Host-Strain-Specific Responses to Pneumonia Virus of Mice Infection: A Study of Lesions, Viral Load, and Cytokine Expression
Abstract
1. Introduction
2. Material and Methods
2.1. Mice
2.2. Viral Infection
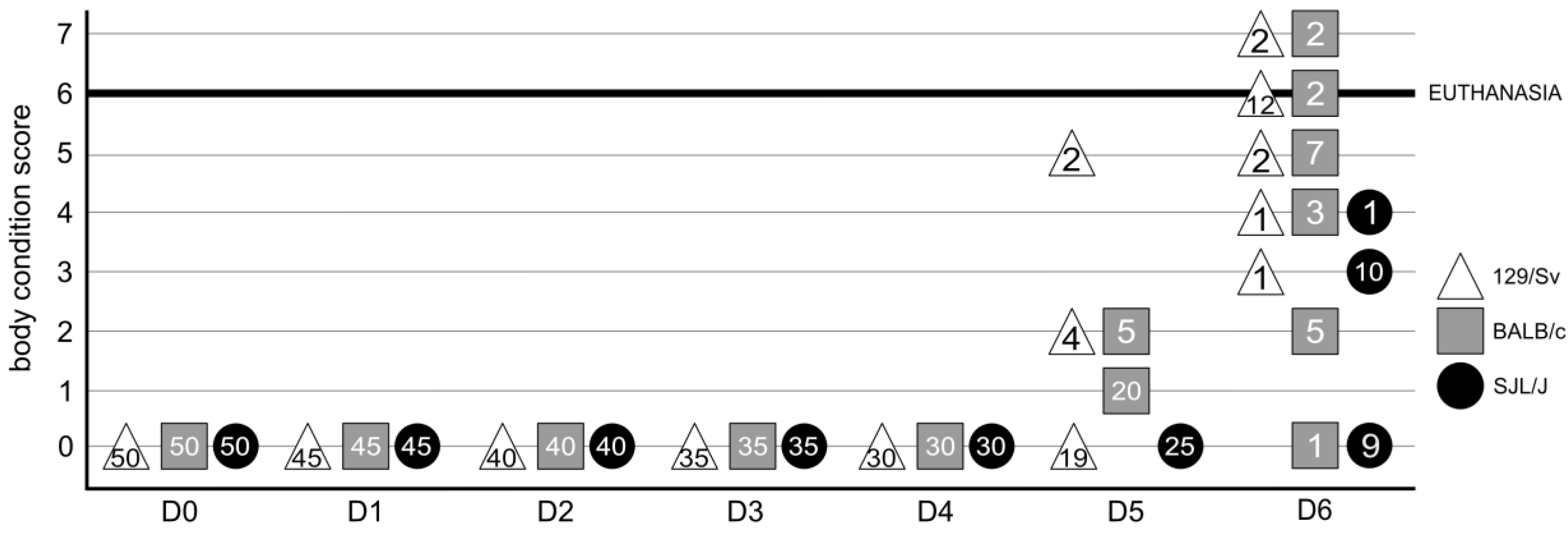
2.3. Clinical Score
2.4. Macroscopic Lesions
2.5. Microscopic Lesions
2.6. qRT-PCR
2.7. Correlation Analyses, Principal Component Analyses (PCA), and Heatmaps
3. Results
3.1. The Genetic Background of Mice Has a Significant Impact on Macroscopic Lesions
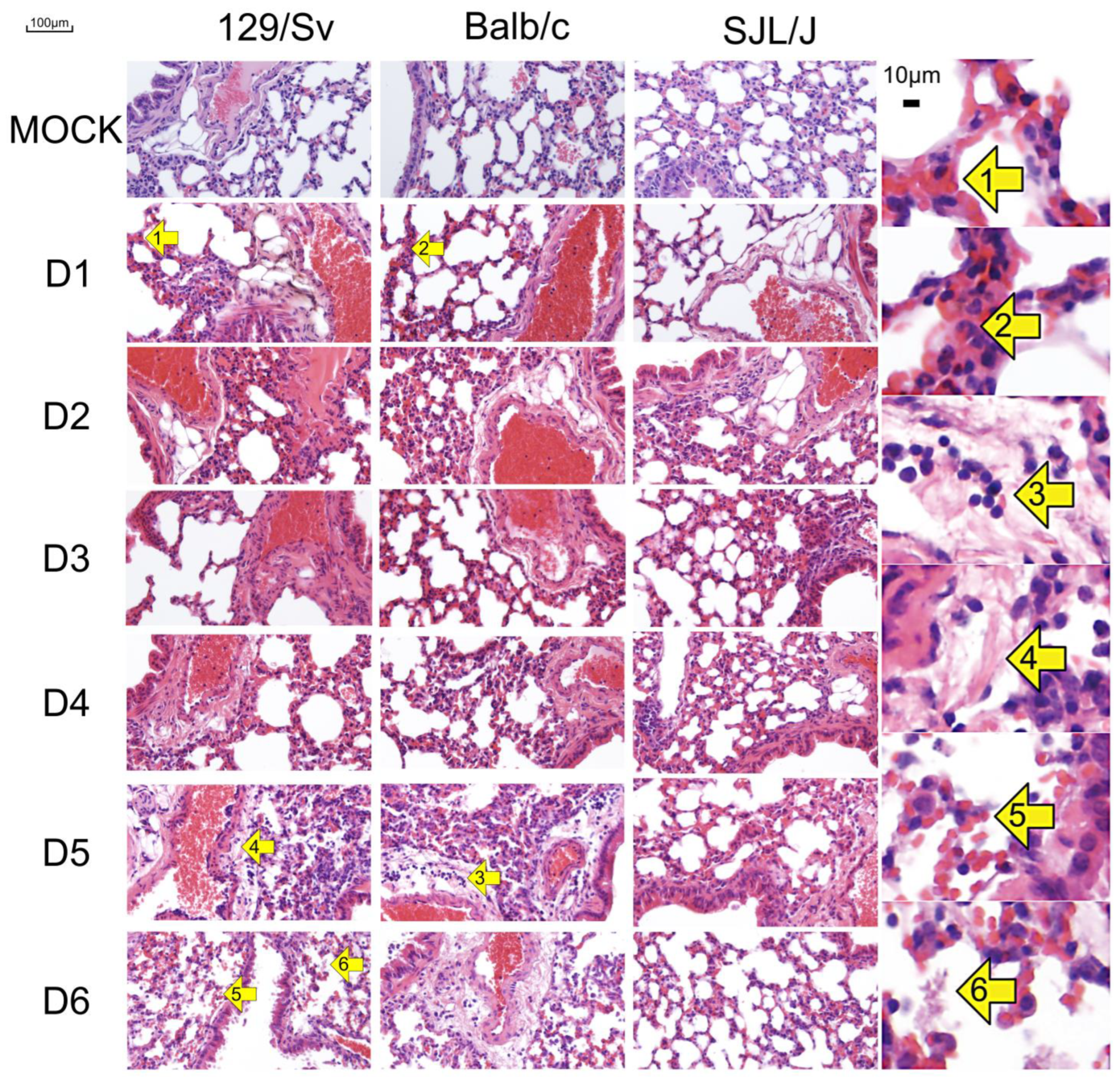
3.2. The Genetic Background of Mice Has a Significant Impact on Microscopic Lesions
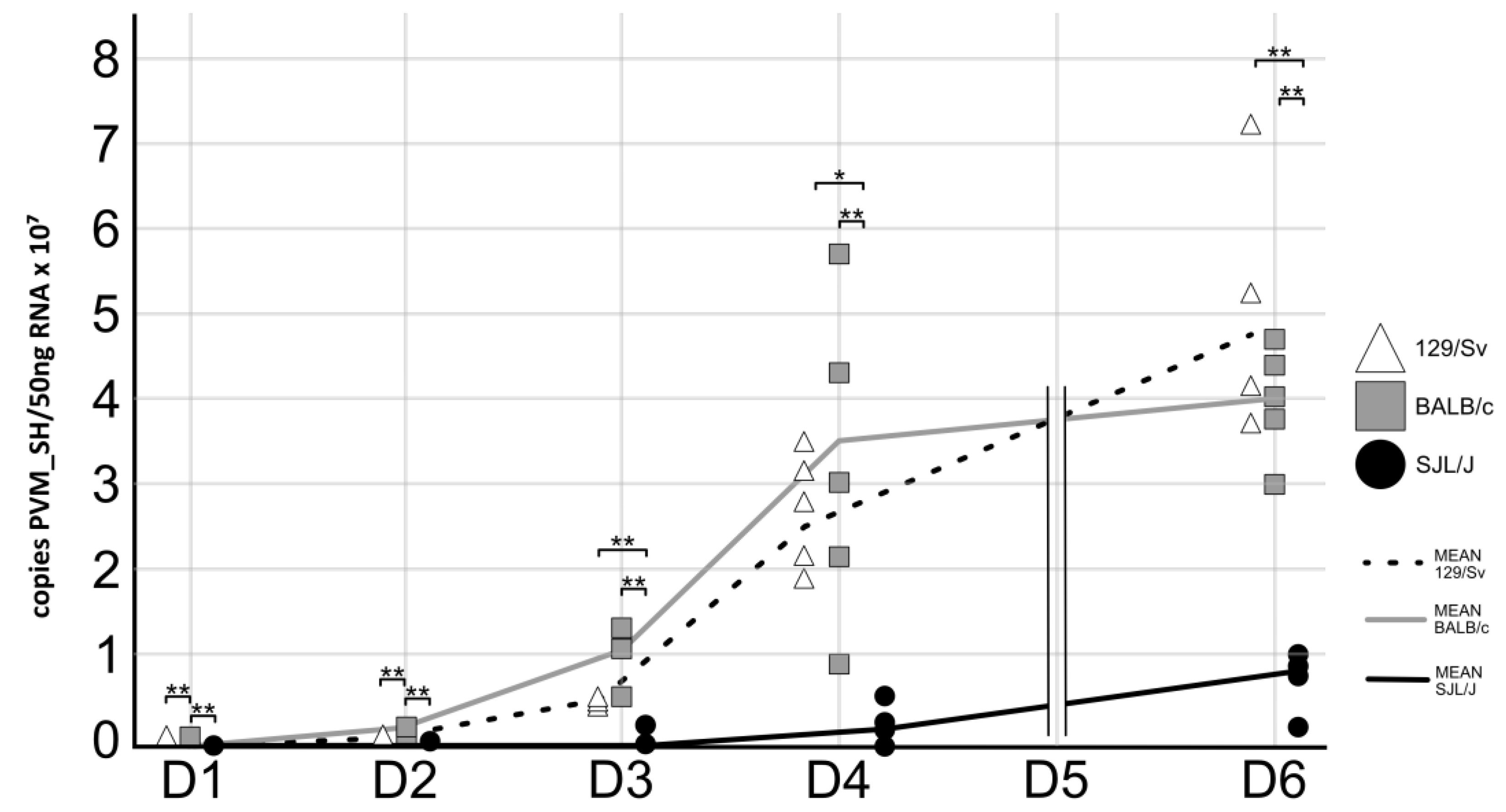
3.3. The Genetic Background of Mice Has a Significant Impact on PVM Virus Replication
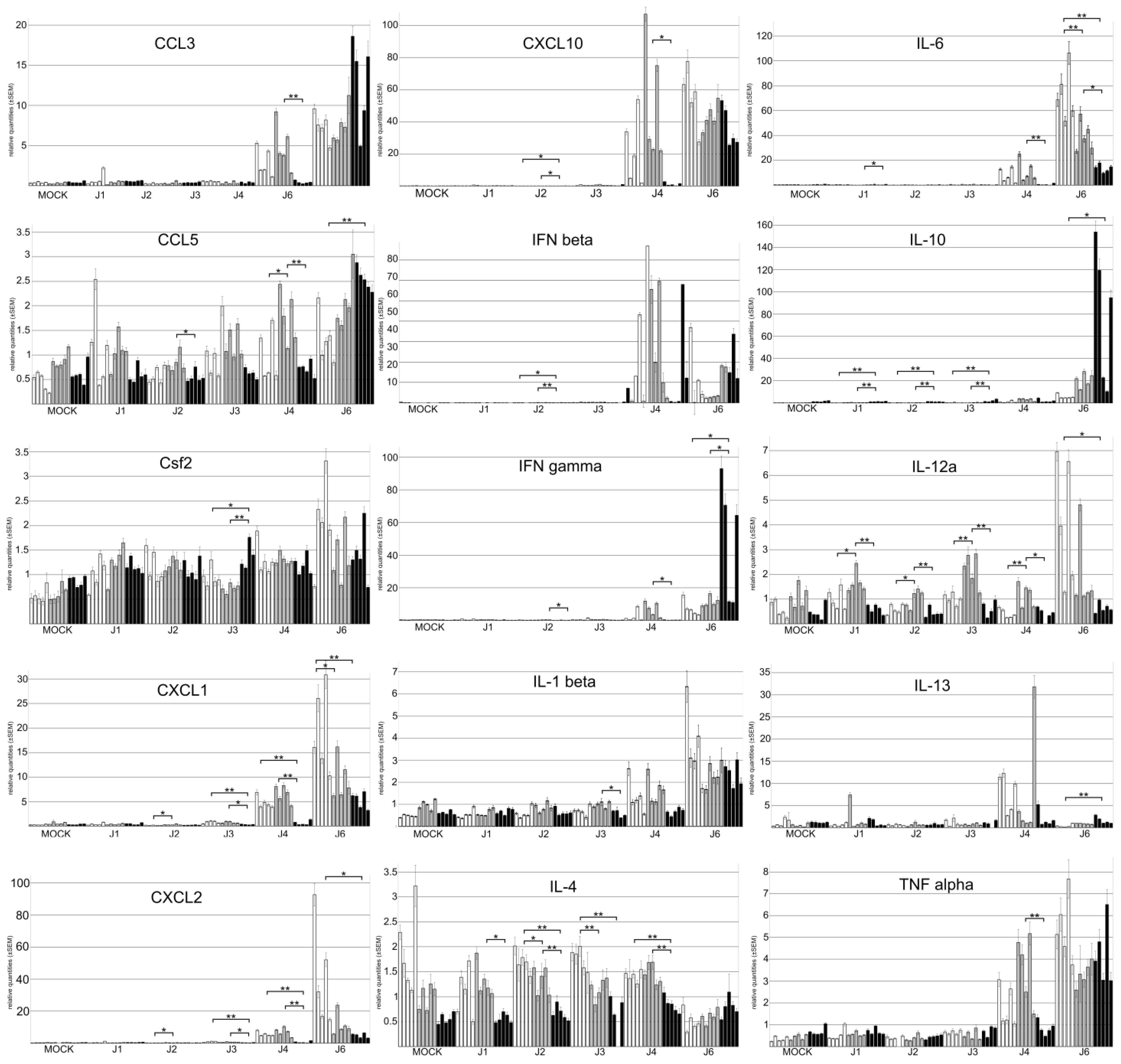
3.4. The Genetic Background of Mice Has a Significant Impact on Cytokine Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munro, A.P.S.; Martinón-Torres, F.; Drysdale, S.B.; Faust, S.N. The disease burden of respiratory syncytial virus in Infants. Curr. Opin. Infect. Dis. 2023, 36, 379–384. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Duan, Y.P.; Tong, X.L.; Huang, Q.R.; Jia, M.M.; Yang, W.Z.; Feng, L.Z. Clinical manifestations of respiratory syncytial virus infection and the risk of wheezing and recurrent wheezing illness: A systematic review and meta-analysis. World J. Pediatr. WJP 2023, 19, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Dyer, K.D.; Garcia-Crespo, K.E.; Glineur, S.; Domachowske, J.B.; Rosenberg, H.F. The Pneumonia Virus of Mice (PVM) model of acute respiratory infection. Viruses 2012, 4, 3494–3510. [Google Scholar] [CrossRef] [PubMed]
- Krempl, C.D.; Lamirande, E.W.; Collins, P.L. Complete Sequence of the RNA Genome of Pneumonia Virus of Mice (PVM). Virus Genes 2005, 30, 237–248. [Google Scholar] [CrossRef]
- Nicklas, W.; Bleich, A.; Mähler, M. Viral Infections of Laboratory Mice. In The Laboratory Mouse, 2nd ed.; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Vicencio, A.G. Susceptibility to bronchiolitis in infants. Curr. Opin. Pediatr. 2010, 22, 302–306. [Google Scholar] [CrossRef]
