Experimental Efficacy of an Alphavirus Vectored RNA Particle Vaccine Against Porcine Parainfluenza Virus-1 in Conventional Weaned Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. PPIV1 Cell Culture and Inoculation
2.2. Animals
2.3. Experimental Design
2.4. Clinical Observations
2.5. Sample Processing
2.6. Nucleic Acid Extraction
2.7. PPIV1 RT-qPCR Assay
2.8. Detection of Viral Antigen by Immunohistochemistry (IHC)
2.9. Gross and Microscopic Lesions
2.10. Serum Virus Neutralization Assay
2.11. Detection of PPIV Antibodies by wvELISA
2.12. Statistical Analysis
3. Results
3.1. Average Daily Gain
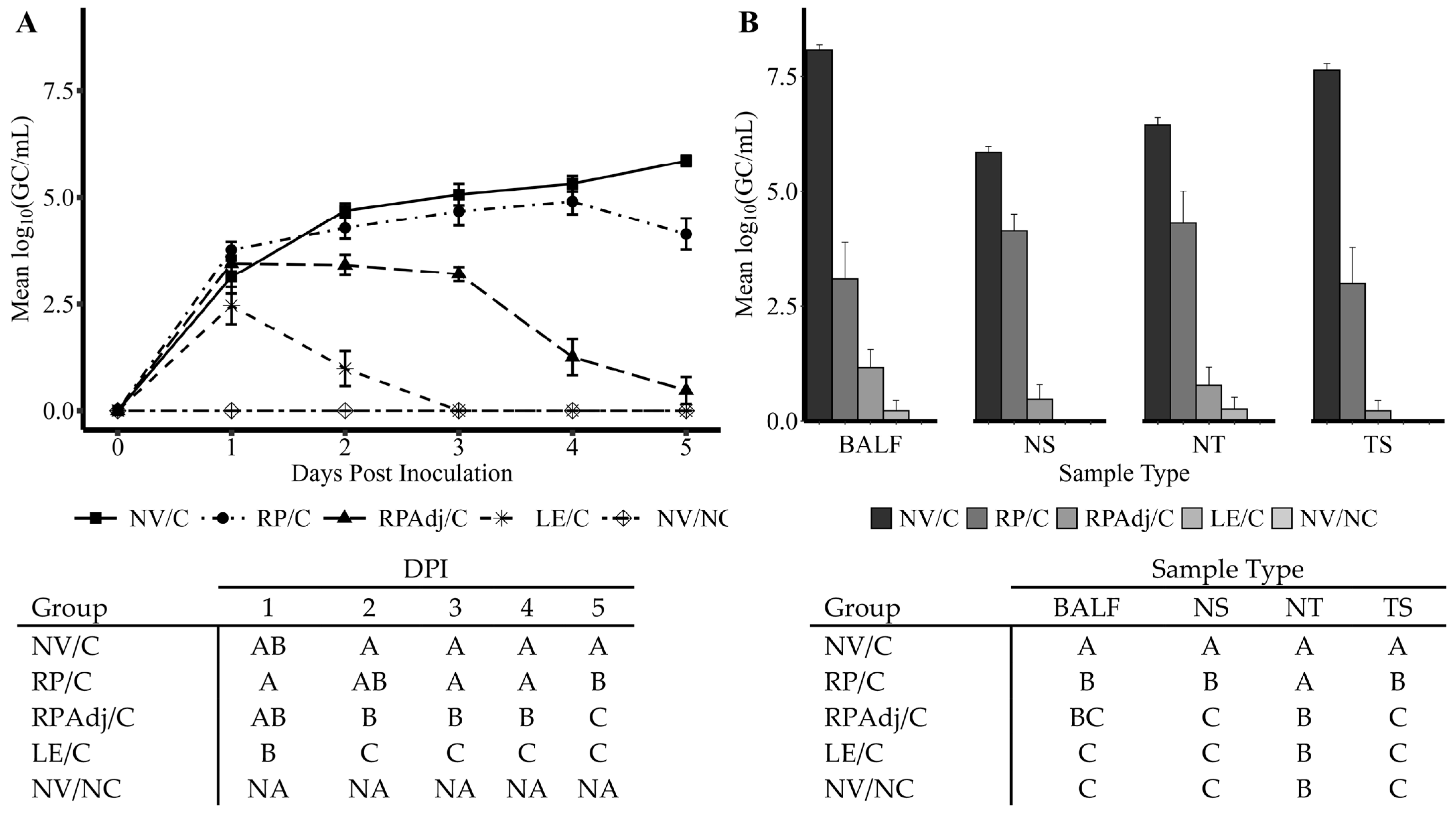
3.2. PPIV1 Detection of Viral Shedding in NS by RT-qPCR
3.3. PPIV1 Detection in Tissues by RT-qPCR
3.4. Macroscopic Lung Lesions
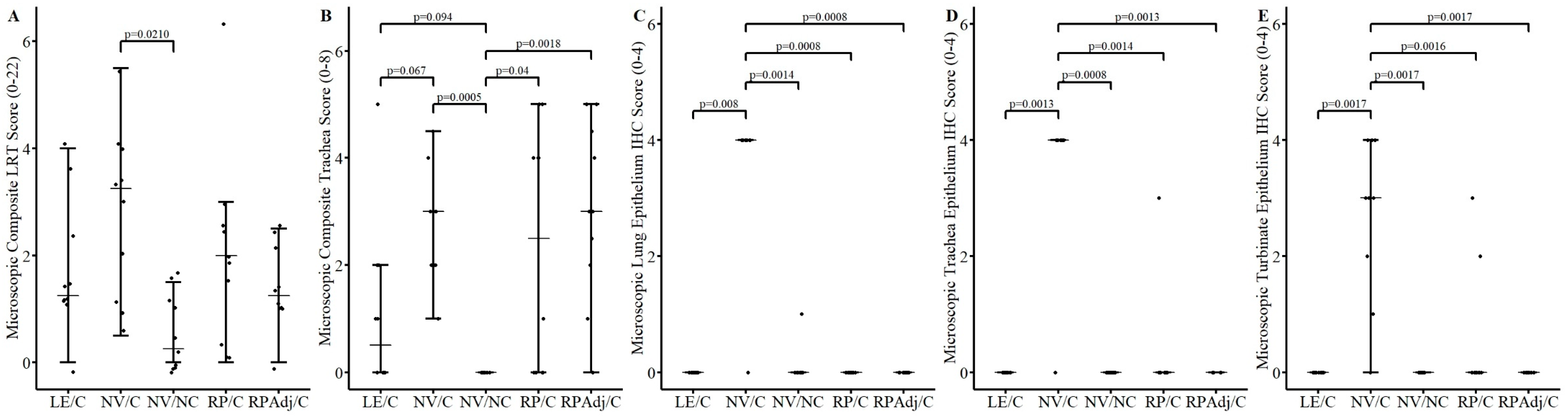
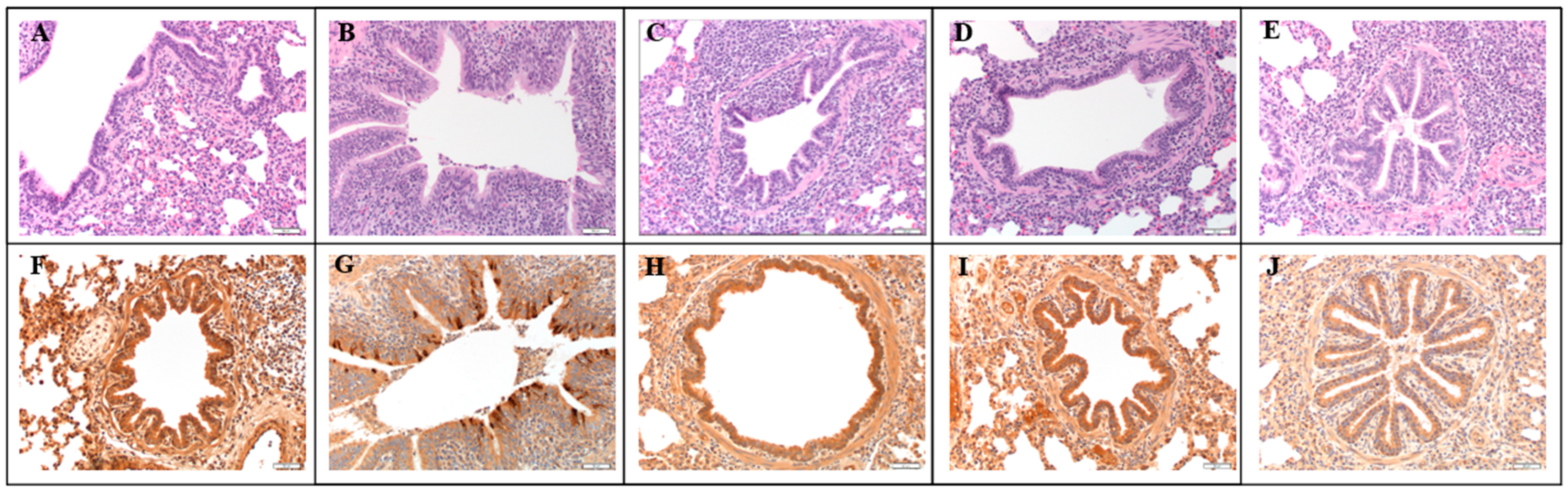
3.5. Microscopic Lesions and Immunohistochemistry
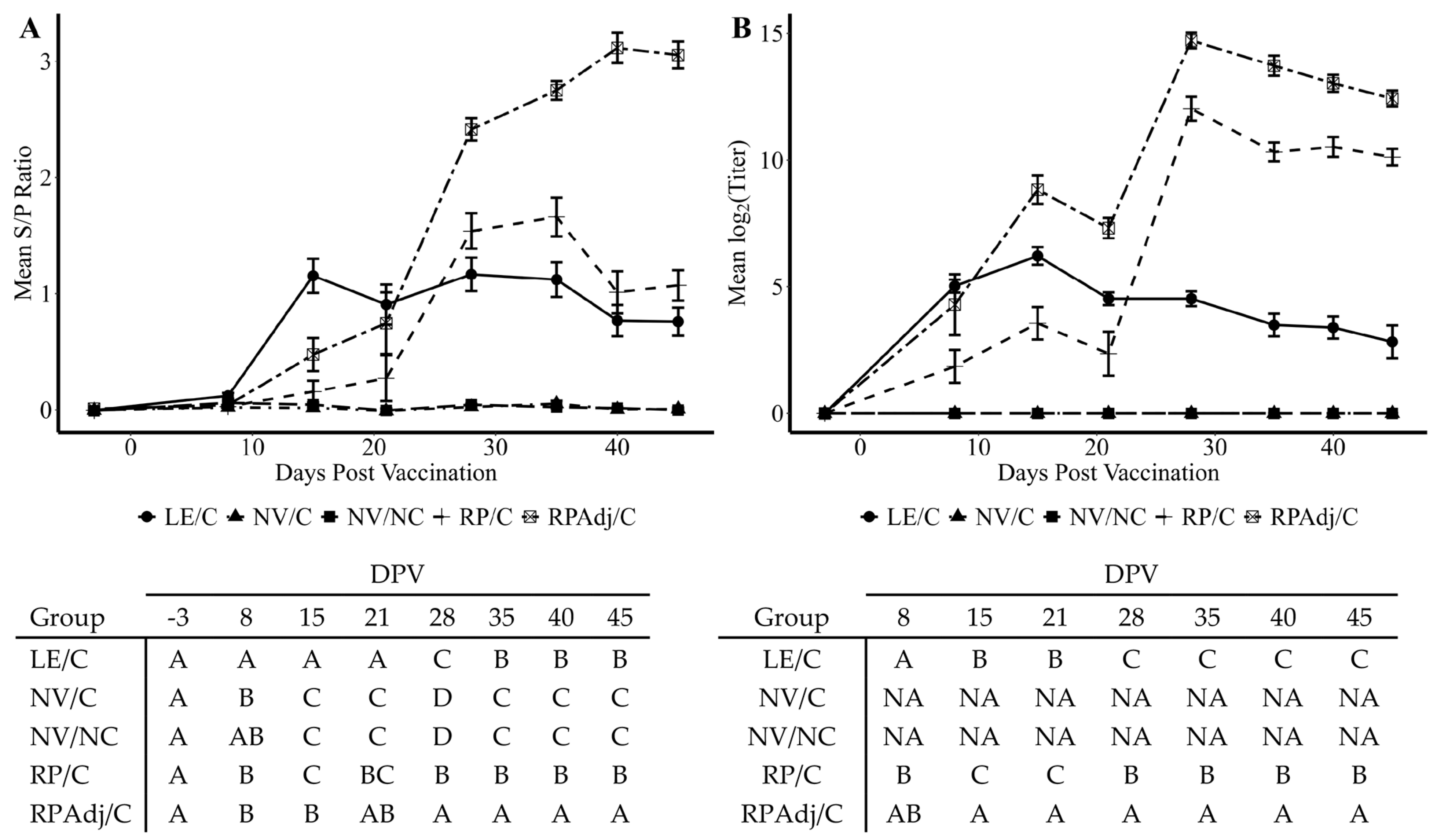
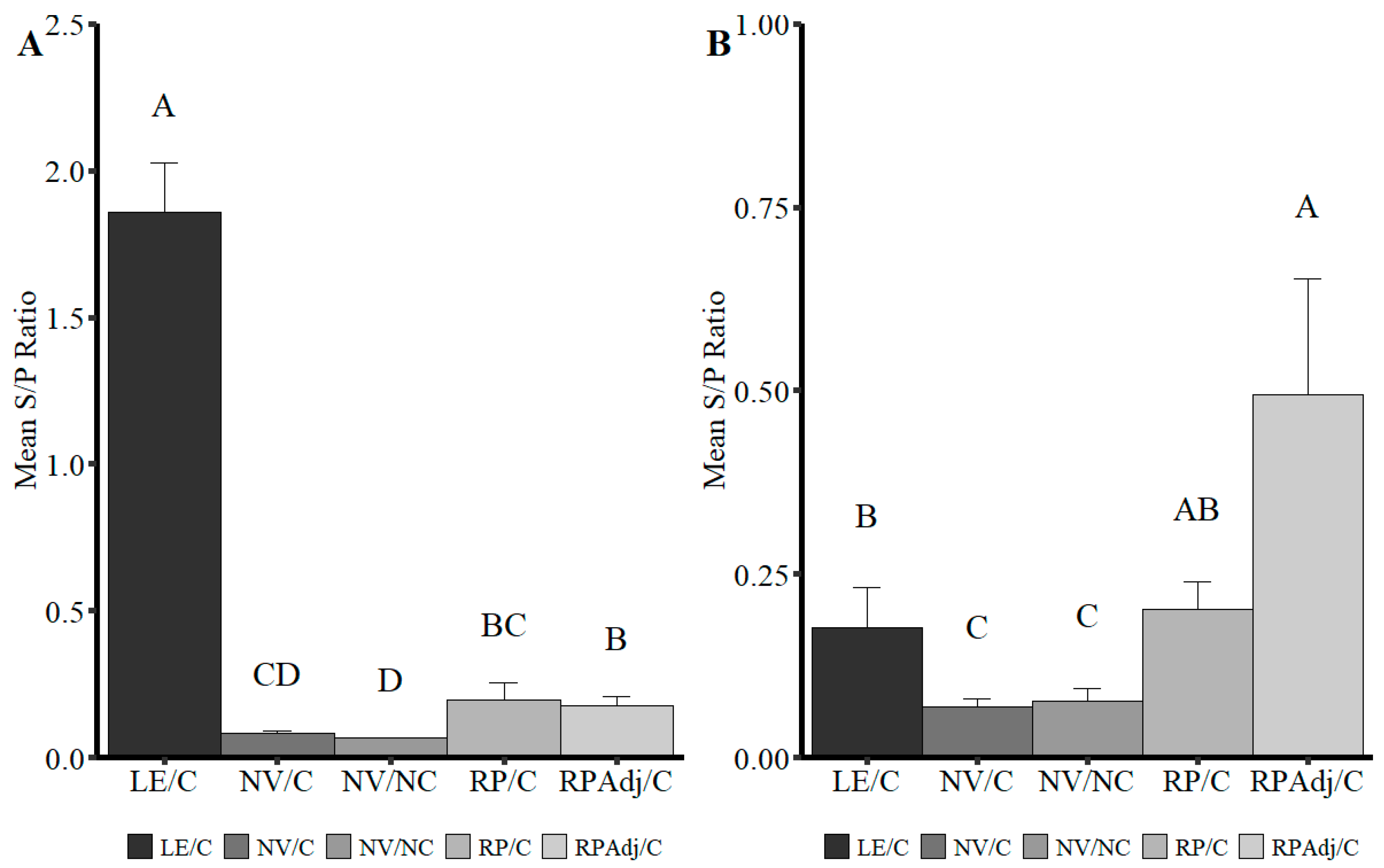
3.6. Detection of IgG Antibody by wvELISA in Serum
3.7. Serum Virus Neutralization Antibody Titers
3.8. Detection of IgG and IgA in BALF
