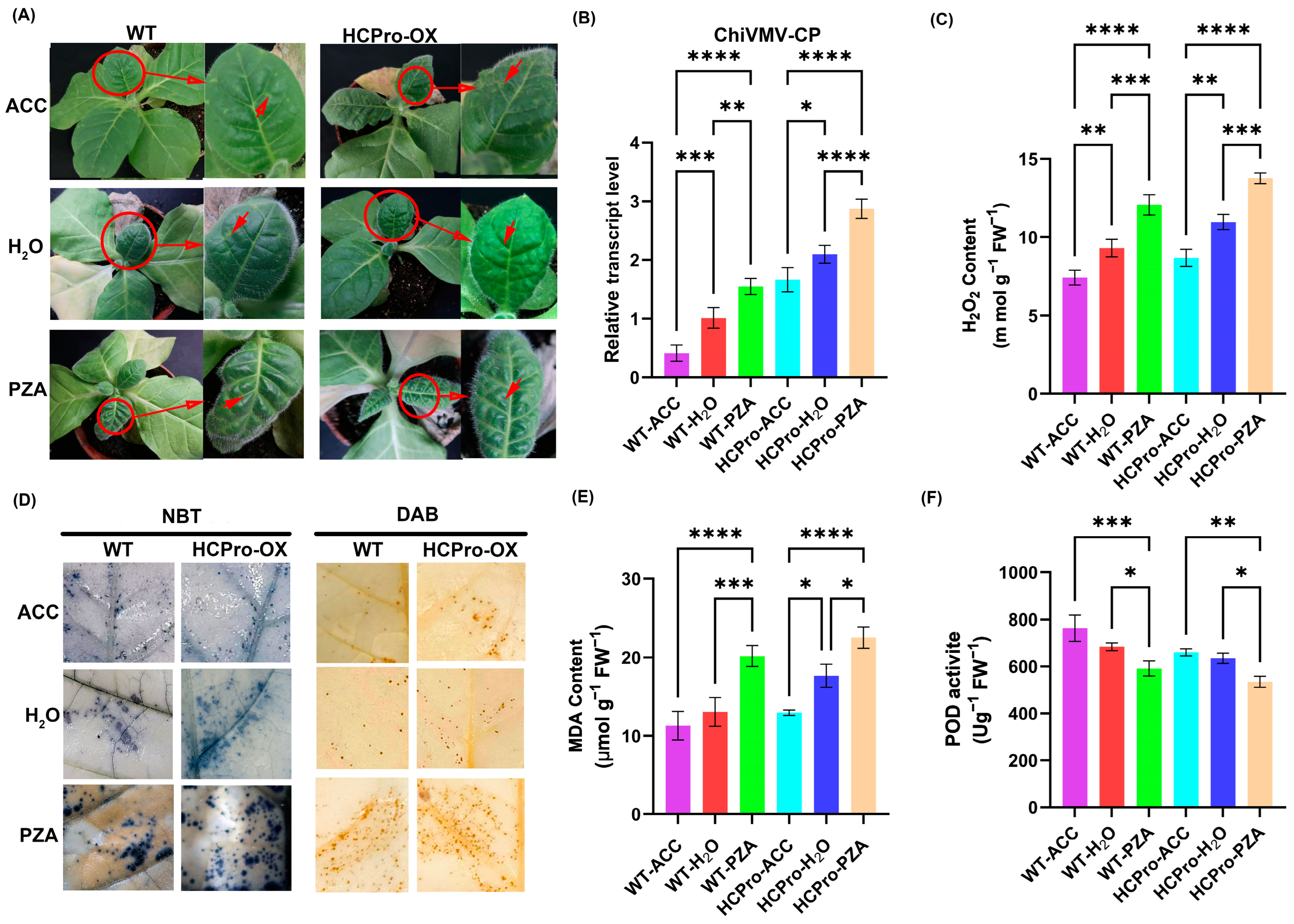
3.6. ET Plays a Positive Role in the Response of N. tabacum to ChiVMV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Jin, J.; Zou, W.; Liao, F.; Shen, J. Geographically Driven Adaptation of Chilli Veinal Mottle Virus Revealed by Genetic Diversity Analysis of the Coat Protein Gene. Arch. Virol. 2016, 161, 1329–1333. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Falk, B.W. Virus-Vector Interactions Mediating Nonpersistent and Semipersistent Transmission of Plant Viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Vijayapalani, P.; Maeshima, M.; Nagasaki-Takekuchi, N.; Miller, W.A. Interaction of the Trans-Frame Potyvirus Protein P3N-PIPO with Host Protein PCaP1 Facilitates Potyvirus Movement. PLoS Pathog. 2012, 8, e1002639. [Google Scholar] [CrossRef]
- Chung, B.Y.-W.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An Overlapping Essential Gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Z.; Lv, R.; Zhu, L.; Peng, Q.; Qiu, L.; Tian, Z.; Lin, H.; Xi, D. N Gene Enhances Resistance to Chilli Veinal Mottle Virus and Hypersensitivity to Salt Stress in Tobacco. J. Plant Physiol. 2018, 230, 92–100. [Google Scholar] [CrossRef]
- Zhao, F.-F.; Xi, D.-H.; Liu, J.; Deng, X.-G.; Lin, H.-H. First Report of Chilli Veinal Mottle Virus Infecting Tomato (Solanum lycopersicum) in China. Plant Dis. 2014, 98, 1589. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Deng, X.-G.; Peng, X.-J.; Zhu, T.; Xi, D.-H.; Lin, H.-H. Turnip Crinkle Virus with Nonviral Gene Cancels the Effect of Silencing Suppressors of P19 and 2b in Arabidopsis Thaliana. Physiol. Mol. Plant Pathol. 2014, 88, 94–100. [Google Scholar] [CrossRef]
- Valli, A.A.; Gallo, A.; Rodamilans, B.; López-Moya, J.J.; García, J.A. The HCPro from the Potyviridae Family: An Enviable Multitasking Helper Component That Every Virus Would like to Have. Mol. Plant Pathol. 2017, 19, 744–763. [Google Scholar] [CrossRef]
- Hu, S.-F.; Wei, W.-L.; Hong, S.-F.; Fang, R.-Y.; Wu, H.-Y.; Lin, P.-C.; Sanobar, N.; Wang, H.-P.; Sulistio, M.; Wu, C.-T.; et al. Investigation of the Effects of P1 on HC-pro-Mediated Gene Silencing Suppression through Genetics and Omics Approaches. Bot. Stud. 2020, 61, 22. [Google Scholar] [CrossRef]
