Advances in the Drug Development and Quality Evaluation Principles of Oncolytic Herpes Simplex Virus
Abstract
1. Introduction
2. Viruses: From Toxicity to Oncolysis
3. Mechanism of Action
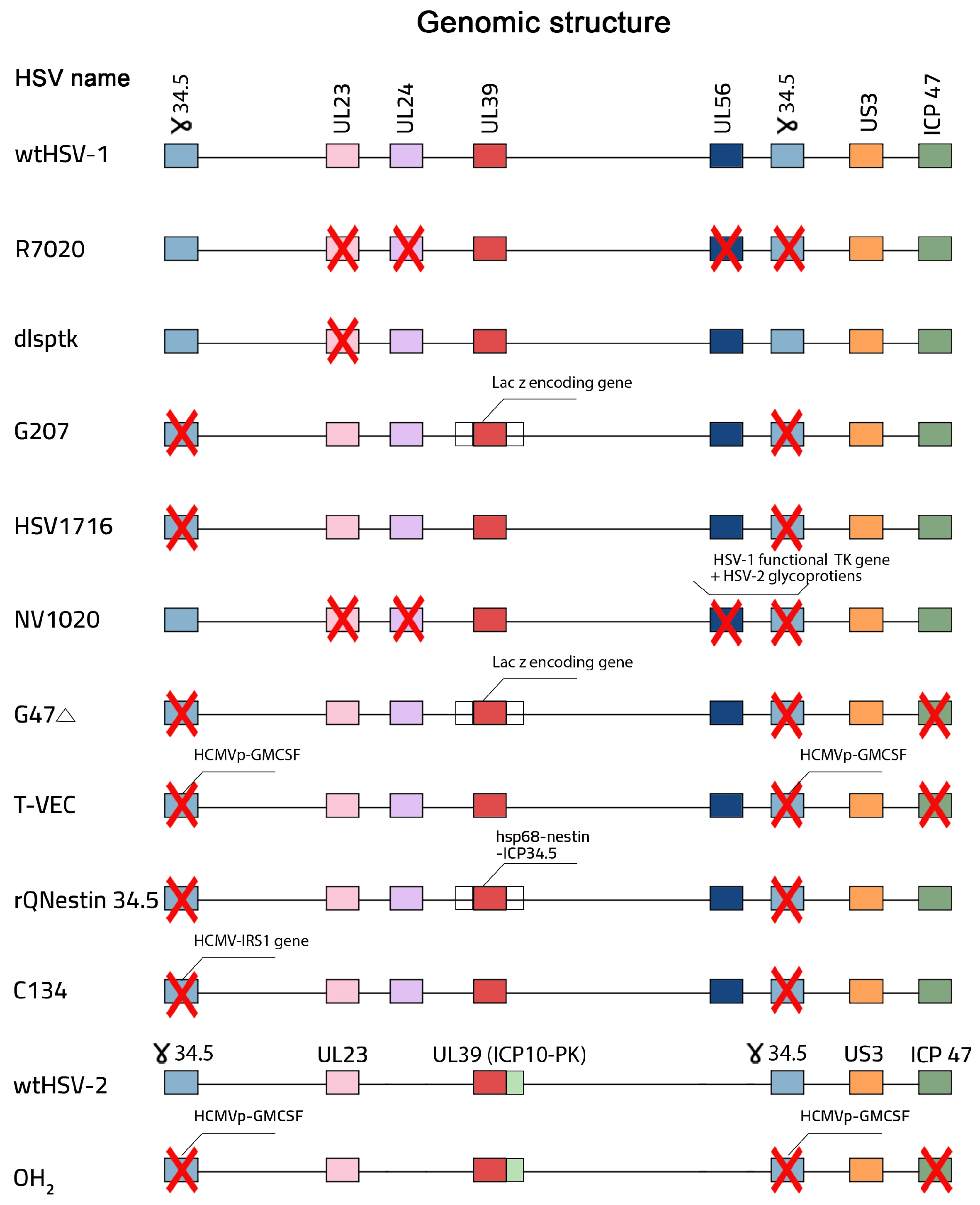
4. Design and Construction of oHSV
5. Preclinical and Clinical Trials of oHSVs
5.1. Talimogene Laherparevec (T-VEC, or Imlygic)
5.2. Delytact (Teserpaturev, or G47Δ)
5.3. HSV1716 (Seprehvir)
6. Combining oHSV with Other Therapies
7. Quality Control and Evaluation Principles of Oncolytic Drugs
7.1. OV Identification and Purification
7.2. OV Safety
7.3. OV Potency and Efficacy
7.4. OV Manufacturing Consistency
7.5. OV Regulatory Compliance
7.6. OV Storage and Stability
7.7. Preclinical and Clinical Studies
8. Limitations and Strategies to Overcome Them
9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Sinkovics, J.; Horvath, J. New developments in the virus therapy of cancer: A historical review. Intervirology 1993, 36, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 2023, 9, 122–139. [Google Scholar] [CrossRef]
- Jahan, N.; Ghouse, S.M.; Martuza, R.L.; Rabkin, S.D. In situ cancer vaccination and immunovirotherapy using oncolytic HSV. Viruses 2021, 13, 1740. [Google Scholar] [CrossRef]
- Donina, S.; Strele, I.; Proboka, G.; Auzinš, J.; Alberts, P.; Jonsson, B.; Venskus, D.; Muceniece, A. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 2015, 25, 421–426. [Google Scholar] [CrossRef]
- Alberts, P.; Tilgase, A.; Rasa, A.; Bandere, K.; Venskus, D. The advent of oncolytic virotherapy in oncology: The Rigvir® story. Eur. J. Pharmacol. 2018, 837, 117–126. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Oncolytic Viruses: Newest Frontier for Cancer Immunotherapy. Cancers 2021, 13, 5452. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, P.; Li, Z.; Xiao, S. Clinical Advances and Future Directions of Oncolytic Virotherapy for Head and Neck Cancer. Cancers 2023, 15, 5291. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Liu, Z.; Wang, J.; Shi, F.; Su, J.; Wang, T.; Wang, F. Efficacy and safety of recombinant human adenovirus type 5 (H101) in persistent, recurrent, or metastatic gynecologic malignancies: A retrospective study. Front. Oncol. 2022, 12, 877155. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Su, C.; Qin, L. Current landscape and perspective of oncolytic viruses and their combination therapies. Transl. Oncol. 2022, 25, 101530. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Shalhout, S.Z.; Iodice, G. Talimogene laherparepvec: Moving from first-in-class to best-in-class. Front. Mol. Biosci. 2022, 9, 834841. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Berkeley, R.; Barr, T.; Ilett, E.; Errington-Mais, F. Past, present and future of oncolytic reovirus. Cancers 2020, 12, 3219. [Google Scholar] [CrossRef]
- Uche, I.K.; Kousoulas, K.G.; Rider, P.J. The effect of herpes simplex virus-type-1 (HSV-1) oncolytic immunotherapy on the tumor microenvironment. Viruses 2021, 13, 1200. [Google Scholar] [CrossRef]
- Johnson, D.B.; Puzanov, I.; Kelley, M.C. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 2015, 7, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccines Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef]
- Greig, S.L. Talimogene laherparepvec: First global approval. Drugs 2016, 76, 147–154. [Google Scholar] [CrossRef]
- Rider, P.J.F.; Uche, I.K.; Sweeny, L.; Kousoulas, K.G. Anti-viral immunity in the tumor microenvironment: Implications for the rational design of herpes simplex virus type 1 oncolytic virotherapy. Curr. Clin. Microbiol. Rep. 2019, 6, 193–199. [Google Scholar] [CrossRef]
- Liu, X.Q.; Xin, H.Y.; Lyu, Y.N.; Ma, Z.W.; Peng, X.C.; Xiang, Y.; Wang, Y.Y.; Wu, Z.J.; Cheng, J.T.; Ji, J.F.; et al. Oncolytic herpes simplex virus tumor targeting and neutralization escape by engineering viral envelope glycoproteins. Drug Deliv. 2018, 25, 1950–1962. [Google Scholar] [CrossRef]