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Antibiotics |
| BALF | Bronchioalveolar lavage fluid |
| BPIV3 | Bovine parainfluenza virus 3 |
| BCS | Body condition score |
| DPV | Days post vaccination |
| DAB | 3,3′-diaminobenzidine |
| ELISA | Enzyme-linked immunosorbent assay |
| FFPE | Formalin-fixed, paraffin-embedded |
| FBS | Fetal bovine serum |
| HRP | Horseradish peroxidase |
| IAV-S | Influenza A virus in swine |
| IHC | Immunohistochemistry |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| ISU VDL | Iowa State University Veterinary Diagnostic Laboratory |
| LE/C | Live exposure, challenged |
| LRT | Lower respiratory tract |
| MEM | Minimum essential medium |
| MHP | Mycoplasma hyopneumoniae |
| NV/C | Non-vaccinated, challenged |
| NV/NC | Non-vaccinated, non-challenged |
| PCV2 | Porcine circovirus type 2 |
| PIM | Post inoculation medium |
| PCR | Polymerase chain reaction |
| PRDC | Porcine respiratory disease complex |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PPIV1 | Porcine parainfluenza virus 1 |
| RP/C | RNA-particle vaccinated, challenged |
| RPAdj/C | Adjuvanted RNA particle vaccinated, challenged |
| rtPCR | Real-time polymerase chain reaction |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| SeV | Sendai virus |
| ST | Swine testicular |
| TCID50 | 50% tissue culture infectious dose |
| TPCK | Tosyl phenylalanyl chloromethyl ketone |
| TS | Tracheal swab |
| NT | Nasal turbinate |
| NS | Nasal swab |
| SVN | Serum virus neutralization |
| wvELISA | Whole virus enzyme-linked immunosorbent assay |
| XIPC | Exogenous internal positive control |
References
- The ICTV Report. Available online: https://talk.ictvonline.org/ (accessed on 12 July 2021).
