Abstract
People living with HIV (PLWH) develop cardiovascular diseases (CVDs) about a decade earlier and at rates 2–3 times higher than the general population. At present, pharmacological strategies to delay the onset of CVDs in PLWH are unavailable, in part because of an incomplete understanding of its molecular causes. We and others recently uncovered elevated levels of the toxic glycolysis and inflammation-induced byproduct methylglyoxal (MG) in plasma from PLWH and from HIV-infected humanized mice (Hu-mice). We also found a reduction in expression of the primary MG-degrading enzyme glyoxalase I (Glo-I) in autopsied cardiac tissues from HIV-1-infected individuals and HIV-1-infected Hu-mice. Increasing the expression of Glo-I in HIV-1-infected Hu-mice not only attenuated heart failure but also reduced endothelial cell damage, increased the density of perfused microvessels, prevented microvascular leakage and micro-ischemia, and blunted the expression of the inflammation-induced protein vascular protein-1 (VAP-1), key mediators of CVDs. In this narrative review, we posit that elevated MG is a contributing cause for the early onset of CVDs in PLWH. Pharmacological strategies to prevent MG accumulation and delay the development of early-onset CVDs in PLWH are also discussed.
1. Introduction
In 2014, the United Nations General Assembly’s Declaration on Ending the AIDS epidemic committed countries to the 95–95–95 targets. Two key elements of this declaration were to provide HIV testing and treatment for 90% of people infected with HIV-1 by the end of 2024 [1], and significant progress has been made in achieving these goals globally [2,3,4,5,6,7,8,9]. However, new challenges have emerged, as people with HIV-1 infection on antiretroviral drugs (ARDs) are developing cardiovascular diseases (CVDs) at least a decade earlier than that of the general population and at rates 2–3 times higher [10,11,12,13,14]. Not surprisingly, the prevalence of CVDs is also higher in women living with HIV-1, since female sex is a risk factor for CVDs [9,10,11,12,13,14,15,16,17]. Starting ARDs soon after HIV-1 diagnosis and without interruption [18,19,20]; reducing behavioral/lifestyle risk factors, including smoking, illicit drug use, and alcohol; and treating health risks, including metabolic syndrome, dyslipidemia, hypertension, and renal dysfunction, soon after diagnosis have delayed the onset of CVDs and blunted major adverse cardiovascular events, including acute myocardial infarction, stroke, peripheral artery disease, and sudden cardiac death, in PLWH [18,19,20,21,22,23]. However, why middle-aged HIV-1-infected individuals on ARDs are developing early-onset CVDs remains puzzling.
To date, studies attribute early-onset CVDs in PLWH to “premature/accelerated aging” arising from persistent activation of immune system and inflammation by HIV-1 proteins and dead cell fragments and chronic ARD use [24,25,26]. However, pharmacological strategies to suppress immune activation and inflammation have yielded mixed results, probably because several mechanisms contribute to immune activation and inflammation, and individually targeting each of these inflammatory pathways is not adequate to completely suppress immune activation and inflammation. Moreover, while ARDs efficiently suppress plasma HIV-1 viremia, the tissue distribution of the various classes of ARDs varies, differentially impacting immune activation, inflammation, and oxidative stress in the various tissues/organs. Improvements in tissue bioavailability by altering routes of administration, dosing frequencies, and longer durations of action have also been tested to address these deficiencies [14,27,28,29,30]. These and other data, led others to conclude that the degree of cardiovascular risk in HIV-1 infection cannot be fully explained by persistent immune activation, inflammation, and health- and lifestyle-related risk factors. Unidentified factor(s) are likely playing roles in the development of early-onset CVDs in PLWH [14]. Recently, Ntsekhe and Baker suggested that clonal hematopoiesis of indeterminate potential (CHIP) and the expansion of somatic mutations in leukocytes could worsen the absolute burden of excess CVD risks among PLWH with advancing age [31]. However, since CHIP is age-dependent, it is unlikely to be the underlying cause of early-onset CVDs in PLWH.
We and others recently uncovered elevated levels of the toxic glycolysis and inflammation-induced byproduct methylglyoxal (MG) in plasma from people living with HIV and HIV-infected humanized mice (Hu-mice) [32,33]. We also found decreased levels of the primary MG-degrading enzyme glyoxalase I (Glo-I) in autopsied cardiac tissues from HIV-1 infected individuals and HIV-1 infected Hu-mice. Increasing expression of Glo-I in HIV-1-infected Hu-mice not only blunted heart failure development but also minimized endothelial cell damage, increased the density of perfused microvessels, and prevented microvascular leakage and micro-ischemia [33]. To our surprise, increasing Glo-I expression in HIV-1-infected Hu-mice also decreased expression of inflammation-induced and MG-synthesizing enzyme vascular adhesion protein-1 (VAP-1), suggesting a link between elevated MG, inflammation, and CVDs. In this narrative review, we propose that elevated levels of MG could be an elusive risk factor that is contributing to early-onset CVDs in PLWH. This increase in MG in HIV-1 infection is arising from an increase in its synthesis (via glycolysis and upregulation of VAP-1) and from a decrease in its degradation due to the downregulation of MG-degrading enzymes and co-factors. In this brief review, we also discuss pharmacological strategies to lower MG levels that could blunt early-onset CVDs in PLWH.
2. Life Cycle of HIV
HIV-1 primarily infects cells of the immune system that contain the CD4 protein, including CD4+-T cells, monocytes, macrophages, dendritic cells, and microglia [34,35,36]. HIV-1 DNA has also been found inside other cells, including epithelial cells (renal and gut), endothelial cells, cardiac myocytes, astrocytes, smooth muscle cells, and pericytes. However, the extent to which the latter cell types support the productive replication of HIV-1 remains unclear [37,38,39,40,41]. HIV-1 also preferentially infects activated CD4+-T cells over quiescent CD4+-T cells, since activated cells have higher levels of Glut1, higher levels of glycolysis, and increased levels of the HIV-1 co-receptor CCR5 on their plasma membranes [42,43]. For cellular entry, the HIV-1 envelope glycoproteins gp120 and gp41 attach themselves to the CD4 protein, and the co-receptors CCR5/CXCR4 on the plasma membrane of the cell. After entry, HIV-1 capsid uncoats and releases HIV-1 RNA, reverse transcriptase, and HIV-1 integrase into the cytoplasm of the cell. The reverse transcriptase converts the HIV-RNA into HIV-1 DNA, which then enters the nucleus and integrates with the DNA of the infected cell (Figure 1; left side) [44]. HIV subtypes that use CCR5 for cell entry are termed the R5 HIV subtype, those that use CXCR4 are termed the X4 HIV subtype, and those that use both co-receptors are called R5X4 HIV [45,46]. CCR5 is expressed on memory T lymphocytes, macrophages, microglia, dendritic cells, and vascular smooth muscle cells [47], while CXCR4 is found on astrocytes, pericytes, cardiac myocytes, and endothelial cells [48,49]. After integration, HIV-1 genes (Gag and GagPol precursor polyproteins, viral envelope glycoproteins, and regulatory and accessory viral proteins) are transcribed in the Golgi and exported to the cytoplasm and plasma membrane for assembly and release (Figure 1; right side) [44]. Every step of these processes requires energy from the infected cell.
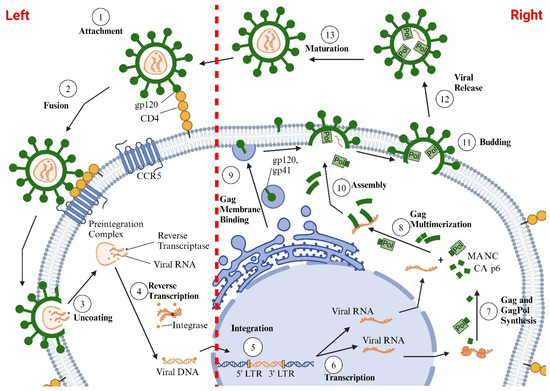
Figure 1.
Illustration of the HIV-1 life cycle, which is divided into two main phases: an early phase and a late phase. The early phase consists of several sequential steps: (i) HIV initially attaches to the cell via its envelope glycoproteins, gp120 and gp41, which bind to the CD4 receptor on the cell surface; (ii) gp120 and gp41 then engage with chemokine receptors, CXCR4 and CCR5, facilitating fusion with the cell membrane and viral entry; (iii) once inside, the HIV-1 capsid uncoats in the cytoplasm, releasing HIV-1 RNA, reverse transcriptase (to convert HIV RNA into HIV DNA), and integrase (to integrate HIV DNA into the host cell’s DNA) (left side). The late phase includes the steps following the integration of HIV-1 DNA into the host DNA: (i) transcription of HIV genes; (ii) export of HIV-1 RNAs from the nucleus to the cytoplasm and translation of these RNAs to produce Gag and GagPol precursor polyproteins, envelope glycoproteins, and regulatory and accessory proteins; (iii) transport of Gag, GagPol, and envelope glycoproteins to the plasma membrane; (iv) assembly of the Gag and GagPol polyproteins on the host cell’s plasma membrane; (v) encapsidation of the viral RNA genome by the forming Gag lattice; (vi) incorporation of viral Env glycoproteins; and (vii) budding of new virions from the host cell, followed by particle maturation (right side).