- Ghose, J.; Dona, A.; Murtadha, M.; Gunes, E.G.; Caserta, E.; Yoo, J.Y.; Russell, L.; Jaime-Ramirez, A.C.; Barwick, B.G.; Gupta, V.A.; et al. Oncolytic herpes simplex virus infects myeloma cells in vitro and in vivo. Mol. Ther. Oncolytics 2021, 20, 519–531. [Google Scholar] [CrossRef]
- Woo, Y.; Reid, V.; Kelly, K.J.; Carlson, D.; Yu, Z.; Fong, Y. Oncolytic Herpes Simplex Virus Prevents Premalignant Lesions from Progressing to Cancer. Mol. Ther. Oncolytics 2020, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Borlongan, M.; Kaufman, H.L.; Le, U.; Nauwynck, H.J.; Rabkin, S.D.; Saha, D. Cytokine-armed oncolytic herpes simplex viruses: A game-changer in cancer immunotherapy? J. Immunother. Cancer 2024, 12, e008025. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Teserpaturev/G47Δ: First approval. BioDrugs 2022, 36, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Rabkin, S.D. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002, 9, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma. Immunotherapy 2018, 10, 779–786. [Google Scholar] [CrossRef]
- Ottolino-Perry, K.; Diallo, J.S.; Lichty, B.D.; Bell, J.C.; McCart, J.A. Intelligent design: Combination therapy with oncolytic viruses. Mol. Ther. 2010, 18, 251–263. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Uchida, E. Regulatory aspects of oncolytic virus products. Curr. Cancer Drug Targets 2007, 7, 203–208. [Google Scholar] [CrossRef]
- Lauer, U.M.; Beil, J. Oncolytic viruses: Challenges and considerations in an evolving clinical landscape. Future Oncol. 2022, 18, 2713–2732. [Google Scholar] [CrossRef]
- Zhang, S.; Rabkin, S.D. The discovery and development of oncolytic viruses: Are they the future of cancer immunotherapy? Expert. Opin. Drug Discov. 2021, 16, 391–410. [Google Scholar] [CrossRef]
- Zhu, X.; Fan, C.; Xiong, Z.; Chen, M.; Li, Z.; Tao, T.; Liu, X. Development and application of oncolytic viruses as the nemesis of tumor cells. Front. Microbiol. 2023, 14, 1188526. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.K.; Peters, C.; Saha, D.; Rabkin, S.D.; Kaufman, H.L. Oncolytic herpes simplex viruses as a paradigm for the treatment of cancer. Annu. Rev. Cancer Biol. 2018, 2, 155–173. [Google Scholar] [CrossRef]
- Sokolowski, N.A.; Rizos, H.; Diefenbach, R.J. Oncolytic virotherapy using herpes simplex virus: How far have we come? Oncolytic Virother. 2015, 4, 207–219. [Google Scholar] [PubMed]
- Russell, L.; Peng, K.W. The emerging role of oncolytic virus therapy against cancer. Chin. Clin. Oncol. 2018, 7, 16. [Google Scholar] [CrossRef]
- Carson, J.; Haddad, D.; Bressman, M.; Fong, Y. Oncolytic Herpes Simplex Virus 1 (HSV-1) Vectors: Increasing Treatment Efficacy and Range Through Strategic Virus Design. Drugs Future 2010, 35, 183–195. [Google Scholar] [CrossRef]
- Menotti, L.; Avitabile, E. Herpes simplex virus oncolytic immunovirotherapy: The blossoming branch of multimodal therapy. Int. J. Mol. Sci. 2020, 21, 8310. [Google Scholar] [CrossRef]
- Aldrak, N.; Alsaab, S.; Algethami, A.; Bhere, D.; Wakimoto, H.; Shah, K.; Alomary, M.N.; Zaidan, N. Oncolytic herpes simplex virus-based therapies for cancer. Cells 2021, 10, 1541. [Google Scholar] [CrossRef]
- Dolan, A.; Jamieson, F.E.; Cunningham, C.; Barnett, B.C.; McGeoch, D.J. The genome sequence of herpes simplex virus type 2. J. Virol. 1998, 72, 2010–2021. [Google Scholar] [CrossRef]
- Peters, C.; Rabkin, S.D. Designing herpes viruses as oncolytics. Mol. Ther. Oncolytics 2015, 2, 15010. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Makvandi, M.; Abbasi, S.; Azadmanesh, K.; Teimoori, A. Developing oncolytic Herpes simplex virus type 1 through UL39 knockout by CRISPR-Cas9. Iran. J. Basic Med. Sci. 2020, 23, 937–944. [Google Scholar]