- Lau, S.K.P.; Woo, P.C.Y.; Wu, Y.; Wong, A.Y.P.; Wong, B.H.L.; Lau, C.C.Y.; Fan, R.Y.Y.; Cai, J.-P.; Tsoi, H.-W.; Chan, K.-H.; et al. Identification and characterization of a novel paramyxovirus, porcine parainfluenza virus 1, from deceased pigs. J. Gen. Virol. 2013, 94, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.M.; Chen, Z.; Henningson, J.N.; Lang, Y.; Rowland, R.R.; Fang, Y.; Prickett, J.; Gauger, P.C.; Hause, B.M. Widespread detection and characterization of porcine parainfluenza virus 1 in pigs in the USA. J. Gen. Virol. 2016, 97, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Welch, M.W.; Harmon, K.M.; Zhang, J.; Pineyro, P.E.; Li, G.; Hause, B.M.; Gauger, P.C. Detection, isolation, and in vitro characterization of porcine parainfluenza virus type 1 isolated from respiratory diagnostic specimens in swine. Vet. Microbiol. 2019, 228, 219–225. [Google Scholar] [CrossRef]
- Agüero, B.; Mena, J.; Berrios, F.; Tapia, R.; Salinas, C.; Dutta, J.; van Bakel, H.; Mor, S.K.; Brito, B.; Medina, R.A.; et al. First report of porcine respirovirus 1 in South America. Vet. Microbiol. 2020, 246, 108726. [Google Scholar] [CrossRef]
- Dénes, L.; Cságola, A.; Schönhardt, K.; Halas, M.; Solymosi, N.; Balka, G. First report of porcine parainfluenza virus 1 (species Porcine respirovirus 1) in Europe. Transbound. Emerg. Dis. 2021, 68, 1731–1735. [Google Scholar] [CrossRef]
- Lower, A. Porcine Parainfluenza Virus—Practitioner Perspective. In Proceedings of the AASV 49th Annual Meeting, San Diego, CA, USA, 3–6 March 2018. [Google Scholar]
- Welch, M.; Park, J.; Harmon, K.; Zhang, J.; Pineyro, P.E.; Giminez-Lirola, L.; Gauger, P. Pathogenesis of a porcine parainfluenza virus-1 isolate (USA/MN25890NS/2016) in conventional and CDCD piglets. In Proceedings of the AASV 49th Annual Meeting, San Diego, CA, USA, 3–6 March 2018. [Google Scholar]
- Welch, M.; Krueger, K.; Zhang, J.; Piñeyro, P.; Patterson, A.; Gauger, P. Pathogenesis of an experimental coinfection of porcine parainfluenza virus 1 and influenza A virus in commercial nursery swine. Vet. Microbiol. 2023, 285, 109850. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Brownlie, J. The challenges in developing effective canine infectious respiratory disease vaccines. J. Pharm. Pharmacol. 2015, 67, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.C.; Schaap-nutt, A.; Bartlett, E.J.; Schomacker, H.; Boonyaratanakornkit, J.; Karron, R.A.; Collins, P.L. Progress in the development of human parainfluenza virus vaccines. Expert. Rev. Respir. Med. 2012, 5, 515–526. [Google Scholar] [CrossRef]
- Smith, C.B.; Bellanti, J.A.; Chanock, R.M. Immunoglobulins in serum and nasal secretions following infection with type 1 parainfluenza virus and injection of inactivated vaccines. J. Immunol. 1967, 99, 133–141. [Google Scholar] [CrossRef]
- Smith, C.B.; Purcell, R.H.; Bellanti, J.A.; Chanock, R.M. Protective effect of antibody to parainfluenza type 1 virus. N. Engl. J. Med. 1966, 275, 1145–1152. [Google Scholar] [CrossRef]
- Glezen, W.P.; Frank, A.L.; Taber, L.H.; Kasel, J.A. Parainfluenza Virus Type 3: Seasonality and Risk of Infection and Reinfection in Young Children. J. Infect. Dis. 1984, 150, 851–857. [Google Scholar] [CrossRef]
- Bosworth, B.; Erdman, M.M.; Stine, D.L.; Harris, I.; Irwin, C.; Jens, M.; Loynachan, A.; Kamrud, K.; Harris, D.L. Replicon particle vaccine protects swine against influenza. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e99–e103. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.; Mogler, M.; Hicks, J.; Harris, D. Protective efficacy of a replicon particle vaccine in both naïve and previously exposed gilts against porcine epidemic diarrhea virus. In Proceedings of the AASV 47th Annual Meeting, New Orleans, LA, USA, 27 February–1 March 2016. [Google Scholar]