- Zeng, R.; Li, C.; Li, N.; Wei, L.; Cui, Y. The role of cytokines and chemokines in severe respiratory syncytial virus infection and subsequent asthma. Cytokine 2011, 53, 1–7. [Google Scholar] [CrossRef]
- Gilliaux, G.; Desmecht, D. Gammaherpesvirus Alters Alveolar Macrophages According to the Host Genetic Background and Promotes Beneficial Inflammatory Control over Pneumovirus Infection. Viruses 2022, 14, 98. [Google Scholar] [CrossRef]
- Djabirska, I.; Delaval, L.; Tromme, A.; Blomet, J.; Desmecht, D.; Van Laere, A.S. Longitudinal quantitative assessment of TMEV-IDD-induced MS phenotypes in two inbred mouse strains using automated video tracking technology. Exp. Neurol. 2024, 379, 114851. [Google Scholar] [CrossRef]
- Anh, D.B.; Faisca, P.; Desmecht, D.J. Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L426–L435. [Google Scholar] [CrossRef]
- Glineur, S.; Tran Anh, D.B.; Sarlet, M.; Michaux, C.; Desmecht, D. Characterization of the resistance of SJL/J mice to pneumonia virus of mice, a model for infantile bronchiolitis due to a respiratory syncytial virus. PLoS ONE 2017, 7, e44581. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Riedel, N.; Grittner, U.; Endres, M.; Banneke, S.; Emmrich, J.V. Body temperature measurement in mice during acute illness: Implantable temperature transponder versus surface infrared thermometry. Sci. Rep. 2018, 8, 3526. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Domachowske, J.B. Pneumonia Virus of Mice (PVM): Exploring Novel Therapeutic Options in a Severe Respiratory Disease Model. In National Institute of Allergy and Infectious Diseases, NIH; Humana Press: Totowa, NJ, USA, 2010; pp. 353–359. [Google Scholar] [CrossRef]
- Feldman, D.B.; Gupta, B.N. Histopathologic changes in laboratory animals resulting from various methods of euthanasia. Lab. Anim. Sci. 1976, 26 Pt 1, 218–221. [Google Scholar] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Tonacci, A.; Isola, S.; Allegra, A.; Gangemi, S. The Potential Role of Cytokine Storm Pathway in the Clinical Course of Viral Respiratory Pandemic. Biomedicines 2021, 9, 1688. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. MMBR 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Hammoudeh, S.M.; Hammoudeh, A.M.; Bhamidimarri, P.M.; Al Safar, H.; Mahboub, B.; Künstner, A.; Busch, H.; Halwani, R.; Hamid, Q.; Rahmani, M.; et al. Systems Immunology Analysis Reveals the Contribution of Pulmonary and Extrapulmonary Tissues to the Immunopathogenesis of Severe COVID-19 Patients. Front. Immunol. 2021, 12, 595150. [Google Scholar] [CrossRef]
- Crosse, K.M.; Monson, E.A.; Beard, M.R.; Helbig, K.J. Interferon-Stimulated Genes as Enhancers of Antiviral Innate Immune Signaling. J. Innate Immun. 2017, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- García-Sastre, A.; Biron, C.A. Type 1 interferons and the virus-host relationship: A lesson in détente. Science 2006, 312, 879–882. [Google Scholar] [CrossRef]
- Parekh, N.J.; Winship, D.; Van Dis, E.; Stetson, D.B. Regulation and Dynamics of IFN-β Expression Revealed with a Knockin Reporter Mouse. J. Immunol. 2024, 213, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar]
- Bonville, C.A.; Percopo, C.M.; Dyer, K.D.; Gao, J.; Prussin, C.; Foster, B.; Rosenberg, H.F.; Domachowske, J.B. Interferon-gamma coordinates CCL3-mediated neutrophil recruitment in vivo. BMC Immunol. 2009, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; Hu, X.; Li, W.P.; Samuels, S.; Sharif, M.N.; Kotenko, S.; Ivashkiv, L.B. Reprogramming of IL-10 activity and signaling by IFN-gamma. J. Immunol. 2003, 171, 5034–5041. [Google Scholar] [CrossRef]
- Glineur, S.F.; Bowen, A.B.; Percopo, C.M.; Garcia-Crespo, K.E.; Dyer, K.D.; Ochkur, S.I.; Lee, N.A.; Lee, J.J.; Domachowske, J.B.; Rosenberg, H.F. Sustained inflammation and differential expression of interferons type I and III in PVM-infected interferon-gamma (IFNγ) gene-deleted mice. Virology 2014, 468–470, 140–149. [Google Scholar] [CrossRef]
- Percopo, C.M.; Ma, M.; Brenner, T.A.; Krumholz, J.O.; Break, T.J.; Laky, K.; Rosenberg, H.F. Critical Adverse Impact of IL-6 in Acute Pneumovirus Infection. J. Immunol. 2019, 202, 871–882. [Google Scholar] [CrossRef]