- Uchida, H.; Hamada, H.; Nakano, K.; Kwon, H.; Tahara, H.; Cohen, J.B.; Glorioso, J.C. Oncolytic herpes simplex virus vectors fully retargeted to tumor-associated antigens. Curr. Cancer Drug Targets 2018, 18, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Wakimoto, H.; Rabkin, S.D. Oncolytic herpes simplex virus interactions with the host immune system. Curr. Opin. Virol. 2016, 21, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shen, Z. Advanced progress in the genetic modification of the oncolytic HSV-1 virus. Front. Oncol. 2024, 14, 1525940. [Google Scholar] [CrossRef]
- Martuza, R.L.; Malick, A.; Markert, J.M.; Ruffner, K.L.; Coen, D.M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991, 252, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Coen, D.M.; Kosz-Vnenchak, M.; Jacobson, J.G.; Leib, D.A.; Bogard, C.L.; Schaffer, P.A.; Tyler, K.L.; Knipe, D.M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 1989, 86, 4736–4740. [Google Scholar] [CrossRef]
- Chou, J.; Kern, E.R.; Whitley, R.J.; Roizman, B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 1990, 250, 1262–1266. [Google Scholar] [CrossRef]
- He, B.; Gross, M.; Roizman, B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 843–848. [Google Scholar]
- Kanai, R.; Zaupa, C.; Sgubin, D.; Antoszczyk, S.J.; Martuza, R.L.; Wakimoto, H.; Rabkin, S.D. Effect of γ34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J. Virol. 2012, 86, 4420–4431. [Google Scholar] [CrossRef]
- Friedman, G.K.; Nan, L.; Haas, M.C.; Kelly, V.M.; Moore, B.P.; Langford, C.P.; Xu, H.; Han, X.; Beierle, E.A.; Markert, J.M.; et al. γ134.5-deleted HSV-1-expressing human cytomegalovirus IRS1 gene kills human glioblastoma cells as efficiently as wild-type HSV-1 in normoxia or hypoxia. Gene Ther. 2015, 22, 348–355. [Google Scholar] [CrossRef]
- Cassady, K.A.; Gross, M. The herpes simplex virus type 1 U(S)11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 2002, 76, 2029–2035. [Google Scholar] [CrossRef]
- Goldsmith, K.; Chen, W.; Johnson, D.C.; Hendricks, R.L. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J. Exp. Med. 1998, 187, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Cassady, K.A.; Gross, M.; Roizman, B. The herpes simplex virus US11 protein effectively compensates for the γ134. 5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J. Virol. 1998, 72, 8620–8626. [Google Scholar] [CrossRef]
- Aghi, M.; Visted, T.; Depinho, R.A.; Chiocca, E.A. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene 2008, 27, 4249–4254. [Google Scholar] [CrossRef]
- Totsch, S.K.; Ishizuka, A.S.; Kang, K.-D.; Gary, S.E.; Rocco, A.; Fan, A.E.; Zhou, L.; Valdes, P.A.; Lee, S.; Li, J.; et al. Combination Immunotherapy with Vaccine and Oncolytic HSV Virotherapy Is Time Dependent. Mol. Cancer Ther. 2024, 23, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Martuza, R.L.; Rabkin, S.D.; Johnson, P.A. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl. Acad. Sci. USA 2001, 98, 6396–6401. [Google Scholar] [CrossRef]
- Ayele, K.; Wakimoto, H.; Nauwynck, H.J.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Understanding the Interplay between oHSV and the Host Immune System: Implications for Therapeutic Oncolytic Virus Development. Mol. Ther. 2024, 33, 1327–1343. [Google Scholar] [CrossRef] [PubMed]
- Qi, X. Advances in antitumour therapy with oncolytic herpes simplex virus combinations. Discov. Oncol. 2024, 15, 302. [Google Scholar] [CrossRef]
- Glorioso, J.C.; Cohen, J.B.; Goins, W.F.; Hall, B.; Jackson, J.W.; Kohanbash, G.; Amankulor, N.; Kaur, B.; Caligiuri, M.A.; Chiocca, E.A. Oncolytic HSV vectors and anti-tumor immunity. Curr. Issues Mol. Biol. 2021, 41, 381–468. [Google Scholar] [CrossRef]