3. Energy Substrates
Glucose, glutamine, and fatty acids are the primary substrates utilized by immune cells (CD4+T, monocytes, macrophages, dendritic cells, and microglia); vascular cells (endothelial cells, smooth muscle cells, and pericytes); lymphatic cells (lymph endothelial cells and lymphatic smooth muscles); cardiac myocytes; and astrocytes to generate the ATP [50,51,52,53]. Resting immune cells, vascular cells, cardiac myocytes, and astrocytes take up glucose via plasma membrane glucose transporter proteins, including Glut1, and via glycolysis, convert each glucose molecule into two molecules of pyruvate. Two molecules of adenosine triphosphate (ATP) are also generated during the process. In oxygen-rich environments, pyruvate is shuttled to the tricarboxylic acid (TCA) cycle of the mitochondria to generate NADH and FADH2 that fuel oxidative phosphorylation (OXPHOS) in the electron transport chain. In mitochondria, each molecule of pyruvate (in the presence of oxygen) generates 16 molecules of ATP (Figure 2) [51,52]. It should be mentioned that glycolysis produces ATP 100× faster than OXPHOS [53]. Alpha-ketoglutarate (α-KG) is another substrate that is utilized by the TCA cycle for the production of NADH/FADH2 and ATP [52]. α-KG is generated from the breakdown of glutamine, the most abundant circulating amino acid by two sequential enzymatic reactions. Each molecule of α-KG that enters the TCA cycle generates 24 ATP molecules [52]. Beta oxidation of fatty acids also generates and shuttles acetyl CoA to the TCA cycle to generate NADH and FADH2.
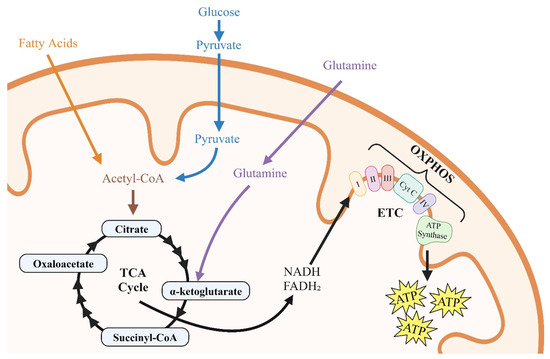
Figure 2.
Illustration of the roles of glycolysis, fatty acids, and glutamine in cellular metabolism. In CD4+-T cells, glucose uptake initiates glycolysis, producing two molecules of ATP and two molecules of pyruvate. The pyruvate is then transported into the mitochondria, entering the tricarboxylic acid (TCA) cycle, where it generates NADH and FADH2. These molecules drive oxidative phosphorylation (OXPHOS) and the electron transport chain (ETC), resulting in the production of up to 16 molecules of ATP per molecule of pyruvate.
Studies have shown that glucose uptake and glycolysis are upregulated in infected CD4+-T cells to rapidly provide the substrates needed for HIV-1 integration, replication, assembly, and release [42,54,55]. Others have also shown that glycolysis remains elevated in CD4+-T cells isolated from PLWH with low HIV-1 viremia [56]. Following infection, glucose is shunted from glucose-storing organs like skeletal muscles and the liver to accommodate the high glucose demand needed for HIV-1 replication [57]. Naïve CD4+-T cells constantly traffic between the blood, peripheral tissues, and lymphatic system in search of foreign antigens. After infection, these cells are responsible for the establishment of HIV-1 reservoirs in lymph nodes [58]. These lymph node reservoirs are usually maintained due to the clonal expansion of latently infected CD4+-T cells and limited ARD penetrance [59].
4. Methylglyoxal Production
In addition to generating ATP, pyruvate, and other substrates needed for HIV-1 replication, glycolysis also generates the diffusible and highly reactive α-oxoaldehyde methylglyoxal (MG) from the breakdown of the triose intermediates, glyceraldehyde 3-phosphate (G-3-P), and dihydroxyacetone phosphate (DHAP) [60,61]. About 0.1% of all glucose flux is converted into MG [62]. Glycolytic bottlenecks, arising from a reduction in GAPDH and impairment of pathways, that typically shunt glycolytic intermediates, including the hexose monophosphate shunt and polyol pathway, also result in higher levels of G-3-P and DHAP and, by extension, more MG production [63,64]. MG is also synthesized from the breakdown of aminoacetone by the inflammation-induced MG-generating ectoenzyme vascular adhesion protein 1 (VAP-1) [65,66]. The latter is especially important, since VAP-1 is upregulated in vasculature smooth muscle cells (SMCs) during inflammation, resulting in a localized source of MG in proximity to vascular endothelial cells (ECs) (Figure 3) [67,68]. Earlier, we reported that ECs have half as much of Glo-I per μg of cells compared to SMCs [68]. Smaller amounts of MG are also generated from fatty acid oxidation; however, the extent to which this occurs in HIV-1 infection with and without ART remains poorly characterized.
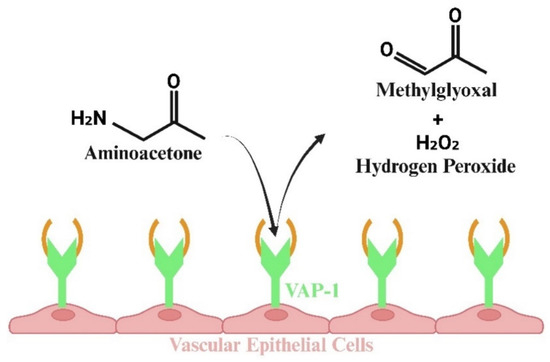
Figure 3.
Illustration showing that HIV replication results in the synthesis of methylglyoxal (MG) via the glycolysis pathway. Additionally, MG is produced by the inflammation-induced ectoenzyme vascular adhesion protein 1 (VAP-1) through the breakdown of aminoacetone. This process is particularly important, as VAP-1 is upregulated in the vasculature, particularly in vascular smooth muscle cells, leading to elevated localized concentrations of MG near vascular endothelial cells.
5. Degradation of Methylglyoxal
In eucaryotes, MG is degraded via three mechanisms: the glyoxalase system (glyoxalase I, Glo-I, and glyoxalase I, Glo-II), with co-factor-reduced glutathione (GSH) and water [69]; aldehyde dehydrogenases (ALDHs; oxidation reaction), with nicotinamide adenine dinucleotide as a co-factor; and aldo-keto reductases, with GSH and/or nicotinamide adenine dinucleotide phosphate as co-factors [69,70]. The expression of Glo1, ALDHs, and AKR and the enzymes that synthesize their co-factors are regulated by the antioxidant transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2)–antioxidant response element (ARE) [71,72,73,74]. It should be mentioned that Glo-I/Glo-II are more widely distributed than ALDHs and AKRs, and the kinetics of degradation of MG by Glo-I/Glo-II are faster. Studies have also linked the downregulation/knockout of these enzymes to impairment in cardiovascular functions [75,76,77].
5.1. MG Degradation via Glyoxalase System
The primary pathway for the degradation of MG is the two-enzyme glyoxalase system. In the first step, the rate-limiting Glo-I converts a hemithioacetal formed between MG and reduced glutathione (MG-GSH) into S,D-lactoylglutathione [69]. In the second step, Glo-II, in the presence of water, degrades S,D-lactoylglutathione into D-lactic acid and GSH (Figure 4). Giacco et al. showed that the knockdown of Glo-I mimics diabetic nephropathy in nondiabetic mice [77]. Dobariya et al. showed that the deletion of Glo-I exacerbates acetaminophen-induced hepatotoxicity in mice [78]. The knockdown of Glo-I in mouse aortic endothelial cells resulted in impairment of angiogenesis [79]. Glo1 knockout reduces anxiety-like behavior but increases depression-like behavior in mice [75]. Others have shown that overexpression of Glo-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress and improves neurovascular coupling and endothelial dysfunction in diabetic rats [80,81]. Others have shown that Glo-I knockout in zebrafish results in upregulated ALDH activity [82]. In yeast, the loss of Glo1 results in hypersensitivity to MG and death [83].