- Poque, S.; Wu, H.-W.; Huang, C.-H.; Cheng, H.-W.; Hu, W.-C.; Yang, J.-Y.; Wang, D.; Yeh, S.-D. Potyviral Gene-Silencing Suppressor HCPro Interacts with Salicylic Acid (SA)-Binding Protein 3 to Weaken SA-Mediated Defense Responses. Mol. Plant-Microbe Interact. MPMI 2018, 31, 86–100. [Google Scholar] [CrossRef]
- Tian, M.; Sasvari, Z.; Gonzalez, P.A.; Friso, G.; Rowland, E.; Liu, X.-M.; van Wijk, K.J.; Nagy, P.D.; Klessig, D.F. Salicylic Acid Inhibits the Replication of Tomato Bushy Stunt Virus by Directly Targeting a Host Component in the Replication Complex. Mol. Plant-Microbe Interact. MPMI 2015, 28, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Rezzonico, E.; Flury, N.; Meins, F.; Beffa, R. Transcriptional Down-Regulation by Abscisic Acid of Pathogenesis-Related Beta-1,3-Glucanase Genes in Tobacco Cell Cultures. Plant Physiol. 1998, 117, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hong, W.; Wu, J.; Wang, Y.; Ji, S.; Zhu, S.; Wei, C.; Zhang, J.; Li, Y. A Viral Protein Promotes Host SAMS1 Activity and Ethylene Production for the Benefit of Virus Infection. eLife 2017, 6, e27529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xi, D.-H.; Deng, X.-G.; Peng, X.-J.; Tang, H.; Chen, Y.-J.; Jian, W.; Feng, H.; Lin, H.-H. The Chilli Veinal Mottle Virus Regulates Expression of the Tobacco Mosaic Virus Resistance Gene N and Jasmonic Acid/Ethylene Signaling Is Essential for Systemic Resistance Against Chilli Veinal Mottle Virus in Tobacco. Plant Mol. Biol. Rep. 2014, 32, 382–394. [Google Scholar] [CrossRef]
- Yang, T.; Qiu, L.; Huang, W.; Xu, Q.; Zou, J.; Peng, Q.; Lin, H.; Xi, D. Chilli Veinal Mottle Virus HCPro Interacts with Catalase to Facilitate Virus Infection in Nicotiana Tabacum. J. Exp. Bot. 2020, 71, 5656–5668. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Liu, M.; Ma, Z.; Zheng, T.; Sun, W.; Zhang, Y.; Jin, W.; Zhan, J.; Cai, Y.; Tang, Y.; Wu, Q.; et al. Insights into the Correlation between Physiological Changes in and Seed Development of Tartary Buckwheat (Fagopyrum Tataricum Gaertn.). BMC Genomics 2018, 19, 648. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New Perspectives on Genomes, Pathways, Diseases and Drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zou, J.; Zhou, J.; Zeng, M.; Zheng, D.; Yuan, X.; Xi, D. The Small GTPase NtRHO1 Negatively Regulates Tobacco Defense Response to Tobacco Mosaic Virus by Interacting with NtWRKY50. J. Exp. Bot. 2022, 73, 366–381. [Google Scholar] [CrossRef]