- Calvillo-Rodríguez, K.M.; Lorenzo-Anota, H.Y.; Rodríguez-Padilla, C.; Martínez-Torres, A.C.; Scott-Algara, D. Immunotherapies inducing immunogenic cell death in cancer: Insight of the innate immune system. Front. Immunol. 2023, 14, 1294434. [Google Scholar] [CrossRef]
- Guo, Z.S.; Liu, Z.; Kowalsky, S.; Feist, M.; Kalinski, P.; Lu, B.; Storkus, W.J.; Bartlett, D.L. Oncolytic immunotherapy: Conceptual evolution, current strategies, and future perspectives. Front. Immunol. 2017, 8, 555. [Google Scholar] [CrossRef]
- Santos Apolonio, J.; Lima de Souza Gonçalves, V.; Cordeiro Santos, M.L.; Silva Luz, M.; Silva Souza, J.V.; Rocha Pinheiro, S.L.; de Souza, W.R.; Sande Loureiro, M.; de Melo, F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Xu, Z.; Li, F.; Zhao, J.; Jian, Z.; Deng, H.; Lai, S.; Sun, X.; Geng, Y.; Zhu, L. Insights on the cGAS-STING signaling pathway during herpesvirus infections. Front. Immunol. 2022, 13, 931885. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, S.K.; Geller, D.A.; de Haan, H.A.; Hörer, M.; Knoll, A.E.; Mescheder, A.; Nemunaitis, J.; Reid, T.R.; Sze, D.Y.; Tanabe, K.K. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum. Gene Ther. 2010, 21, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Advani, S.J.; Bradley, J.D.; Kataoka, Y.; Vashistha, K.; Yan, S.Y.; Markert, J.M.; Gillespie, G.Y.; Whitley, R.J.; Roizman, B.; et al. The use of a genetically engineered herpes simplex virus (R7020) with ionizing radiation for experimental hepatoma. Gene Ther. 2002, 9, 75–80. [Google Scholar] [CrossRef]
- Meignier, B.; Longnecker, R.; Roizman, B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: Construction and evaluation in rodents. J. Infect. Dis. 1988, 158, 602–614. [Google Scholar] [CrossRef]
- Kelly, K.J.; Wong, J.; Fong, Y. Herpes simplex virus NV1020 as a novel and promising therapy for hepatic malignancy. Expert Opin. Investig. Drugs 2008, 17, 1105–1113. [Google Scholar] [CrossRef]
- Hu, M.; Liao, X.; Tao, Y.; Chen, Y. Advances in oncolytic herpes simplex virus and adenovirus therapy for recurrent glioma. Front. Immunol. 2023, 14, 1285113. [Google Scholar]
- Foreman, P.M.; Friedman, G.K.; Cassady, K.A.; Markert, J.M. Oncolytic Virotherapy for the Treatment of Malignant Glioma. Neurotherapeutics 2017, 14, 333–344. [Google Scholar] [CrossRef]
- Cinatl Jr, J.; Cinatl, J.; Michaelis, M.; Kabickova, H.; Kotchetkov, R.; Vogel, J.-U.; Doerr, H.W.; Klingebiel, T.; Driever, P.H. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res. 2003, 63, 1508–1514. [Google Scholar]
- Aghi, M.K.; Chiocca, E.A. Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol. Ther. 2009, 17, 8–9. [Google Scholar] [CrossRef]
- Zhang, S.X. Turning killer into cure—The story of oncolytic herpes simplex viruses. Discov. Med. 2015, 20, 303–309. [Google Scholar] [PubMed]
- Peters, C.; Paget, M.; Tshilenge, K.-T.; Saha, D.; Antoszczyk, S.; Baars, A.; Frost, T.; Martuza Robert, L.; Wakimoto, H.; Rabkin Samuel, D. Restriction of Replication of Oncolytic Herpes Simplex Virus with a Deletion of γ34.5 in Glioblastoma Stem-Like Cells. J. Virol. 2018, 92, 10.1128–jvi.00246. [Google Scholar] [CrossRef] [PubMed]
- Mineta, T.; Rabkin, S.D.; Yazaki, T.; Hunter, W.D.; Martuza, R.L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995, 1, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat. Commun. 2022, 13, 4119. [Google Scholar] [CrossRef]
- Sugawara, K.; Iwai, M.; Ito, H.; Tanaka, M.; Seto, Y.; Todo, T. Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition via dynamic intratumoral immune modulation. Mol. Ther. Oncolytics 2021, 22, 129–142. [Google Scholar] [CrossRef]
- Amgen buys oncolytic virus company. Nat. Rev. Drug Discov. 2011, 10, 166. [CrossRef]