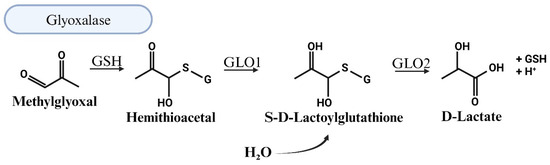
Figure 4.
Illustration of the detoxification of methylglyoxal (MG) through the glyoxalase system. The main pathway for methylglyoxal (MG) degradation involves the two-enzyme glyoxalase system. In the initial step, the rate-limiting enzyme glyoxalase-1 (Glo1) catalyzes the conversion of a hemithioacetal formed between MG and reduced glutathione (MG-GSH) into S,D-lactoylglutathione. Subsequently, the second enzyme, glyoxalase-2 (Glo2), acts in the presence of water to degrade S,D-lactoylglutathione into D-lactic acid and glutathione (GSH).
Earlier, we reported downregulation of Glo1 in the hearts of humanized mice infected with HIV-1 (NOD.Cg-Prkdc(scid)Il2rg(tm1Wjl)/SzJ), in plasma from PLWH, and in autopsied cardiac tissues from deceased HIV seropositive individuals [33]. These findings should not come as a surprise, since several groups have reported the downregulation of Nrf2 in HIV-1 infection [84,85,86,87]. For example, Fan et al. showed that HIV-1-related proteins downregulate Nrf2 expression and/or activity within the alveolar epithelium, which in turn impairs antioxidant defenses and barrier function, rendering the lungs susceptible to oxidative stress and injury [84]. Han et al. also found that Nrf2 activation blocks HIV replication in macrophages before the integration phase and inhibits the expression of apoptotic pathways in virus-exposed macrophages [85]. Staitieh et al. also showed that HIV-1 infection and exposure to HIV-1-related proteins inhibit Nrf2–ARE activity in alveolar macrophages and impair their phagocytic function [86]. Davinelli et al. also found a significant reduction in the protein levels of Nrf2 and hemooxygenase-1 (HO-1) in HIV-1 transgenic rats, suggesting a weakening in the protection exerted by the Nrf2/HO-1 system [87].
5.2. MG Degradation via Oxidation
ALDHs are another class of enzymes that is responsible for the NAD(P)-dependent oxidation of aldehydes, including MG, to carboxylic acids [69]. The E1, E2, and E3 isozymes of ALDHs oxidize MG into pyruvate in a NAD-dependent manner (Figure 5). Murine models with a loss of ALDHs have reported elevated aldehydic adducts, cardiovascular and motor dysfunction, and tissue damage. Single-nucleotide polymorphisms in ALDH2 and mutations in ALDH1/2 are also linked to increases in risk of CVDs [76,88]. Conversely, overexpression of ALDHs attenuates the effects of oxidative stress and reactive oxygen species (ROS) in various organs, conditions that are often upregulated in response to MG accumulation [89,90]. The knockout of the Glo1 protein in zebrafish also results in ALDH activity. However, MG levels remain moderately elevated in Glo1 knockout zebrafish, suggesting that ALDHs and AKRs are not sufficient replacements for Glo1 [82]. Vander Jagt and Hunsaker indicated that ALDHs and AKRs have lower affinities for MG compared to Glo1 [70]. To date, the effects of HIV-1 on the expression of ALDHs remain poorly characterized.
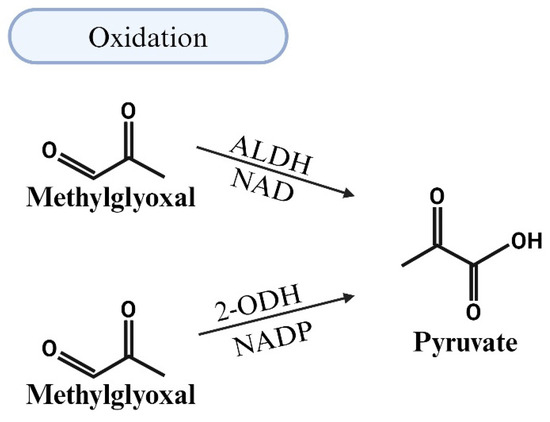
Figure 5.
Illustration of the detoxification of methylglyoxal (MG) through oxidation by aldehyde dehydrogenases (ALDHs). This class of enzymes is responsible for the NAD(P)-dependent oxidation of aldehydes, including MG, into carboxylic acids. ALDHs facilitate the oxidation of MG to pyruvate in a NAD-dependent manner.
5.3. MG Degradation via Reduction
AKRs are a group of enzymes that reduce aldehydes and ketones into their corresponding alcohols [91,92]. AKR-mediated MG degradation operates through two pathways: a GSH-dependent pathway, where the hemithioacetal formed between GSH and MG is converted by an AKR and NADPH to lactaldehyde, and a GSH-independent pathway, where MG reacts with the AKR and NADPH to form acetol [92,93]. Further metabolism of lactaldehyde and acetol by the AKR leads to the production of propanediol (Figure 6). In the presence of GSH, the efficiency of reduction in MG by ALDHs increases but the site of reduction switches from the aldehyde to the ketone carbonyl. The latter arises because glutathione converts ALDHs from an aldehyde reductase to a ketone reductase [94].
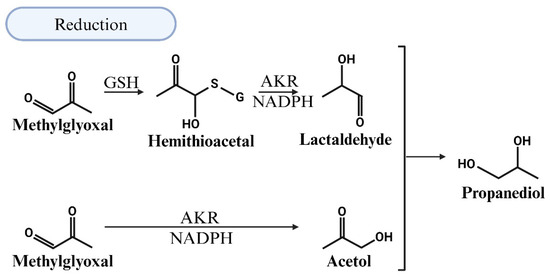
Figure 6.
Illustration of the detoxification of methylglyoxal (MG) through reduction in the presence of reduced glutathione (GSH). The efficiency of MG reduction by aldehyde dehydrogenases (ALDHs) increases, but the reduction site shifts from the aldehyde to the ketone carbonyl. This shift occurs because glutathione modifies ALDHs, converting them from aldehyde reductases to ketone reductases. Further metabolism of the resulting lactaldehyde and acetol by aldo-keto reductase (AKR) leads to the production of propanediol.
AKRs can also increase MG levels by increasing the production of the triose phosphate intermediate dihydroxyacetone phosphate [93,94]. Aldose reductase is the first enzyme of the polyol pathway, and it reduces glucose to sorbitol, which can then be converted to fructose by sorbitol dehydrogenase. Fructose is then converted to fructose-1-phosphate and, eventually, to DHAP and MG (Figure 7). To date, the effects of HIV-1 on the expression of AKRs remain poorly characterized.
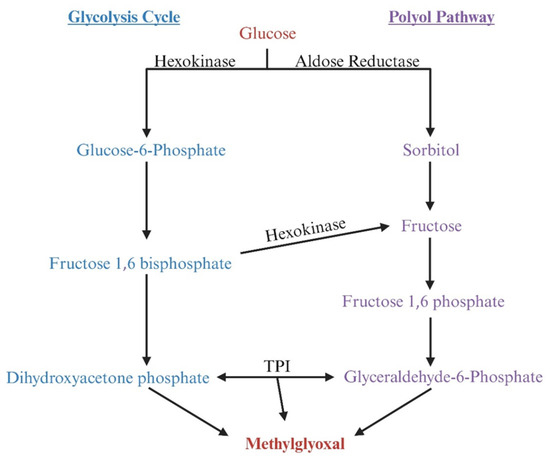
Figure 7.
Illustration demonstrating that aldo-keto reductase (AKR) can degrade methylglyoxal (MG) but also indirectly promotes MG production by increasing the production of the triose phosphate intermediate dihydroxyacetone phosphate (DHAP). Aldose reductase (AR), the first enzyme in the polyol pathway, reduces glucose to sorbitol, which is subsequently converted to fructose by sorbitol dehydrogenase. Fructose is then phosphorylated to fructose-1-phosphate by ketohexokinase, which is further converted to DHAP by fructose bisphosphate aldolase. Alternatively, fructose can be phosphorylated to fructose-6-phosphate by hexokinase, which is then converted to fructose-1,6-bisphosphate by phosphofructokinase-1; this compound is subsequently converted to DHAP by fructose bisphosphate aldolase. DHAP can yield MG.