- Roy, M.; Richard, J.F.; Dumas, A.; Vallières, L. CXCL1 can be regulated by IL-6 and promotes granulocyte adhesion to brain capillaries during bacterial toxin exposure and encephalomyelitis. J. Neuroinflamm. 2012, 9, 18. [Google Scholar] [CrossRef]
- Kaiser, K.; Prystaz, K.; Vikman, A.; Haffner-Luntzer, M.; Bergdolt, S.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; Ignatius, A. Pharmacological inhibition of IL-6 trans-signaling improves compromised fracture healing after severe trauma. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 523–536. [Google Scholar] [CrossRef]
- Hou, S.M.; Chen, P.C.; Lin, C.M.; Fang, M.L.; Chi, M.C.; Liu, J.F. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res. Ther. 2020, 22, 251. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Gąssowska-Dobrowolska, M.; Wójcik, J.; Szatkowska, I.; Barczak, K.; Chlubek, M.; Baranowska-Bosiacka, I. The Importance of CXCL1 in Physiology and Noncancerous Diseases of Bone, Bone Marrow, Muscle and the Nervous System. Int. J. Mol. Sci. 2022, 23, 4205. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Descriptions | Score |
|---|---|---|
| Appearance | Bright eyes, shiny and maintained coat | 0 |
| Shaggy, dull coat | 1 | |
| Hunched, pilo-erection, disheveled (nape) | 2 | |
| Hands-off monitoring | Mouse is active, interacts with its environment | 0 |
| Slight decrease in activity, less interaction | 1 | |
| Pronounced decrease in activity, mouse is isolated | 2 | |
| Labored breathing | 2 | |
| Tremors | 2 | |
| Hyperactivity or immobility | 3 | |
| Discordant respiratory pattern | 6 | |
| Self-harm | 6 | |
| Response to external stimuli | Mouse moves away quickly | 0 |
| Mouse moves slowly or reacts excessively | 1 | |
| Delayed movement or reaction | 2 | |
| No movement, no reaction | 6 | |
| Weight | Normal | 0 |
| Loss > 10% of base weight | 1 | |
| Loss > 20% of base weight | 6 | |
| Temperature | <36 °C | 3 |
| <32 °C | 6 |
| Gene | Reference | Primer1 | Primer2 | Probe |
|---|---|---|---|---|
| Ccl3 | Mm.PT.58.29283216 | CGATGAATTGGCGTGGAATC | CCTTGCTGTTCTTCTCTGTACC | /5SUN/ACTGCCTGC/ZEN/TGCTTCTCCTACAG/3IABkFQ/ |