- Liu, B.L.; Robinson, M.; Han, Z.Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Kambara, H.; Okano, H.; Chiocca, E.A.; Saeki, Y. An oncolytic HSV-1 mutant expressing ICP34. 5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005, 65, 2832–2839. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Nakashima, H.; Kasai, K.; Fernandez, S.A.; Oglesbee, M. Preclinical Toxicology of rQNestin34.5v.2: An Oncolytic Herpes Virus with Transcriptional Regulation of the ICP34.5 Neurovirulence Gene. Mol. Ther. Methods Clin. Dev. 2020, 17, 871–893. [Google Scholar] [CrossRef]
- Cassady, K.A.; Bauer, D.F.; Roth, J.; Chambers, M.R.; Shoeb, T.; Coleman, J.; Prichard, M.; Gillespie, G.Y.; Markert, J.M. Pre-clinical assessment of C134, a chimeric oncolytic herpes simplex virus, in mice and non-human primates. Mol. Ther. Oncolytics 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Shah, A.C.; Parker, J.N.; Gillespie, G.Y.; Lakeman, F.D.; Meleth, S.; Markert, J.M.; Cassady, K.A. Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses. Gene Ther. 2007, 14, 1045–1054. [Google Scholar] [CrossRef]
- Patel, D.M.; Foreman, P.M.; Nabors, L.B.; Riley, K.O.; Gillespie, G.Y.; Markert, J.M. Design of a phase I clinical trial to evaluate M032, a genetically engineered HSV-1 expressing IL-12, in patients with recurrent/progressive glioblastoma multiforme, anaplastic astrocytoma, or gliosarcoma. Hum. Gene Ther. Clin. Dev. 2016, 27, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Ji, Q.; Fang, A.; Song, L.; Xu, X.; Lin, Y.; Peng, Y.; Yu, J.; Xie, L.; et al. OH2 oncolytic virus: A novel approach to glioblastoma intervention through direct targeting of tumor cells and augmentation of anti-tumor immune responses. Cancer Lett. 2024, 589, 216834. [Google Scholar] [CrossRef] [PubMed]
- Nasar, R.T.; Uche, I.K.; Kousoulas, K.G. Targeting Cancers with oHSV-Based Oncolytic Viral Immunotherapy. Curr. Issues Mol. Biol. 2024, 46, 5582–5594. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, J.; Tiep, L.; Zhou, J.; Beers, C. Talilmogene Iaherparepvec generates systemic T-cell-mediated anti-tumor immunity. J. Immunother. Cancer 2013, 1, P198. [Google Scholar] [CrossRef]
- Cooke, K.; Estrada, J.; Zhan, J.; Mitchell, P.; Bulliard, Y.; Beltran, P.J. Development of a B16F10 cell line expressing mNectin1 to study the activity of OncoVEXmGM-CSF in murine syngeneic melanoma models. Cancer Res. 2016, 76 (Suppl. S14), 2351. [Google Scholar] [CrossRef]
- Hu, J.C.; Coffin, R.S.; Davis, C.J.; Graham, N.J.; Groves, N.; Guest, P.J.; Harrington, K.J.; James, N.D.; Love, C.A.; McNeish, I. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006, 12, 6737–6747. [Google Scholar] [CrossRef]
- Senzer, N.N.; Kaufman, H.L.; Amatruda, T.; Nemunaitis, M.; Reid, T.; Daniels, G.; Gonzalez, R.; Glaspy, J.; Whitman, E.; Harrington, K. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor–encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009, 27, 5763–5771. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Streby, K.A.; Geller, J.I.; Currier, M.A.; Warren, P.S.; Racadio, J.M.; Towbin, A.J.; Vaughan, M.R.; Triplet, M.; Ott-Napier, K.; Dishman, D.J. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.L.; Fraser, N.W. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol. Ther. 2003, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wang, P.-Y.; Hutzen, B.; Sprague, L.; Swain, H.M.; Love, J.K.; Stanek, J.R.; Boon, L.; Conner, J.; Cripe, T.P. Cooperation of oncolytic herpes virotherapy and PD-1 blockade in murine rhabdomyosarcoma models. Sci. Rep. 2017, 7, 2396. [Google Scholar] [CrossRef]
- Tazzyman, S.; Stewart, G.R.; Yeomans, J.; Linford, A.; Lath, D.; Conner, J.; Muthana, M.; Chantry, A.D.; Lawson, M.A. HSV1716 Prevents Myeloma Cell Regrowth When Combined with Bortezomib In Vitro and Significantly Reduces Systemic Tumor Growth in Mouse Models. Viruses 2023, 15, 603. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef]