6. Consequences of Elevated MG
Under non-oxidative/non-inflammatory environments, the majority of MG generated in cells is reversibly bound to the SH moieties on GSH and cysteine residues of proteins [95]. The hemithioacetal formed between MG and GSH (MG-GSH) is also targeted for degradation by Glo-I. Under oxidative/inflammatory conditions, as is the case in HIV-1 infection, where Nrf2 expression is downregulated and steady state levels of Glo-I and GSH are reduced, MG will accumulate. This MG will irreversibly react with accessible basic moieties on proteins, DNA/RNA, and lipids. On proteins, MG reacts with arginine, lysine, and histidine residues. On arginine residues, the primary adduct formed is the hydroimidazolone MG-H1 (>90% of all MG adducts). Lesser amounts of MG-H2, MG-H3, [96] and Nδ-(5-hydroxy-4,6-dimethylpyramidine-2-yl)-l-ornithine argpyrimidine (AP) have also been detected [97] (Figure 8). When MG reacts to lysine residues, Nε-(1-carboxyethyl) lysine (CEL) and the lysine dimer 1,3-di(Nε-lysino)-4-methyl-imidazolium (MOLD) are formed [98]. These adducts have been identified on proteins in the cytoplasm, and inside the Golgi, mitochondria, lysosomes, and endoplasmic reticulum, as MG, can diffuse from the cytoplasm into these organelles. NH moieties on certain lipids, including ceramide and sphingosine, may also be chemically modified by MG. MG will also diffuse from the cytoplasm into the nucleus to form adducts with basic moieties on DNA. The N2 position of the deoxyguanosine (dG) nucleotide is most susceptible to MG modification, leading to the formation of dihydroimidazolone, N2-(1,2-dihydroxy-2-methyl) ethano-dG (cMG-dG) and N2-(1-carboxyethyl)-7-1-hydroxy-2-oxopropyl-dG (MG-CEdG) [99]. MG-CEdG is a good marker for long-term MG accumulation. No enzymes have been identified in eukaryote cells that are capable of breaking MG adducts after they are formed.
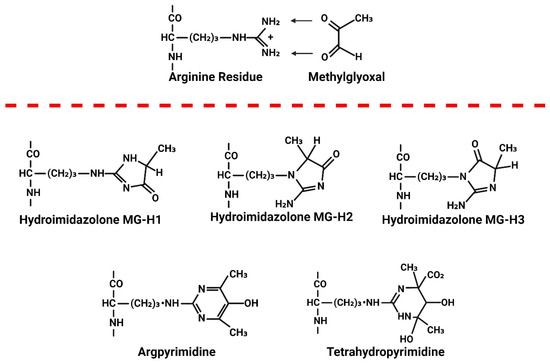
Figure 8.
Illustration showing the formation of methylglyoxal (MG) adducts on proteins. When MG levels are elevated, it irreversibly reacts with accessible arginine, lysine, and histidine residues on proteins. This reaction with arginine results in the formation of three hydroimidazolone adducts: MG-H1, MG-H2, and MG-H3. Methylglyoxal (MG) also reacts with arginine to form Nδ-(5-hydroxy-4,6-dimethylpyrimidine-2-yl)-L-ornithine, known as argpyrimidine (AP), and Nδ-(4-carboxy-4,6-dimethyl-5,6-dihydroxy-1,4,5,6-tetrahydropyrimidine-2-yl)-L-ornithine, known as tetrahydropyrimidine (THP).
The Link Between Elevated MG and CVDs in HIV-1 Infection
Heart failure, essential hypertension, pulmonary hypertension, and stroke are common CVDs in PLWH [100,101,102,103]. Studies suggest that these diseases are due, in part, to the dysregulation of vasculature ECs and the build up of plaques inside arteries arising from immune activation, inflammation, oxidative stress, and certain classes of ARDs. To date, clinical trials to suppress immune activation and inflammation to suppress CVDs have yielded mixed results, in part because of an incomplete understanding of their molecular triggers [104,105,106,107,108,109,110,111,112]. As a result, pharmacological strategies to protect the endothelium and reduce arterosclerosis in PLWH remain limited.
We and others showed earlier that exposing vascular ECs to MG reduces the expression of tight junction proteins and induced apoptosis. Bathing cerebral pial arterioles of anesthetized rats with MG decreased their response to an EC-mediated vasodilator [68]. Exposing ECs to MG also decreases the phosphorylation of eNOS at Ser1177, causing eNOS to switch from the generation of nitric oxide (NO) to superoxide anion [113]. The dysregulation of vascular ECs will lead to microvascular leakage, a reduction in the density of perfused microvessels, and micro-ischemia [68,114,115,116]. Tissue micro-ischemia will induce the expression of hypoxia-inducible factor 1-alpha (HIF-1α) to upregulate glycolysis genes, MG production, and tissue fibrosis. Others have shown that MG will (i) induce lipid oxidation and accumulation in macrophages (foam cell formation) [117]; (ii) trigger the proliferation of vascular smooth muscle cells (vSMCs), decreasing blood vessel diameter [118]; (iii) induce intima thickening and increase arterial wall stiffness [119,120]; and (iv) induce platelet hyperaggregation and reduce thrombus stability [121]. Thus, elevated MG could be contributing to atherosclerosis plaque buildup and hypertension in PLWH.
MG is an established activator of multiple inflammation pathways, including NOD-like receptor protein inflammasomes, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), activator protein-1 (AP-1), and the receptor for advanced glycation end products (RAGE) [101,122,123,124,125,126,127,128,129]. These data could help explain why mono-pharmacology to attenuate inflammatory mediators, including interleukin-1β (IL-1β) and tumor necrotic factor-alpha (TNF-α), have yielded mixed results. Our group also showed that MG adducts can impair cardiac contraction and relaxation by forming adducts on the type 2 ryanodine receptor calcium release channel (RyR2), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA 2), and myosin heavy chains (MHC—alpha and beta) [130,131,132]. These adducts dose-dependently increase and then reduce the activity of RyR2, triggering the depletion of Ca2+ from the sarcoplasmic reticulum (SR), delaying the return of Ca2+ into the SR, and decreasing the rate and force of myocyte contraction. Others have shown that MG can impair the function of other ion channels, including Na+ and K+ channels. We also showed that elevated MG was responsible, in part, for exacerbated ischemia–reperfusion injury in the brains of rats following mid-cerebral artery occlusion [68]. Although the specific reason for this was not clear, we suspect that MG increases ROS generation in mitochondria via complex I and complex III. Studies have linked elevated MG to kidney disease, certain cancers, retinopathy, and sepsis [20,130,133,134,135,136,137,138,139,140,141,142]. Recent data suggest that elevated MG may also contribute to the high incidence of pulmonary arterial hypertension (PAH) and right ventricular dysfunction in PLWH by increasing arterial stiffening via the cross-linking of collagen within the extracellular matrix (ECM) [143].
7. Therapeutic Strategies to Lower Methylglyoxal Levels
Recently, we and others uncovered elevated MG in the plasma of PLWH, autopsied tissues from HIV-infected individuals, and tissues and plasma from HIV-1-infected Hu-mice [32,33]. We also found an increase in the expression of the inflammation-induced and MG-synthesizing enzyme VAP-1 and a decrease in the expression of Glo-I in autopsied cardiac tissues from HIV-1-infected individuals and HIV-1-infected Hu-mice. We also showed that increasing the expression of Glo-I in HIV-1-infected Hu-mice using a custom-engineered adeno-associated virus designed to express Glo-I under inflammatory/oxidative conditions not only blunted heart failure, but it also attenuated cardiac endothelial damage, increased the density of perfused microvessels, attenuated microvascular leakage and micro-ischemia, and decreased the expression of VAP-1. The latter is consistent with a reduction in inflammation [33]. Taken collectively, these data suggest that lower MG could attenuate early-onset CVDs in PLWH.
Several strategies have been tested in preclinical models and clinical trials to lower MG, attenuate inflammation, and blunt CVDs in other diseases. These include treatment with agents that contain nucleophilic moieties (arginine/arginine analogs) to which MG can preferentially react and prevent the formation of adducts on proteins, lipids and DNA; treatment with agents to prevent or reduce the formation of MG by diverting early glycolysis substrates to the polyol and other pathways; treatment with agents that can increase the expression of Nrf2 and MG-degrading enzymes; and treatment with agents that increase the levels of the co-factors needed by MG-degrading enzymes, including reduced glutathione, nicotinamide adenine dinucleotide, and nicotinamide adenine dinucleotide phosphate, including polyphenols and flavonoids/isoflavonoids (Figure 9 and Table 1). Recently, we also replaced the endogenous promoter of AAV2/9 with the promoter of preproendothelin1 to be able to drive the expression of Glo-I under inflammatory conditions [33,68,144].
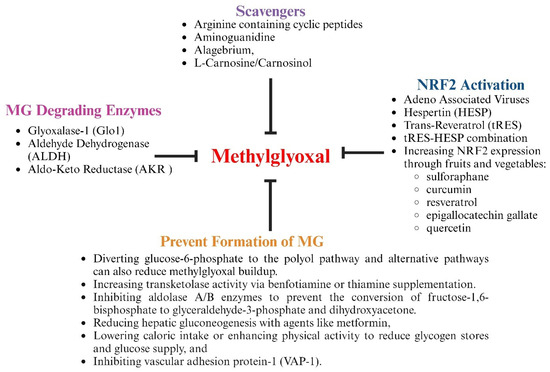
Figure 9.