- McHugh, M.L. Multiple Comparison Analysis Testing in ANOVA. Biochem. Medica 2011, 21, 203–209. [Google Scholar] [CrossRef]
- Bronkhorst, A.W.; van Rij, R.P. The Long and Short of Antiviral Defense: Small RNA-Based Immunity in Insects. Curr. Opin. Virol. 2014, 7, 19–28. [Google Scholar] [CrossRef]
- Pumplin, N.; Voinnet, O. RNA Silencing Suppression by Plant Pathogens: Defence, Counter-Defence and Counter-Counter-Defence. Nat. Rev. Microbiol. 2013, 11, 745–760. [Google Scholar] [CrossRef]
- Kasschau, K.D.; Carrington, J.C. A Counterdefensive Strategy of Plant Viruses: Suppression of Posttranscriptional Gene Silencing. Cell 1998, 95, 461–470. [Google Scholar] [CrossRef]
- Anandalakshmi, R.; Pruss, G.J.; Ge, X.; Marathe, R.; Mallory, A.C.; Smith, T.H.; Vance, V.B. A Viral Suppressor of Gene Silencing in Plants. Proc. Natl. Acad. Sci. USA 1998, 95, 13079–13084. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Casteel, C.L. Effector-Mediated Plant–Virus–Vector Interactions. Plant Cell 2022, 34, 1514–1531. [Google Scholar] [CrossRef]
- Deng, Z.; Ma, L.; Zhang, P.; Zhu, H. Small RNAs Participate in Plant–Virus Interaction and Their Application in Plant Viral Defense. Int. J. Mol. Sci. 2022, 23, 696. [Google Scholar] [CrossRef]
- Atabekova, A.K.; Solovieva, A.D.; Chergintsev, D.A.; Solovyev, A.G.; Morozov, S.Y. Role of Plant Virus Movement Proteins in Suppression of Host RNAi Defense. Int. J. Mol. Sci. 2023, 24, 9049. [Google Scholar] [CrossRef] [PubMed]
- Soitamo, A.J.; Jada, B.; Lehto, K. HC-Pro Silencing Suppressor Significantly Alters the Gene Expression Profile in Tobacco Leaves and Flowers. BMC Plant Biol. 2011, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Jiao, B.; Liu, S.; Yang, Y.; Peng, Q.; Huang, W.; Xi, D. HCPro Affects Heterologous Virus Infection through Salicylic Acid and Auxin Pathways. Plant Pathol. 2023, 72, 1416–1427. [Google Scholar] [CrossRef]
- Endres, M.W.; Gregory, B.D.; Gao, Z.; Foreman, A.W.; Mlotshwa, S.; Ge, X.; Pruss, G.J.; Ecker, J.R.; Bowman, L.H.; Vance, V. Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing. PLoS Pathog. 2010, 6, e1000729. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Gutterson, N.; Reuber, T.L. Regulation of Disease Resistance Pathways by AP2/ERF Transcription Factors. Curr. Opin. Plant Biol. 2004, 7, 465–471. [Google Scholar] [CrossRef]