- Cody, J.J.; Markert, J.M.; Hurst, D.R. Histone deacetylase inhibitors improve the replication of oncolytic herpes simplex virus in breast cancer cells. PLoS ONE 2014, 9, e92919. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, M.; Yang, C.; Wei, W.; He, X.; Cheng, G.; Wang, S. Combination therapy with oncolytic viruses and immune checkpoint inhibitors in head and neck squamous cell carcinomas: An approach of complementary advantages. Cancer Cell Int. 2023, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Groeneveldt, C.; van Hall, T.; van der Burg, S.H.; Ten Dijke, P.; van Montfoort, N. Immunotherapeutic potential of TGF-β inhibition and oncolytic viruses. Trends Immunol. 2020, 41, 406–420. [Google Scholar] [CrossRef]
- Puzanov, I.; Milhem, M.M.; Minor, D.; Hamid, O.; Li, A.; Chen, L.; Chastain, M.; Gorski, K.S.; Anderson, A.; Chou, J.; et al. Talimogene Laherparepvec in Combination with Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J. Clin. Oncol. 2016, 34, 2619–2626. [Google Scholar] [CrossRef]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients with Advanced, Unresectable Melanoma. J. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef]
- Moesta, A.K.; Cooke, K.; Piasecki, J.; Mitchell, P.; Rottman, J.B.; Fitzgerald, K.; Zhan, J.; Yang, B.; Le, T.; Belmontes, B. Local delivery of OncoVEXmGM-CSF generates systemic antitumor immune responses enhanced by cytotoxic T-lymphocyte–associated protein blockade. Clin. Cancer Res. 2017, 23, 6190–6202. [Google Scholar] [CrossRef]
- Eissa, I.R.; Naoe, Y.; Bustos-Villalobos, I.; Ichinose, T.; Tanaka, M.; Zhiwen, W.; Mukoyama, N.; Morimoto, T.; Miyajima, N.; Hitoki, H. Genomic signature of the natural oncolytic herpes simplex virus HF10 and its therapeutic role in preclinical and clinical trials. Front. Oncol. 2017, 7, 149. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Tang, J.; Hu, S.; Luo, S.; Luo, Z.; Zhou, F.; Tan, S.; Ying, J.; Chang, Q. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: A multicenter, phase I/II clinical trial. J. Immunother. Cancer 2021, 9, e002224. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e5. [Google Scholar] [CrossRef]
- Jennings, V.A.; Scott, G.B.; Rose, A.M.S.; Scott, K.J.; Migneco, G.; Keller, B.; Reilly, K.; Donnelly, O.; Peach, H.; Dewar, D.; et al. Potentiating Oncolytic Virus-Induced Immune-Mediated Tumor Cell Killing Using Histone Deacetylase Inhibition. Mol. Ther. 2019, 27, 1139–1152. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Aspromonte, S.; Zloza, A.; Rabkin, S.D.; Kaufman, H.L. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci. Transl. Med. 2018, 10, eaau0417. [Google Scholar] [CrossRef]
- Crespo-Rodriguez, E.; Bergerhoff, K.; Bozhanova, G.; Foo, S.; Patin, E.C.; Whittock, H.; Buus, R.; Haider, S.; Muirhead, G.; Thway, K. Combining BRAF inhibition with oncolytic herpes simplex virus enhances the immune-mediated antitumor therapy of BRAF-mutant thyroid cancer. J. Immunother. Cancer 2020, 8, e000698. [Google Scholar] [CrossRef]
- Hutzen, B.; Chen, C.Y.; Wang, P.Y.; Sprague, L.; Swain, H.M.; Love, J.; Conner, J.; Boon, L.; Cripe, T.P. TGF-β Inhibition Improves Oncolytic Herpes Viroimmunotherapy in Murine Models of Rhabdomyosarcoma. Mol. Ther. Oncolytics 2017, 7, 17–26. [Google Scholar] [CrossRef]
- Han, J.; Chen, X.; Chu, J.; Xu, B.; Meisen, W.H.; Chen, L.; Zhang, L.; Zhang, J.; He, X.; Wang, Q.-E. TGFβ treatment enhances glioblastoma virotherapy by inhibiting the innate immune response. Cancer Res. 2015, 75, 5273–5282. [Google Scholar] [CrossRef]
- Esaki, S.; Nigim, F.; Moon, E.; Luk, S.; Kiyokawa, J.; Curry Jr, W.; Cahill, D.P.; Chi, A.S.; Iafrate, A.J.; Martuza, R.L. Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int. J. Cancer 2017, 141, 2348–2358. [Google Scholar] [CrossRef]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-and-analysis-shedding-studies-virus-or-bacteria-based-gene-therapy-and-oncolytic-products (accessed on 1 April 2025).