Illustration showing pharmacological strategies to blunt MG accumulation.

Table 1.
Therapeutic strategies to lower methylglyoxal levels.
7.1. Treatment with Agents Containing Nucleophilic Groups to Scavenge MG
MG is an electrophile and, when elevated, chemically reacts with amino moieties on proteins, lipids, and DNA/RNA to form adducts. Agents that contain amino moieties were tested in animal models to determine their ability to scavenge MG. Agents tested in diabetes models include aminoguanidine, pyridoxamine, L-carnosinol, and arginine-containing linear and cyclic peptides. Aminoguanidine was shown to lower AGE formation and prevent diabetic nephropathy retinopathy, neuropathy, and heart failure in preclinical models but was not as less effective in clinical trials [166]. Pyridoxamine, a naturally occurring B vitamin, was also found to attenuate diabetic nephropathy and preserves renal function [145,146] in rodent models of diabetes and patients with Type 2 diabetes. It also decreased the formation of AGEs, inflammation, and pain in patients with osteoarthritis. The naturally occurring histidine dipeptide l-carnosine also lowers MG levels in rodent models of metabolic syndrome and normalized insulin sensitivity, insulin secretion, and glucose tolerance in overweight and obese subjects [147,167,168,169]. However, its therapeutic potential is limited due to a rapid hydrolysis of the peptide by carnosinases. Carnosinol, a derivative of L-carnosine that is resistant to metabolism by carnosinase [147,148], dose-dependently reduced carbonyl stress, normalized glycemic control, and mitigated inflammation and steatohepatitis [147]. The 40-amino acid arginine-containing peptide (GERP10) lowered MG. However, GERP10 was rapidly degraded by endogenous peptidases. To improve the half-life, the cyclic arginine-containing peptide CycK(Myr)R4E was developed [149]. CycK(Myr)R4E was found to have comparable MG scavenging activity as GERP10 and prevented MG-induced adducts and pain in mice [149]. The widely used antidiabetic agent metformin contains a guanidine, and its ability to scavenge MG could help explain, in part, its ability to reduce vascular complications in animal and clinical studies [170]. A major limitation of MG-scavenging molecules is that their use must be continuous, as they are unlikely to get rid of the underlying inflammation and oxidative stress. Because high does of these agents are needed, additional benefits could be obtained from non-MG scavenging mechanisms. Inadequate biodistribution/bioavailability could limit the usefulness of MG-scavenging approaches.
7.2. Treatment with Agents to Prevent or Reduce the Formation of MG
Benfotiamine is an activator of transketolase, which directs glucose-6-phosphate to the pentose phosphate pathway (hexose monophosphate shunt). Studies have shown that treatment of diabetic rodents with benfotiamine decreased plasma MG and MG-derived glycation and microvascular complications [152]. In individuals with Type 2 diabetes, treatment with a high dose of thiamine (the parent compound) both prevented and reversed early-stage diabetic nephropathy [171]. Benfotiamine also attenuated NF-κB activation [150]. Since activated NF-κB suppresses Nrf2 mRNA expression, reducing NF-κB activation could enhance Nrf2 levels and the expression of antioxidant enzymes. Wu and colleagues showed earlier that the upregulation of aldolase B, a key enzyme in fructose metabolism, increased vascular MG production [172]. They also showed that insulin-enhanced MG overproduction in insulin-sensitive adipocytes was due to upregulating aldolase A. Thus, it is possible that the inhibition of aldolase A and aldolase B could suppress MG production and CVDs. Caloric restriction and exercise training have also been shown to reduce MG and improve vascular function in individuals with Type 2 diabetes and diabetic rats [153,154,155]. The MG-lowering effect of caloric restriction and exercise was attributed to better metabolic control. Studies have also shown that metformin suppresses MG production by reducing hepatic gluconeogenesis.
VAP-1 is an inflammation-induced enzyme that catalyzes the deamination of aminoacetone to produce MG, hydrogen peroxide, and ammonia. Several VAP-1 inhibitors have been evaluated in preclinical and clinical studies, including MDL72974A, PXS-4728A, SZE5302, and LJP1586. These studies demonstrated that the inhibition of VAP-1 attenuated inflammation, intracerebral hemorrhagic stroke in mice, neurologic dysfunction in rats subjected to subarachnoid hemorrhage, atherosclerosis, diabetic macular edema, nephropathy and retinopathy, and ischemic reperfusion injury [156,157,158]. In diabetic rat models, the inhibition of VAP-1 attenuated the development of atherosclerotic lesions independent of serum glucose levels. There are also ongoing clinical studies evaluating the effects of the VAP-1 inhibitor on reducing inflammation associated with non-alcoholic steatohepatitis (TT-01025-CL) [159]; primary sclerosing cholangitis (BTT1023, a monoclonal antibody that blocks VAP-1) [173]; and chronic kidney disease in patients with Type 2 diabetes (ASP8232) [157]. However, to the best of our knowledge, MG levels or MG adducts in SMCs have not been measured in VAP-1 inhibition studies. Since the expression of VAP-1 is triggered by inflammation and many inflammatory pathways are activated in PLWH, including AP-1, NF-κB, NRLP inflammasomes, and the receptor for advanced glycation end products (RAGE), it is not clear whether VAP-1 inhibitors would be sufficient for lowering inflammation/oxidative stress in PLWH.
7.3. Treatment with Agents to Increase Expression of Nrf2 and MG-Degrading Enzymes
Nrf2 is the principal transcription factor that drives the expression of the antioxidant and MG-degrading enzymes Glo1, ALDHs, and AKRs and their co-factors. In healthy cells, Nrf2 is continuously synthesized and released into the cytoplasm. Keap1 is a thiol-rich E3 ubiquitin ligase that tightly regulates the activity of Nrf2 by binding to and ubiquitinating it for proteasome-dependent degradation. Under oxidative stress, the cysteine residues on Keap1 become oxidized, allowing Nrf2 to escape ubiquitination and translocate to the nucleus, and induces the expression of antioxidant genes.
Sulfur-containing compounds, polyphenols, and flavonoids/isoflavanoids, including sulforaphane (broccoli, Brussels sprouts, and kale); curcumin (turmeric); resveratrol (grapes, red wine, and berries); epigallocatechin gallate (green tea); and quercetin (apples, onions, and berries) (Figure 9) have been identified as “Nrf2 inducers” [160,161,162,174,175]. In cells, these nucleophiles are converted into electrophiles by metabolic enzymes, including polyphenol oxidases (PPOs) [176]. The “electrophiles” then covalently react with the cysteine residues in Keap1, causing it to dissociate from Nrf2 [177]. Nrf2 then translocates to the nucleus where it binds to the antioxidant response element (ARE) of DNA to trigger the expression of antioxidant genes that protect against oxidative stress. The perseverant tert-butylhydroquinone and the FDA-approved drugs dimethyl fumarate, used to treat sclerosis and psoriasis; bardoxolone methyl, used to treat chronic kidney disease and certain cancers; and carbamazepine, used to treat epilepsy and bipolar disorders, also dissociate Keap1 from Nrf2 [178,179]. A drawback of “Nrf2 enhancers” is that under inflammatory conditions, as is the case in HIV-1, activated NF-κB will suppress Nrf2 mRNA translation, resulting in a reduction in Nrf2 expression [180,181]. Thus, it is likely that the beneficial effects of Nrf2 enhancers will be significantly reduced over time in chronic inflammatory conditions. Another drawback could be the limited bioavailability and tissue distribution of polyphenols and flavonoids/isoflavanoids
Overexpressing ALDHs and AKRs has also been shown to attenuate MG levels [151,182]. Thornalley and colleagues also showed that treating overweight and obese subjects with trans-resveratrol and hesperetin (tRES-HESP) to induce the expression of Glo-I also attenuated MG. They also found that while the tRES-HESP combination reversed insulin resistance, this improvement was not seen in individuals treated with with tRES and HESP individually, suggesting that pharmacological synergism between tRES-HESP and that HESP was likely inhibiting intestinal glucuronosyl transferase [183]. Recently, we replaced the endogenous promoter of AAV2/9 with the promoter of preproendothelin1 and show that we can induce the expression of Glo-I under inflammatory conditions. Using this strategy, we were not only able to blunt heart failure in HIV-1-infected Hu-mice and a rat model of Type 1 diabetic rats but also attenuated cardiac endothelial damage, increased the density of perfused microvessels, attenuated microvascular leakage and micro-ischemia, and decreased the expression of VAP-1 [33,68,144].