| Ccl5 | Mm.PT.58.43548565 | CCTCTATCCTAGCTCATCTCCA | GCTCCAATCTTGCAGTCGT | /5SUN/TCTTCTCTG/ZEN/GGTTGGCACACACTT/3IABkFQ/ |
| Csf2 | Mm.PT.58.9186111 | CCTTGAGTTTGGTGAAATTGCC | GTCTCTAACGAGTTCTCCTTCA | /56-FAM/CGAATATCT/Zen/TCAGGCGGGTCTGCA/3IABkFQ/ |
| Cxcl1 | Mm.PT.58.42076891 | GTGCCATCAGAGCAGTCT | CCAAACCGAAGTCATAGCCA | /56-FAM/AGGTGTCCC/Zen/CAAGTAACGGAGAAAGA/3IABkFQ/ |
| Cxcl10 | Mm.PT.58.43575827 | TGATTTCAAGCTTCCCTATGGC | ATTTTCTGCCTCATCCTGCT | /56-FAM/ATCCCTCTC/Zen/GCAAGGACGGTC/3IABkFQ/ |
| Cxcl2 | Mm.PT.58.10456839 | CTTTCCAGGTCAGTTAGCCTT | CAGAAGTCATAGCCACTCTCAAG | /56-FAM/CCCCCTGGT/Zen/TCAGAAAATCATCCAAAAG/3IABkFQ/ |
| Gapdh | Mm.PT.39a.1 | GTGGAGTCATACTGGAACATGTAG | AATGGTGAAGGTCGGTGTG | /56-FAM/TGCAAATGG/Zen/CAGCCCTGGTG/3IABkFQ/ |
| IFNβ | designed in house | AGTTACACTGCCTTTGCCATC | GTCTGCTGGTGGAGTTCATC | /56-FAM/ACAATTTCT/ZEN/CCAGCACTGGGTGGA/3IABkFQ/ |
| IFNγ | Mm.PT.58.41769240 | TCCACATCTATGCCACTTGAG | CTGAGACAATGAACGCTACACA | /56-FAM/TCTTGGCTT/Zen/TGCAGCTCTTCCTCA/3IABkFQ/ |
| IL-4 | Mm.PT.58.32703659 | TCTTTAGGCTTTCCAGGAAGTC | GAGCTGCAGAGACTCTTTCG | /56-FAM/AGCTGCACC/ZEN/ATGAATGAGTCCAAGT/3IABkFQ/ |
| IL-10 | Mm.PT.58.13531087 | ATGGCCTTGTAGACACCTTG | GTCATCGATTTCTCCCCTGTG | /56-FAM/ATCACTCTT/Zen/CACCTGCTCCACTGC/3IABkFQ/ |
| IL-12a | Mm.PT.58.13818295 | CTCTCGTTCTTGTGTAGTTCCA | ACAGATGACATGGTGAAGACG | /5SUN/TGGTTTGGT/ZEN/CCCGTGTGATGTCTT/3IABkFQ/ |
| IL-1β | Mm.PT.58.41616450 | CTCTTGTTGATGTGCTGCTG | GACCTGTTCTTTGAAGTTGACG | /5SUN/TTCCAAACC/ZEN/TTTGACCTGGGCTGT/3IABkFQ/ |
| IL-6 | Mm.PT.58.10005566 | TCCTTAGCCACTCCTTCTGT | AGCCAGAGTCCTTCAGAGA | /56-FAM/CCTACCCCA/Zen/ATTTCCAATGCTCTCCT/3IABkFQ/ |
| IL-13 | Mm.PT.58.11338747 | GAATCCAGGGCTACACAGAAC | AACATCACACAAGACCAGACTC | /56-FAM/TCCACACTC/ZEN/CATACCATGCTGCC/3IABkFQ/ |
| Rplp0 | Mm.PT.58.43894205 | CGCTTGTACCCATTGATGATG | TTATAACCCTGAAGTGCTCGAC | /56-FAM/AGGCCCTGC/Zen/ACTCTCGCTT/3IABkFQ/ |
| Tnf | Mm.PT.58.12575861 | TCTTTGAGATCCATGCCGTTG | AGACCCTCACACTCAGATCA | /5SUN/CCACGTCGT/ZEN/AGCAAACCACCAAGT/3IABkFQ/ |
| Lesion | Mock | D1 | D2 | D3 | D4 | D5 | D6 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Congestion | 0.5 ±0 | 0.5 ±0 | 0.5 ±0 | 0.8 ±0.6 | 0.5 ±0.4 | 0.5 ±0.3 | 1.7 ±0.7 | 1.1 ±0.6 | 0.6 ±0.2 | 1.4 ±0.7 | 1.3 ±0.4 | 0.9 ±0.3 | 2.4 ±0.5 | 1.8 ±0.4 | 2 ±0 | 2.1 ±0.3 | 1.4 ±0.9 | 1.5 ±0.4 | 2.1 ±0.6 | 1.7 ±0.6 | 1.3 ±0.9 | |