- Rehman, S.; Mahmood, T. Functional Role of DREB and ERF Transcription Factors: Regulating Stress-Responsive Network in Plants. Acta Physiol. Plant. 2015, 37, 178. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Li, D.; Wang, F.; Yu, D. WRKY8 Transcription Factor Functions in the TMV-Cg Defense Response by Mediating Both Abscisic Acid and Ethylene Signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, E1963-71. [Google Scholar] [CrossRef]
- Wang, F.; Cui, X.; Sun, Y.; Dong, C.-H. Ethylene Signaling and Regulation in Plant Growth and Stress Responses. Plant Cell Rep. 2013, 32, 1099–1109. [Google Scholar] [CrossRef]
- Frederickson Matika, D.E.; Loake, G.J. Redox Regulation in Plant Immune Function. Antioxid. Redox Signal. 2014, 21, 1373–1388. [Google Scholar] [CrossRef]
- Mehlhorn, H. Ethylene-Promoted Ascorbate Peroxidase Activity Protects Plants against Hydrogen Peroxide, Ozone and Paraquat. Plant Cell Environ. 1990, 13, 971–976. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Zhao, K.; Yao, W.; Li, R.; Zhou, B.; Jiang, T. Over-Expression of ERF38 Gene Enhances Salt and Osmotic Tolerance in Transgenic Poplar. Front. Plant Sci. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Argandoña, V.H.; Chaman, M.; Cardemil, L.; Muñoz, O.; Zúñiga, G.E.; Corcuera, L.J. Ethylene Production and Peroxidase Activity in Aphid-Infested Barley. J. Chem. Ecol. 2001, 27, 53–68. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Dong, L.; Wan, R.; Zeng, C.; Yang, T. Investigation of HCPro-Mediated Ethylene Synthesis Pathway Through RNA-Seq Approaches. Viruses 2025, 17, 602. https://doi.org/10.3390/v17050602
Jiang X, Dong L, Wan R, Zeng C, Yang T. Investigation of HCPro-Mediated Ethylene Synthesis Pathway Through RNA-Seq Approaches. Viruses. 2025; 17(5):602. https://doi.org/10.3390/v17050602
Chicago/Turabian StyleJiang, Xinpeng, Lan Dong, Renjing Wan, Changli Zeng, and Ting Yang. 2025. "Investigation of HCPro-Mediated Ethylene Synthesis Pathway Through RNA-Seq Approaches" Viruses 17, no. 5: 602. https://doi.org/10.3390/v17050602
APA StyleJiang, X., Dong, L., Wan, R., Zeng, C., & Yang, T. (2025). Investigation of HCPro-Mediated Ethylene Synthesis Pathway Through RNA-Seq Approaches. Viruses, 17(5), 602. https://doi.org/10.3390/v17050602