7.4. Treatment to Increase Co-Factors for MG-Degrading Enzymes
Oxidative stress is elevated in PLWH due to impairment in the antioxidant defense system and from the downregulation of Nrf2 [184,185]. The downregulation of Nrf2 will not only decrease the expression of antioxidant enzymes but also decreases the levels of co-factors needed by MG-degrading enzymes, including reduced glutathione, nicotinamide adenine dinucleotide, and nicotinamide adenine dinucleotide phosphate, and enzymes involved in their synthesis, including a in reduction γ-glutamate cysteine ligase glutathione synthetase and NAD(P)H:quinone oxidoreductase 1 (NQO1) and nicotinamide N-methyltransferase and NAD kinase [186]. Several over-the-counter (OTC) antioxidant supplements are available either as a single agent or as a combination with antioxidants, including N-acetylcysteine (a precursor to GSH), vitamin C, vitamin E, lipoic acid, polyphenols, flavonoids/isoflavanoids, green tea extract, Coenzyme Q10, curcumin (turmeric), pomegranate, etc. Vegetable and fruit extracts that contain compounds that activate Nrf2 (see Section 7.3 above) are also available OTC. Selective FDA-approved drugs to lower CVD risks, such as statins and angiotensin receptor blockers, also have antioxidant properties [163,164]. Kumar et al. [165] found that PLWH treated with a combination of glycine and N-acetyl cysteine (GlyNAC) for 12 weeks improved several outcomes, including red-blood cell and muscle GSH concentrations, mitochondrial function, mitophagy and autophagy, oxidative stress, inflammation, endothelial function, genomic damage, insulin resistance, glucose production, muscle protein breakdown rates, body composition, physical function, and cognition. Juruga et al. [187] also found that supplementation of vitamins A, C, and E in PLWH decreased modified DNA bases and partially restored antioxidant enzymes (superoxide dismutase and catalase). In another study, Silva et al. [188] found serum triglyceride levels were increased after curcumin supplementation in PLWH. Erdos et al. [189] indicated that available data are not sufficient to conclude whether supplementation with glutathione would attenuate HIV-associated neurocognitive impairment in PLWH. Wilkinson et al. [190] also concluded that antioxidant micronutrients, alone or in combination, in PLWH have yielded mostly null results, with a few studies observing mild benefits of supplementation with zinc [191,192,193] and selenium [194,195,196]. The poor absorption of antioxidants from the gut, low levels of other micronutrients (selenium and zinc), and a low dose of the antioxidant may be reasons for variations in efficacies. It is also possible that the reduction in endogenously generated antioxidants is the consequence of other changes that are occurring in PLWH and that supplementation with exogenous antioxidants is not sufficient to alleviate the antioxidant deficit.
8. Conclusions
Highly active antiretroviral drugs have transformed HIV-1 infection from a literal death sentence to a chronic manageable disease. However, new challenges have emerged as PLWH are developing an array of early-onset CVDs for which the underlying causes remain poorly understood. Here, we posit that an elevation in glycolysis and the inflammation-induced byproduct MG could be an elusive risk factor that is triggering early-onset CVDs in PLWH. The increase in MG is triggering (i) a dysregulation of vascular endothelial cells and pericytes, resulting in microvascular leakage, a reduction in the density of perfused microvessels, micro-ischemia, and fibrosis; (ii) a proliferation of vascular smooth muscle cells that could decrease the blood vessel diameter, leading to hypertension; (iii) intima thickening, leading to an increase in arterial wall stiffness; (iv) lipid oxidation and accumulation in macrophages (foam cell formation), leading to vascular plaques; (v) platelet hyper-aggregation and reduced thrombus stability; (vi) impairment in cellular ionic homeostasis; and (vii) an enhancement in oxidative stress and inflammation. We also theorize that elevated MG could impair the function of lymphatic endothelial cells and lymphatic smooth muscles and by extension impair the function of the lymphatic vasculature. Although clinically approved interventions for MG-associated complications are not currently available, some treatments are available as nutritional supplements which could be used to lower MG levels and reduce CVD risks in PLWH.
Author Contributions
K.R.B. conceived the idea that methylglyoxal could be an elusive factor contributing to early-onset CVDs in PLWH and planned the review along with all other authors. B.E., S.G. discussed the idea of methylglyoxal being an elusive factor for CVD in PLWH. M.R. wrote the first draft of the manuscript, and Z.L.V. drew the figures. F.A.A., A.N., S.G. and K.R.B. assisted with the outlining, proofreading, and referencing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported, in part, by NIH (R56HL151602-01A1, 1R01HL164306-01 and R21NS139920-01) (K.R.B. and S.G.) and a Neuroscience Alliance Award (37-5160-2001-011) (B.E.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frescura, L.; Godfrey-Faussett, P.; Feizzadeh, A.; El-Sadr, W.; Syarif, O.; Ghys, P.D.; on and behalf of the 2025 testing treatment target Working Group. Achieving the 95 95 95 targets for all: A pathway to ending AIDS. PLoS ONE 2022, 17, e0272405. [Google Scholar] [CrossRef] [PubMed]
- Raymond, H.F.; Scheer, S.; Santos, G.M.; McFarland, W. Examining progress toward the UNAIDS 90-90-90 framework among men who have sex with men, San Francisco, 2014. AIDS Care 2016, 28, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Granich, R.; Gupta, S.; Hall, I.; Aberle-Grasse, J.; Hader, S.; Mermin, J. Status and methodology of publicly available national HIV care continua and 90-90-90 targets: A systematic review. PLoS Med. 2017, 14, e1002253. [Google Scholar] [CrossRef] [PubMed]
- Grobler, A.; Cawood, C.; Khanyile, D.; Puren, A.; Kharsany, A.B. Progress of UNAIDS 90-90-90 targets in a district in KwaZulu-Natal, South Africa, with high HIV burden, in the HIPSS study: A household-based complex multilevel community survey. Lancet HIV 2017, 4, e505–e513. [Google Scholar] [CrossRef]
- Labhardt, N.; Ringera, I.; Lejone, T.; Cheleboi, M.; Wagner, S.; Muhairwe, J.; Klimkait, T. When patients fail UNAIDS’last 90-the. J. Int. AIDS Soc. 2017. [Google Scholar]
- Zhao, Y.; Han, M.; Ma, Y.; Li, D. Progress Towards the 90-90-90 Targets for Controlling HIV—China, 2018. China CDC Wkly 2019, 1, 5–7. [Google Scholar]
- Green, D.; Kharono, B.; Tordoff, D.M.; Akullian, A.; Bershteyn, A.; Morrison, M.; Garnett, G.; Duerr, A.; Drain, P. Demographic and risk group heterogeneity across the UNAIDS 90-90-90 targets: A systematic review and meta-analysis protocol. Syst. Rev. 2019, 8, 110. [Google Scholar]
- Ghazy, R.M.; Al Awaidy, S.; Taha, S.H.N. Trends of HIV indicators in Egypt from 1990 to 2021: Time-series analysis and forecast toward UNAIDS 90-90-90 targets. BMC Public Health 2023, 23, 625. [Google Scholar]
- de Bree, G.J.; van Sighem, A.; Zuilhof, W.; van Bergen, J.; Prins, M.; Heidenrijk, M.; van der Valk, M.; Brokx, P.; Reiss, P.; Initiative, H.I.V.T.E.A. Is reaching 90-90-90 enough to end AIDS? Lessons from Amsterdam. Curr. Opin. HIV AIDS 2019, 14, 455–463. [Google Scholar] [CrossRef]
- Suleman, M.; Khan, S.U.; Hussain, T.; Khan, M.U.; Shamsul Hassan, S.; Majid, M.; Khan, S.U.; Shehzad Khan, M.; Shan Ahmad, R.U.; Arif, M.; et al. Cardiovascular challenges in the era of antiretroviral therapy for AIDS/HIV: A comprehensive review of research advancements, pathophysiological insights, and future directions. Curr. Probl. Cardiol. 2024, 49, 102353. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, W.; He, J.; Duan, W.; Ma, X.; Guan, H.; Wu, Y.; Li, S.; Li, Y.; Tian, T. Higher cardiovascular disease risks in people living with HIV: A systematic review and meta-analysis. J. Glob. Health 2024, 14, 04078. [Google Scholar] [PubMed]