| Interstitial inflammation | 0 ±0 | 0 ±0 | 0 ±0 | 0.1 ±0.2 | 0.2 ±0.2 | 0 ±0 | 0.8 ±0.2 | 0.6 ±0.2 | 0.6 ±0.2 | 1.1 ±0.4 | 1.2 ±0.5 | 0.7 ±0.2 | 1.6 ±0.5 | 2 ±0.6 | 1 ±0 | 1.3 ±0.2 | 1 ±0.5 | 0.5 ±0 | 1.9 ±0.4 | 1.9 ±0.5 | 1 ±0 | |
| Perivascular inflammation | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0.1 ±0.2 | 0.2 ±0.2 | 0.2 ±0.4 | 0.4 ±0.3 | 0.6 ±0.3 | 0.4 ±0.3 | 1.8 ±0.4 | 1.4 ±0.5 | 0 ±0 | 0.9 ±0.2 | 1.2 ±0.2 | 0.3 ±0.2 | 1.4 ±0.7 | 1.7 ±0.6 | 0.6 ±0.8 | |
| Perivascular edema | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0.1 ±0.2 | 0.4 ±0.4 | 0.2 ±0.2 | 0.2 ±0.4 | 0.6 ±0.3 | 0.4 ±0.3 | 1 ±0.6 | 1.4 ±0.5 | 0 ±0 | 0.7 ±0.2 | 1.2 ±0.2 | 0.3 ±0.2 | 1.7 ±1 | 2 ±0.5 | 0.2 ±0.4 | |
| Parenchymal inflammation | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0.5 ±0.4 | 0.2 ±0.2 | 0 ±0 | 0.4 ±0.5 | 1.4 ±0.8 | 0 ±0 | 1.3 ±0.2 | 1.9 ±0.5 | 0.3 ±0.2 | 1.5 ±0.6 | 1.7 ±0.6 | 0.4 ±0.5 | |
| Micro hemorrhages | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0.4 ±0.3 | 0.3 ±0.4 | 0 ±0 | 0.6 ±0.5 | 1.4 ±0.8 | 0 ±0 | 1.3 ±0.2 | 1.9 ±0.5 | 0 ±0 | 1.7 ±0.7 | 1.6 ±0.6 | 0.6 ±0.8 | |
| Necrosis | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0 ±0 | 0.3 ±0.4 | 0.3 ±0.4 | 0 ±0 | 0.4 ±0.5 | 1.2 ±0.7 | 0 ±0 | 1 ±0.4 | 1.9 ±0.5 | 0 ±0 | 1.3 ±0.6 | 1.6 ±0.5 | 0.4 ±0.5 | |
| TOTAL | Mean | 0.5 | 0.5 | 0.5 | 0.9 | 0.7 | 0.5 | 2.7 | 2.3 | 1.6 | 4.3 | 4.5 | 2.4 | 8.2 | 10.6 | 3 | 8.6 | 10.5 | 2.9 | 11.5 | 12.1 | 4.5 |
| SD | ±0 | ±0 | ±0 | ±0.6 | ±0.7 | ±0.3 | ±1.2 | ±1.2 | ±1 | ±2.7 | ±2.4 | ±1.2 | ±1.9 | ±3.9 | ±0 | ±1.4 | ±2.7 | ±0.3 | ±2.1 | ±2.9 | ±2 | |
| Day p.i. | PC1 | PC2 | PC1—Largest Loadings | PC2—Largest Loadings |
|---|---|---|---|---|
| 1 | 36 | 30.7 | IL-13 (−0.95) | IL-12a (−0.51) |
| CCL5 (0.20) | IL-10 (0.45) | |||
| IL-10 (−0.14) | IL-4 (−0.43) | |||
| 2 | 52.3 | 18 | IL-10 (0.65) | IL-12a (−0.66) |
| IL-4 (−0.50) | IL-1β (−0.38) | |||
| IFNβ (0.45) | CCL5 (−0.34) | |||
| 3 | 43.8 | 18.7 | IL-10 (−0.68) | IL-12a (−0.59) |