- Yamaguchi, T.; Uchida, E. Oncolytic virus: Regulatory aspects from quality control to clinical studies. Curr. Cancer Drug Targets 2018, 18, 202–208. [Google Scholar] [CrossRef]
- Rasa, A.; Alberts, P. Oncolytic virus preclinical toxicology studies. J. Appl. Toxicol. 2023, 43, 620–648. [Google Scholar] [CrossRef]
- Frequently Asked Questions—Developing Potential Cellular and Gene Therapy Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/frequently-asked-questions-developing-potential-cellular-and-gene-therapy-products (accessed on 1 April 2025).
- Onnockx, S.; Baldo, A.; Pauwels, K. Oncolytic Viruses: An Inventory of Shedding Data from Clinical Trials and Elements for the Environmental Risk Assessment. Vaccines 2023, 11, 1448. [Google Scholar] [CrossRef]
- Dambra, R.; Matter, A.; Graca, K.; Akhand, S.S.; Mehta, S.; Bell-Cohn, A.; Swenson, J.M.; Abid, S.; Xin, D.; Lewis, C.; et al. Nonclinical pharmacokinetics and biodistribution of VSV-GP using methods to decouple input drug disposition and viral replication. Mol. Ther. Methods Clin. Dev. 2023, 28, 190–207. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Qu, Z.; Tian, C.; Wang, T.; Miao, Y.; Jiang, H.; Li, L.; Liu, J.; Zhao, R.; et al. Preclinical safety assessment of toxicity and biodistribution of oncolytic virus HSV-1 expressing human PD-1 antibody in mice. Regul. Toxicol. Pharmacol. 2022, 132, 105166. [Google Scholar] [CrossRef]
- Cyrelle Ornella, M.S.; Kim, J.-J.; Cho, E.; Cho, M.; Hwang, T.-H. Dose Considerations for Vaccinia Oncolytic Virus Based on Retrospective Reanalysis of Early and Late Clinical Trials. Vaccines 2024, 12, 1010. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Y.; Hu, X.; Jiao, J.; Wang, W.; Yao, H. Advances in the clinical development of oncolytic viruses. Am. J. Transl. Res. 2022, 14, 4192–4206. [Google Scholar]
- Harrington, K.J.; Michielin, O.; Malvehy, J.; Pezzani Grüter, I.; Grove, L.; Frauchiger, A.L.; Dummer, R. A practical guide to the handling and administration of talimogene laherparepvec in Europe. Onco Targets Ther. 2017, 10, 3867–3880. [Google Scholar] [CrossRef]
- Baldo, A.; Galanis, E.; Tangy, F.; Herman, P. Biosafety considerations for attenuated measles virus vectors used in virotherapy and vaccination. Hum. Vaccin. Immunother. 2016, 12, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Potency Tests for Cellular and Gene Therapy Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/potency-tests-cellular-and-gene-therapy-products (accessed on 29 March 2025).
- Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). Guidance for Industry. Available online: https://www.fda.gov/media/113760/download (accessed on 1 April 2025).
- Corrigan, P.A.; Beaulieu, C.; Patel, R.B.; Lowe, D.K. Talimogene Laherparepvec: An Oncolytic Virus Therapy for Melanoma. Ann. Pharmacother. 2017, 51, 675–681. [Google Scholar] [CrossRef]
- Todo, T.; Feigenbaum, F.; Rabkin, S.D.; Lakeman, F.; Newsome, J.T.; Johnson, P.A.; Mitchell, E.; Belliveau, D.; Ostrove, J.M.; Martuza, R.L. Viral shedding and biodistribution of G207, a multimutated, conditionally replicating herpes simplex virus type 1, after intracerebral inoculation in aotus. Mol. Ther. 2000, 2, 588–595. [Google Scholar] [CrossRef]
- Harrow, S.; Papanastassiou, V.; Harland, J.; Mabbs, R.; Petty, R.; Fraser, M.; Hadley, D.; Patterson, J.; Brown, S.M.; Rampling, R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: Safety data and long-term survival. Gene Ther. 2004, 11, 1648–1658. [Google Scholar] [CrossRef]
- Cripe, T.P.; Ngo, M.C.; Geller, J.I.; Louis, C.U.; Currier, M.A.; Racadio, J.M.; Towbin, A.J.; Rooney, C.M.; Pelusio, A.; Moon, A.; et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015, 23, 602–608. [Google Scholar] [CrossRef]
- Park, S.H.; Breitbach, C.J.; Lee, J.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Moon, A.; Mun, J.H.; Sommermann, E.M.; Maruri Avidal, L.; et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol. Ther. 2015, 23, 1532–1540. [Google Scholar] [CrossRef]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef]
- Xia, Z.J.; Chang, J.H.; Zhang, L.; Jiang, W.Q.; Guan, Z.Z.; Liu, J.W.; Zhang, Y.; Hu, X.H.; Wu, G.H.; Wang, H.Q.; et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng 2004, 23, 1666–1670. [Google Scholar]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- McKee, T.D.; Grandi, P.; Mok, W.; Alexandrakis, G.; Insin, N.; Zimmer, J.P.; Bawendi, M.G.; Boucher, Y.; Breakefield, X.O.; Jain, R.K. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006, 66, 2509–2513. [Google Scholar] [CrossRef]