- Zimmer, G. RNA replicons—A new approach for influenza virus immunoprophylaxis. Viruses 2010, 2, 413–434. [Google Scholar] [CrossRef]
- Welch, M.; Park, J.; Harmon, K.; Zhang, J.; Piñeyro, P.; Gimenez-Lirola, L.; Zhang, M.; Wang, C.; Patterson, A.; Gauger, P.C. Pathogenesis of a novel porcine parainfluenza virus type 1 isolate in conventional and colostrum deprived/caesarean derived pigs. Virology 2021, 563, 88–97. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method for estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Welch, M.; Krueger, K.; Zhang, J.; Piñeyro, P.; Magtoto, R.; Wang, C.; Giménez-Lirola, L.; Strait, E.; Mogler, M.; Gauger, P. Detection of porcine parainfluenza virus type-1 antibody in swine serum using whole-virus ELISA, indirect fluorescence antibody and virus neutralizing assays. BMC Vet. Res. 2022, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Mengeling, W.L.; Lager, K.M.; Vorwald, A.C. Diagnosis of Porcine Reproductive and Respiratory Syndrome. J. Vet. Diagn. Investig. 1995, 7, 3–16. [Google Scholar] [CrossRef]
- Burri, C.; Allgoewer, M.; Roth, W. The preparation of a sterile tisue homogenate and its evaluation in animal experiments. Z. Fur Die Gesamte Exp. Med. 1964, 138, 92–98. [Google Scholar] [CrossRef]
- Schroeder, M.E.; Bounpheng, M.A.; Rodgers, S.; Baker, R.J.; Black, W.; Naikare, H.; Velayudhan, B.; Sneed, L.; Szonyi, B.; Clavijo, A. Development and performance evaluation of calf diarrhea pathogen nucleic acid purification and detection workflow. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 2012, 24, 945–953. [Google Scholar] [CrossRef]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef]
- Murphy, B.R. Current approaches to the development of vaccines effective against parainfluenza viruses. Bull. World Health Organ. 1988, 66, 391–397. [Google Scholar] [PubMed]
- Polack, F.P.; Auwaerter, P.G.; Lee, S.-H.; Nousari, H.C.; Valsamakis, A.; Leiferman, K.M.; Diwan, A.; Adams, R.J.; Griffin, D.E. Production of atypical measles in rhesus macaques: Evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat. Med. 1999, 5, 629–634. [Google Scholar] [CrossRef]
- Wright, P.F.; Karron, R.A.; Belshe, R.B.; Shi, J.R.; Randolph, V.B.; Collins, P.L.; O’Shea, A.F.; Gruber, W.C.; Murphy, B.R. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 2007, 25, 7372–7378. [Google Scholar] [CrossRef] [PubMed]
- Greer, C.; Zhou, F.; Goodsell, A.; Legg, H.; Tang, Z.; Zur Megede, J.; Uematsu, Y.; Polo, J.; Vajdy, M. Long-term protection in hamsters against human parainfluenza virus type 3 following mucosal or combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles. Scand. J. Immunol. 2007, 66, 645–653. [Google Scholar] [CrossRef]
- Greer, C.E.; Zhou, F.; Legg, H.S.; Tang, Z.; Perri, S.; Sloan, B.A.; Zur Megede, J.; Uematsu, Y.; Vajdy, M.; Polo, J.M. A chimeric alphavirus RNA replicon gene-based vaccine for human parainfluenza virus type 3 induces protective immunity against intranasal virus challenge. Vaccine 2007, 25, 481–489. [Google Scholar] [CrossRef]
- Vander Veen, R.; Mogler, M.; Russell, B.; Loynachan, A.; Harris, D.; Kamrud, K. Haemagglutinin and nucleoprotein replicon particle vaccination of swine protects against the pandemic H1N1 2009 virus. Vet. Rec. 2013, 173, 344. [Google Scholar] [CrossRef]