| IL-12a (0.41) | CXCL10 (−0.43) | |||
| CCL5 (0.26) | IL-10 (−0.41) | |||
| 4 | 78.4 | 13.1 | CXCL10 (0.55) | IL-13 (−0.71) |
| IFNβ (0.50) | IFNβ (0.43) | |||
| IL-6 (0.35) | CXCL2 (−0.25) | |||
| 6 | 60 | 20.2 | IL-10 (−0.54) | IFNβ (−0.94) |
| IFNγ (−0.42) | CXCL2 (−0.21) | |||
| CXCL2 (0.41) | IL-10 (0.18) |
| Micro. | V. load | IFNβ | CCL3 | CCL5 | CXCL1 | CXCL10 | CXCL2 | Csf2 | IL-6 | IL-1β | TNFα | IFNγ | IL-10 | IL-12a | IL-13 | IL-4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Micro. | 1.3 × 10−18 | 6.8 × 10−3 | 5.5 × 10−5 | 1.4 × 10−2 | 6.6 × 10−12 | 3.4 × 10−12 | 1.9 × 10−5 | 2.1 × 10−1 | 2.0 × 10−10 | 4.4 × 10−9 | 2.9 × 10−10 | 1 | 1 | 9.1 × 10−3 | 1 | 1 | |
| V. load | 0.839 | 4.0 × 10−6 | 7.4 × 10−5 | 7.2 × 10−3 | 6.4 × 10−15 | 2.6 × 10−18 | 3.1 × 10−5 | 1.5 × 10−2 | 5.0 × 10−14 | 3.6 × 10−8 | 8.2 × 10−14 | 1 | 1 | 1.4 × 10−2 | 1 | 1 | |
| IFNβ | 0.459 | 0.598 | 4.7 × 10−4 | 5.4 × 10−4 | 8.7 × 10−2 | 1.5 × 10−12 | 2.4 × 10−2 | 1 | 6.0 × 10−1 | 2.6 × 10−3 | 7.1 × 10−8 | 1 | 1 | 1 | 1 | 1 | |
| CCL3 | 0.549 | 0.544 | 0.516 | 2.2 × 10−13 | 1.8 × 10−6 | 4.6 × 10−15 | 8.5 × 10−3 | 8.7 × 10−1 | 5.3 × 10−6 | 1.3 × 10−8 | 2.9 × 10−16 | 7.5 × 10−20 | 1.3 × 10−15 | 1 | 1 | 5.3 × 10−1 | |
| CCL5 | 0.435 | 0.452 | 0.514 | 0.767 | 1.1 × 10−1 | 1.4 × 10−8 | 1 | 1 | 1.7 × 10−1 | 6.0 × 10−4 | 9.0 × 10−8 | 8.2 × 10−7 | 2.4 × 10−5 | 1 | 1 | 1 | |
| CXCL1 | 0.74 | 0.791 | 0.393 | 0.603 | 0.382 | 8.8 × 10−14 | 2.3 × 10−14 | 5.4 × 10−9 | 1.0 × 10−38 | 4.4 × 10−12 | 1.7 × 10−20 | 1 | 1 | 4.7 × 10−10 | 1 | 2.8 × 10−1 | |
| CXCL10 | 0.746 | 0.836 | 0.759 | 0.794 | 0.665 | 0.774 | 6.5 × 10−6 | 5.1 × 10−2 | 5.9 × 10−12 | 5.8 × 10−10 | 6.8 × 10−24 | 4.4 × 10−3 | 1.7 × 10−1 | 3.0 × 10−2 | 1 | 1 | |
| CXCL2 | 0.567 | 0.559 | 0.428 | 0.448 | 0.295 | 0.783 | 0.584 | 3.6 × 10−1 | 4.8 × 10−16 | 2.5 × 10−13 | 7.4 × 10−8 | 1 | 1 | 7.6 × 10−17 | 1 | 1 | |
| Csf2 | 0.361 | 0.435 | 0.148 | 0.314 | 0.105 | 0.676 | 0.403 | 0.345 | 9.4 × 10−7 | 1.1 × 10−1 | 1.7 × 10−6 | 1 | 1 | 1 | 1 | 1 | |
| IL-6 | 0.71 | 0.778 | 0.332 | 0.587 | 0.368 | 0.958 | 0.741 | 0.808 | 0.612 | 1.0 × 10−10 | 6.5 × 10−16 | 1 | 1 | 2.4 × 10−11 | 1 | 1.8 × 10−2 | |