- Cassady, K.A. Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection. J. Virol. 2005, 79, 8707–8715. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Goshima, F. Oncolytic Virotherapy by HSV. Adv. Exp. Med. Biol. 2018, 1045, 63–84. [Google Scholar] [PubMed]
- Chen, Q.; Han, B.; Meng, X.; Duan, C.; Yang, C.; Wu, Z.; Magafurov, D.; Zhao, S.; Safin, S.; Jiang, C.; et al. Immunogenomic analysis reveals LGALS1 contributes to the immune heterogeneity and immunosuppression in glioma. Int. J. Cancer 2019, 145, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, F. Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas. Cell Death Dis. 2020, 11, 485. [Google Scholar] [CrossRef]

| oHSV | Combination Therapy | Type of Cancer | Results |
|---|---|---|---|
| Immune checkpoint inhibitors | |||
| T-VEC | Ipilimumab | Advanced melanoma | T-VEC plus ipilimumab vs. ipilimumab alone: ORR (39% vs. 18%); 52% of the cases exhibited responses in non-injected visceral lesions [99,100]. |
| T-VEC | Pembrolizumab | Advanced melanoma | CRR: 33% with an increase in CD8+ TILs and PD-L1/IFNγ after receiving T-VEC [101]. |
| OncoVEXmGM-CSF (mT-VEC) | Anti-CTLA-4 | In vivo mouse tumor models | Combination therapy increased the survival period vs. that of single treatment. Improved antitumor immunity (tumor-specific splenocytes) [102]. |
| HF10 | Ipilimumab | Advanced melanoma | This combination achieved a BOR of 41% in a phase II clinical trial, which means nearly half of the patients responded [103]. |
| OH2 (T-VEC-like oHSV-2) | Anti-PD-1 (HX008) | Advanced solid tumors | Durable iPR was observed in the patients either receiving OH2 or in combination with anti-PD-1 (HX008) [104]. |
| HSV1716 | Anti-PD-1 | Rhabdomyosarcoma syngeneic mouse models | MHC I controlled the rate of response, as tumors with an elevated expression of MHC I responded to the therapy, while the therapy did not affect tumors with a low expression of MHC I [93]. |
| G47D-mIL12 | Anti-PD-1 and anti-CTLA-4 | GBM | Most mice responded to this triple combination therapy. High expression of macrophages, but low expression of CD4+ Tregs [105]. |
| Histone deacetylase (HDAC) inhibitors | |||
| OncoVEXmGM-CSF (mT-VEC) | Valproic acid | Melanoma | Antitumor immunity was activated by enhancing the expression of IFN, NK cells, and DCs. Virus replication was increased in melanoma cells [106]. |
| MAPK pathway inhibitors | |||
| mT-VEC | Trametinib | BRAFV600E melanoma | Tumor growth inhibition and antitumor immunity activation; 40% of the injected mice showed tumor elimination, while 70% of the treated mice showed rechallenge protection [107]. |
| mRP1 | PLX4720 | Thyroid tumor cell lines with BRAFV600E | Antitumor immunity activation and tumor growth regression [108]. |
| TGFβ inhibitors | |||
| HSV1716 | A8301 | Rhabdomyosarcoma | Antitumor immunity activation and increased survival period. A complete durable response was observed in 20% [109]. |
| rQNestin34.5 | ID11 | Glioblastoma | Prolonged survival period [110]. |
| oHSV MG18L | Galunisertib | Human GBM stem-like cells | A total of 60% were cured, and a prolonged survival period was noted [111]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorab, B.E.A.; Liu, T.; Ying, M.; Wang, P.; Qin, M.; Xing, J.; Wang, H.; Xu, F. Advances in the Drug Development and Quality Evaluation Principles of Oncolytic Herpes Simplex Virus. Viruses 2025, 17, 581. https://doi.org/10.3390/v17040581
Ghorab BEA, Liu T, Ying M, Wang P, Qin M, Xing J, Wang H, Xu F. Advances in the Drug Development and Quality Evaluation Principles of Oncolytic Herpes Simplex Virus. Viruses. 2025; 17(4):581. https://doi.org/10.3390/v17040581
Chicago/Turabian StyleGhorab, Basma Eid Abdullah, Tongtan Liu, Min Ying, Ping Wang, Meirong Qin, Jiayong Xing, Huadong Wang, and Fuqiang Xu. 2025. "Advances in the Drug Development and Quality Evaluation Principles of Oncolytic Herpes Simplex Virus" Viruses 17, no. 4: 581. https://doi.org/10.3390/v17040581
APA StyleGhorab, B. E. A., Liu, T., Ying, M., Wang, P., Qin, M., Xing, J., Wang, H., & Xu, F. (2025). Advances in the Drug Development and Quality Evaluation Principles of Oncolytic Herpes Simplex Virus. Viruses, 17(4), 581. https://doi.org/10.3390/v17040581