- Nazari, I.; Feinstein, M.J. Evolving mechanisms and presentations of cardiovascular disease in people with HIV: Implications for management. Clin. Microbiol. Rev. 2024, 37, e00098-22. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, M.J.; Hsue, P.Y.; Benjamin, L.A.; Bloomfield, G.S.; Currier, J.S.; Freiberg, M.S.; Grinspoon, S.K.; Levin, J.; Longenecker, C.T.; Post, W.S. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: A scientific statement from the American Heart Association. Circulation 2019, 140, e98–e124. [Google Scholar] [PubMed]
- Hsue, P.Y. Mechanisms of Cardiovascular Disease in the Setting of HIV Infection. Can. J. Cardiol. 2019, 35, 238–248. [Google Scholar] [CrossRef]
- Stone, L.; Looby, S.E.; Zanni, M.V. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr. Opin. HIV AIDS 2017, 12, 585–593. [Google Scholar] [CrossRef]
- Kovacs, L.; Kress, T.C.; Belin de Chantemèle, E.J. HIV, combination antiretroviral therapy, and vascular diseases in men and women. Basic. Transl. Sci. 2022, 7, 410–421. [Google Scholar]
- Raffe, S.; Sabin, C.; Gilleece, Y.; Women Against Viruses in Europe, E.A.C.S. Comorbidities in women living with HIV: A systematic review. HIV Med. 2022, 23, 331–361. [Google Scholar]
- Group, T.A.S. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N. Engl. J. Med. 2015, 373, 808–822. [Google Scholar]
- Lundgren, J.D.; Borges, A.H.; Neaton, J.D. Serious Non-AIDS Conditions in HIV: Benefit of Early ART. Curr. HIV/AIDS Rep. 2018, 15, 162–171. [Google Scholar] [CrossRef]
- Strategies for Management of Antiretroviral Therapy Study Group; Emery, S.; Neuhaus, J.A.; Phillips, A.N.; Babiker, A.; Cohen, C.J.; Gatell, J.M.; Girard, P.M.; Grund, B.; Law, M.; et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J. Infect. Dis. 2008, 197, 1133–1144. [Google Scholar] [CrossRef]
- Ruamtawee, W.; Tipayamongkholgul, M.; Aimyong, N.; Manosuthi, W. Prevalence and risk factors of cardiovascular disease among people living with HIV in the Asia-Pacific region: A systematic review. BMC Public Health 2023, 23, 477. [Google Scholar] [CrossRef] [PubMed]
- Pallipamu, N.; Taheri, S.; Thiagaraj, S.S.; Shukla, T.S.; Gutlapalli, S.D.; Farhat, H.; Irfan, H.; Muthiah, K.; Alfonso, M. A systematic review of how to reduce morbidity in hiv patients with cardiovascular diseases. Cureus 2023, 15, e34745. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, W.; Manuel, J.I.; Kariuki, N.; Tuchman, E.; O’Neal, J.; Lalanne, G.A. HIV and smoking: Associated risks and prevention strategies. HIV AIDS 2016, 8, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; de Boer, R.; Brul, S.; Budovskaya, Y.; van Spek, H. Premature and accelerated aging: HIV or HAART? Front. Genet. 2012, 3, 328. [Google Scholar] [CrossRef]
- Cohen, J.; D’Agostino, L.; Tuzer, F.; Torres, C. HIV antiretroviral therapy drugs induce premature senescence and altered physiology in HUVECs. Mech. Ageing Dev. 2018, 175, 74–82. [Google Scholar] [CrossRef]
- Grozdeva, R.; Ivanov, D.; Strashimirov, D.; Kapincheva, N.; Yordanova, R.; Mihailova, S.; Georgieva, A.; Alexiev, I.; Grigorova, L.; Partsuneva, A. Relationship between Modern ART Regimens and Immunosenescence Markers in Patients with Chronic HIV Infection. Viruses 2024, 16, 1205. [Google Scholar] [CrossRef]
- Owen, A.; Rannard, S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv. Drug Deliv. Rev. 2016, 103, 144–156. [Google Scholar] [CrossRef]
- Puri, A.; Sivaraman, A.; Zhang, W.; Clark, M.R.; Banga, A.K. Expanding the domain of drug delivery for HIV prevention: Exploration of the transdermal route. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 551–587. [Google Scholar] [CrossRef]
- Surve, D.H.; Jindal, A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J. Control Release 2020, 324, 379–404. [Google Scholar] [CrossRef]
- Nayan, M.U.; Sillman, B.; Hasan, M.; Deodhar, S.; Das, S.; Sultana, A.; Le, N.T.H.; Soriano, V.; Edagwa, B.; Gendelman, H.E. Advances in long-acting slow effective release antiretroviral therapies for treatment and prevention of HIV infection. Adv. Drug Deliv. Rev. 2023, 200, 115009. [Google Scholar] [CrossRef]
- Ntsekhe, M.; Baker, J.V. Cardiovascular disease among persons living with HIV: New insights into pathogenesis and clinical manifestations in a global context. Circulation 2023, 147, 83–100. [Google Scholar] [PubMed]
- El Kamari, V.; Rodriguez, K.; Moser, C.; Currier, J.S.; Kelesidis, T.; Stein, J.H.; Brown, T.T.; Howell, S.K.; Beisswenger, P.J.; McComsey, G.A. Advanced Glycation End Products Associated With Cardiometabolic Biomarkers in Treated Human Immunodeficiency Virus Infection. Open Forum Infect. Dis. 2021, 8, ofab423. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Alomar, F.A.; Cox, J.L.; McMillan, J.; Hackfort, B.T.; Makarov, E.; Morsey, B.; Fox, H.S.; Gendelman, H.E.; Gorantla, S.; et al. A Link Between Methylglyoxal and Heart Failure During HIV-1 Infection. Front. Cardiovasc. Med. 2021, 8, 792180. [Google Scholar] [CrossRef]
- Douek, D.C.; Brenchley, J.M.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Okamoto, Y.; Casazza, J.P.; Kuruppu, J.; Kunstman, K.; Wolinsky, S.; et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002, 417, 95–98. [Google Scholar] [CrossRef]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef]
- Zink, W.E.; Zheng, J.; Persidsky, Y.; Poluektova, L.; Gendelman, H.E. The neuropathogenesis of HIV-1 infection. FEMS Immunol. Med. Microbiol. 1999, 26, 233–241. [Google Scholar] [CrossRef]
- Naranjo, O.; Torices, S.; Clifford, P.R.; Daftari, M.T.; Osborne, O.M.; Fattakhov, N.; Toborek, M. Pericyte infection by HIV-1: A fatal attraction. Retrovirology 2022, 19, 27. [Google Scholar]
- Li, G.H.; Henderson, L.; Nath, A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr. HIV Res. 2016, 14, 373–381. [Google Scholar] [CrossRef]
- Nottet, H.S. Interactions between macrophages and brain microvascular endothelial cells: Role in pathogenesis of HIV-1 infection and blood—Brain barrier function. J. Neurovirol 1999, 5, 659–669. [Google Scholar] [CrossRef]
- Rodriguez, E.R.; Nasim, S.; Hsia, J.; Sandin, R.L.; Ferreira, A.; Hilliard, B.A.; Ross, A.M.; Garrett, C.T. Cardiac myocytes and dendritic cells harbor human immunodeficiency virus in infected patients with and without cardiac dysfunction: Detection by multiplex, nested, polymerase chain reaction in individually microdissected cells from right ventricular endomyocardial biopsy tissue. Am. J. Cardiol. 1991, 68, 1511–1520. [Google Scholar] [CrossRef]
- Moris, A.; Pajot, A.; Blanchet, F.; Guivel-Benhassine, F.; Salcedo, M.; Schwartz, O. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 2006, 108, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Valle-Casuso, J.C.; Angin, M.; Volant, S.; Passaes, C.; Monceaux, V.; Mikhailova, A.; Bourdic, K.; Avettand-Fenoel, V.; Boufassa, F.; Sitbon, M.; et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4+ T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2019, 29, 611–626.e5. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, A.; Kavanagh Williamson, M.; Huthoff, H. HIV-1 pathogenicity and virion production are dependent on the metabolic phenotype of activated CD4+ T cells. Retrovirology 2014, 11, 98. [Google Scholar] [CrossRef]
- Joshi, A.; Garg, H.; Ablan, S.; Freed, E.O.; Nagashima, K.; Manjunath, N.; Shankar, P. Targeting the HIV entry, assembly and release pathways for anti-HIV gene therapy. Virology 2011, 415, 95–106. [Google Scholar] [CrossRef]
- Church, J.D.; Huang, W.; Mwatha, A.; Toma, J.; Stawiski, E.; Donnell, D.; Guay, L.A.; Mmiro, F.; Musoke, P.; Jackson, J.B.; et al. HIV-1 tropism and survival in vertically infected Ugandan infants. J. Infect. Dis. 2008, 197, 1382–1388. [Google Scholar] [CrossRef]