| IL-1β | 0.678 | 0.654 | 0.481 | 0.666 | 0.505 | 0.744 | 0.699 | 0.766 | 0.381 | 0.716 | 3.5 × 10−12 | 1.7 × 10−1 | 1 | 5.9 × 10−5 | 1 | 1 | |
| TNFα | 0.709 | 0.777 | 0.656 | 0.814 | 0.646 | 0.862 | 0.891 | 0.649 | 0.607 | 0.809 | 0.749 | 5.6 × 10−3 | 1.3 × 10−1 | 1.9 × 10−3 | 1 | 1 | |
| IFNγ | 0.201 | 0.146 | 0.288 | 0.853 | 0.614 | 0.232 | 0.463 | 0.148 | 0.045 | 0.222 | 0.369 | 0.461 | 7.9 × 10−56 | 1 | 1 | 1 | |
| IL-10 | 0.147 | 0.068 | 0.155 | 0.802 | 0.563 | 0.167 | 0.369 | 0.066 | 0.032 | 0.165 | 0.299 | 0.38 | 0.986 | 1 | 1 | 1 | |
| IL-12a | 0.449 | 0.439 | 0.192 | 0.255 | 0.252 | 0.705 | 0.42 | 0.821 | 0.295 | 0.733 | 0.551 | 0.487 | 0.01 | −0.053 | 1 | 1 | |
| IL-13 | 0.197 | 0.108 | 0.054 | 0.005 | 0.002 | 0.019 | 0.071 | −0.038 | 0.024 | −0.069 | 0.156 | 0.014 | −0.012 | −0.014 | −0.140 | 1 | |
| IL-4 | −0.141 | −0.204 | 0.027 | −0.334 | −0.128 | −0.355 | −0.247 | −0.259 | −0.265 | −0.433 | −0.237 | −0.313 | −0.238 | −0.254 | −0.186 | 0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, E.; Gilliaux, G.; Sarlet, M.; Desmecht, D.; Van Laere, A.-S. Host-Strain-Specific Responses to Pneumonia Virus of Mice Infection: A Study of Lesions, Viral Load, and Cytokine Expression. Viruses 2025, 17, 548. https://doi.org/10.3390/v17040548
Levy E, Gilliaux G, Sarlet M, Desmecht D, Van Laere A-S. Host-Strain-Specific Responses to Pneumonia Virus of Mice Infection: A Study of Lesions, Viral Load, and Cytokine Expression. Viruses. 2025; 17(4):548. https://doi.org/10.3390/v17040548
Chicago/Turabian StyleLevy, Etienne, Gautier Gilliaux, Michaël Sarlet, Daniel Desmecht, and Anne-Sophie Van Laere. 2025. "Host-Strain-Specific Responses to Pneumonia Virus of Mice Infection: A Study of Lesions, Viral Load, and Cytokine Expression" Viruses 17, no. 4: 548. https://doi.org/10.3390/v17040548
APA StyleLevy, E., Gilliaux, G., Sarlet, M., Desmecht, D., & Van Laere, A.-S. (2025). Host-Strain-Specific Responses to Pneumonia Virus of Mice Infection: A Study of Lesions, Viral Load, and Cytokine Expression. Viruses, 17(4), 548. https://doi.org/10.3390/v17040548