- Suzuki, T.; Ainai, A.; Hasegawa, H. Functional and structural characteristics of secretory IgA antibodies elicited by mucosal vaccines against influenza virus. Vaccine 2017, 35, 5297–5302. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawaguchi, A.; Ainai, A.; Tamura, S.; Ito, R.; Multihartina, P.; Setiawaty, V.; Pangesti, K.N.; Odagiri, T.; Tashiro, M.; et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc. Natl. Acad. Sci. USA 2015, 112, 7809–7814. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Sano, K.; Suzuki, T.; Ainai, A.; Taga, Y.; Ueno, T.; Tabata, K.; Saito, K.; Wada, Y.; Ohara, Y.; et al. IgA tetramerization improves target breadth but not peak potency of functionality of anti-influenza virus broadly neutralizing antibody. PLoS Pathog. 2019, 15, e1007427. [Google Scholar] [CrossRef]
- Okuya, K.; Yoshida, R.; Manzoor, R.; Saito, S.; Suzuki, T.; Sasaki, M.; Saito, T.; Kida, Y.; Mori-Kajihara, A.; Kondoh, T.; et al. Potential Role of Nonneutralizing IgA Antibodies in Cross-Protective Immunity against Influenza A Viruses of Multiple Hemagglutinin Subtypes. J. Virol. 2020, 94, e00408–e00420. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Phan, S.I.; Chen, Z.; Xu, P.; Li, Z.; Gao, X.; Foster, S.L.; Teng, M.N.; Tripp, R.A.; Sakamoto, K.; He, B. A respiratory syncytial virus (RSV) vaccine based on parainfluenza virus 5 (PIV5). Vaccine 2014, 32, 3050–3057. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mooney, A.J.; Gabbard, J.D.; Gao, X.; Xu, P.; Place, R.J.; Hogan, R.J.; Tompkins, S.M.; He, B. Recombinant parainfluenza virus 5 expressing hemagglutinin of influenza A virus H5N1 protected mice against lethal highly pathogenic avian influenza virus H5N1 challenge. J. Virol. 2013, 87, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zaiser, S.A.; Shang, P.; Heiden, D.L.; Hajovsky, H.; Katwal, P.; DeVries, B.; Baker, J.; Richt, J.A.; Li, Y.; et al. A chimeric influenza hemagglutinin delivered by parainfluenza virus 5 vector induces broadly protective immunity against genetically divergent influenza a H1 viruses in swine. Vet. Microbiol. 2020, 250, 108859. [Google Scholar] [CrossRef]
| Vaccination | Challenge | ||||||
|---|---|---|---|---|---|---|---|
| Group | N | Vaccine Group | Vaccination Route | Priming Dose | Boost Dose | Challenge | Necropsy |
| 1 | 10 | NV/NC | IM | 0 DPV | 21 DPV | 40 DPV/0 DPI | 45 DPV/5 DPI |
| 2 | 10 | NV/C | IM | 0 DPV | 21 DPV | 40 DPV/0 DPI | 45 DPV/5 DPI |
| 3 | 10 | RPadj/C | IM | 0 DPV | 21 DPV | 40 DPV/0 DPI | 45 DPV/5 DPI |
| 4 | 10 | RP/C | IM | 0 DPV | 21 DPV | 40 DPV/0 DPI | 45 DPV/5 DPI |
| 5 | 10 | LE/C | IN | 0 DPV | 21 DPV | 40 DPV/0 DPI | 45 DPV/5 DPI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welch, M.; Krueger, K.; Zhang, J.; Piñeyro, P.; Mogler, M.; Strait, E.; Gauger, P. Experimental Efficacy of an Alphavirus Vectored RNA Particle Vaccine Against Porcine Parainfluenza Virus-1 in Conventional Weaned Pigs. Viruses 2025, 17, 565. https://doi.org/10.3390/v17040565
Welch M, Krueger K, Zhang J, Piñeyro P, Mogler M, Strait E, Gauger P. Experimental Efficacy of an Alphavirus Vectored RNA Particle Vaccine Against Porcine Parainfluenza Virus-1 in Conventional Weaned Pigs. Viruses. 2025; 17(4):565. https://doi.org/10.3390/v17040565
Chicago/Turabian StyleWelch, Michael, Karen Krueger, Jianqiang Zhang, Pablo Piñeyro, Mark Mogler, Erin Strait, and Phillip Gauger. 2025. "Experimental Efficacy of an Alphavirus Vectored RNA Particle Vaccine Against Porcine Parainfluenza Virus-1 in Conventional Weaned Pigs" Viruses 17, no. 4: 565. https://doi.org/10.3390/v17040565
APA StyleWelch, M., Krueger, K., Zhang, J., Piñeyro, P., Mogler, M., Strait, E., & Gauger, P. (2025). Experimental Efficacy of an Alphavirus Vectored RNA Particle Vaccine Against Porcine Parainfluenza Virus-1 in Conventional Weaned Pigs. Viruses, 17(4), 565. https://doi.org/10.3390/v17040565