- Poli, G.; Buonaguro, L. Introducing the issue on “differential use of CCR5 versus CXCR4 by HIV-1. Pathogenic, translational and clinical open questions”. J. Transl. Med. 2011, 9 (Suppl. S1), I1. [Google Scholar] [CrossRef][Green Version]
- Barmania, F.; Pepper, M.S. C-C chemokine receptor type five (CCR5): An emerging target for the control of HIV infection. Appl. Transl. Genom. 2013, 2, 3–16. [Google Scholar] [CrossRef]
- Graumann, U.; Ritz, M.F.; Rivero, B.G.; Hausmann, O. CD133 expressing pericytes and relationship to SDF-1 and CXCR4 in spinal cord injury. Curr. Neurovasc Res. 2010, 7, 144–154. [Google Scholar] [CrossRef]
- Pyo, R.T.; Sui, J.; Dhume, A.; Palomeque, J.; Blaxall, B.C.; Diaz, G.; Tunstead, J.; Logothetis, D.E.; Hajjar, R.J.; Schecter, A.D. CXCR4 modulates contractility in adult cardiac myocytes. J. Mol. Cell Cardiol. 2006, 41, 834–844. [Google Scholar] [CrossRef]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic substrate utilization in stress-induced immune cells. Intensive Care Med. Exp. 2020, 8, 28. [Google Scholar] [CrossRef]
- Kang, S.; Tang, H. HIV-1 Infection and Glucose Metabolism Reprogramming of T Cells: Another Approach Toward Functional Cure and Reservoir Eradication. Front. Immunol. 2020, 11, 572677. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, G.J.; Pearce, E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012, 249, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, L.R.; Maldarelli, F.; Chamoun, G.; Schilling, B.; Chokekijcahi, S.; Staudt, L.; Mitsuya, H.; Simpson, I.A.; Zeichner, S.L. Human immunodeficiency virus type 1 infection of H9 cells induces increased glucose transporter expression. J. Virol. 1996, 70, 7275–7279. [Google Scholar] [CrossRef]
- Kishimoto, N.; Yamamoto, K.; Abe, T.; Yasuoka, N.; Takamune, N.; Misumi, S. Glucose-dependent aerobic glycolysis contributes to recruiting viral components into HIV-1 particles to maintain infectivity. Biochem. Biophys. Res. Commun. 2021, 549, 187–193. [Google Scholar] [CrossRef]
- Palmer, C.S.; Ostrowski, M.; Gouillou, M.; Tsai, L.; Yu, D.; Zhou, J.; Henstridge, D.C.; Maisa, A.; Hearps, A.C.; Lewin, S.R.; et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS 2014, 28, 297–309. [Google Scholar] [CrossRef]
- Palmer, C.S.; Crowe, S.M. The role of glucose and lipid metabolism in the pathogenesis of HIV-1 infection. Immunology 2012, 13, 37–50. [Google Scholar]
- Scholz, E.M.B.; Kashuba, A.D.M. The Lymph Node Reservoir: Physiology, HIV Infection, and Antiretroviral Therapy. Clin. Pharmacol. Ther. 2021, 109, 918–927. [Google Scholar] [CrossRef]
- Simonetti, F.R.; Sobolewski, M.D.; Fyne, E.; Shao, W.; Spindler, J.; Hattori, J.; Anderson, E.M.; Watters, S.A.; Hill, S.; Wu, X.; et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 1883–1888. [Google Scholar] [CrossRef]
- Richard, J.P. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans. 1993, 21, 549–553. [Google Scholar] [CrossRef]
- Phillips, S.A.; Thornalley, P.J. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 1993, 212, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem. J. 1988, 254, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Tajes, M.; Guivernau, B.; Ramos-Fernandez, E.; Bosch-Morato, M.; Palomer, E.; Guix, F.X.; Munoz, F.J. The pathophysiology of triose phosphate isomerase dysfunction in Alzheimer’s disease. Histol. Histopathol. 2013, 28, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, S.; Ibrahim, A.; Rahman, M.D.M.; Eid, A.H.; Munusamy, S. Role of Methylglyoxal in Diabetic Cardiovascular and Kidney Diseases: Insights from Basic Science for Application into Clinical Practice. Curr. Pharm. Des. 2018, 24, 3072–3083. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. VAP-1: An adhesin and an enzyme. Trends Immunol. 2001, 22, 211–216. [Google Scholar]
- Yu, P.H.; Wright, S.; Fan, E.H.; Lun, Z.R.; Gubisne-Harberle, D. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim. Biophys. Acta 2003, 1647, 193–199. [Google Scholar] [CrossRef]
- Jaakkola, K.; Kaunismaki, K.; Tohka, S.; Yegutkin, G.; Vanttinen, E.; Havia, T.; Pelliniemi, L.J.; Virolainen, M.; Jalkanen, S.; Salmi, M. Human vascular adhesion protein-1 in smooth muscle cells. Am. J. Pathol. 1999, 155, 1953–1965. [Google Scholar] [CrossRef]
- Alomar, F.; Singh, J.; Jang, H.S.; Rozanzki, G.J.; Shao, C.H.; Padanilam, B.J.; Mayhan, W.G.; Bidasee, K.R. Smooth muscle-generated methylglyoxal impairs endothelial cell-mediated vasodilatation of cerebral microvessels in type 1 diabetic rats. Br. J. Pharmacol. 2016, 173, 3307–3326. [Google Scholar]
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Vander Jagt, D.L. Methylglyoxal, diabetes mellitus and diabetic complications. Drug Metabol. Drug Interact. 2008, 23, 93–124. [Google Scholar] [CrossRef]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, T.; Yabe-Nishimura, C. Transcription factor Nrf2 regulates promoter activity of mouse aldose reductase (AKR1B3) gene. J. Pharmacol. Sci. 2005, 97, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Abordo, E.A.; Minhas, H.S.; Thornalley, P.J. Accumulation of alpha-oxoaldehydes during oxidative stress: A role in cytotoxicity. Biochem. Pharmacol. 1999, 58, 641–648. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, D.M.; Kwon, K.; Park, C. Generation and characterization of mouse knockout for glyoxalase 1. Biochem. Biophys. Res. Commun. 2017, 490, 460–465. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Lee, Y.S.; Liu, Y.L.; Hsu, W.C.; Ho, W.M.; Huang, Y.H.; Tsai, S.J.; Kuo, P.H.; Chen, Y.C. Association Study of Alcohol Dehydrogenase and Aldehyde Dehydrogenase Polymorphism With Alzheimer Disease in the Taiwanese Population. Front. Neurosci. 2021, 15, 625885. [Google Scholar] [CrossRef]
- Giacco, F.; Du, X.; D’Agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Dobariya, P.; Xie, W.; Rao, S.P.; Xie, J.; Seelig, D.M.; Vince, R.; Lee, M.K.; More, S.S. Deletion of Glyoxalase 1 exacerbates acetaminophen-induced hepatotoxicity in mice. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nigro, C.; Leone, A.; Longo, M.; Prevenzano, I.; Fleming, T.H.; Nicolo, A.; Parrillo, L.; Spinelli, R.; Formisano, P.; Nawroth, P.P.; et al. Methylglyoxal accumulation de-regulates HoxA5 expression, thereby impairing angiogenesis in glyoxalase 1 knock-down mouse aortic endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 73–85. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Geoffrion, M.; Kuraitis, D.; McBane, J.E.; Lochhead, M.; Vanderhyden, B.C.; Korbutt, G.S.; Milne, R.W.; Suuronen, E.J. Glyoxalase-1 overexpression in bone marrow cells reverses defective neovascularization in STZ-induced diabetic mice. Cardiovasc. Res. 2014, 101, 306–316. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.; Miyata, T.; Ostergaard, J.A.; Flyvbjerg, A.; Peutz-Kootstra, C.J.; Sieber, J.; Mundel, P.H.; Brownlee, M.; Janssen, B.J.; et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 2014, 57, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, D.P.; Lou, B.; Middel, C.S.; Morgenstern, J.; Fleming, T.; Sticht, C.; Hausser, I.; Hell, R.; Hammes, H.P.; Szendrodi, J.; et al. Accumulation of acetaldehyde in aldh2.1−/− zebrafish causes increased retinal angiogenesis and impaired glucose metabolism. Redox Biol. 2022, 50, 102249. [Google Scholar] [CrossRef] [PubMed]
- Jana, G.A.; Yaish, M.W. Functional characterization of the Glyoxalase-I (PdGLX1) gene family in date palm under abiotic stresses. Plant Signal Behav. 2020, 15, 1811527. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Staitieh, B.S.; Jensen, J.S.; Mould, K.J.; Greenberg, J.A.; Joshi, P.C.; Koval, M.; Guidot, D.M. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L267–L277. [Google Scholar] [CrossRef]
- Han, D.; Lu, X.; Yin, W.; Fu, H.; Zhang, X.; Cheng, L.; Liu, F.; Jin, C.; Tian, X.; Xie, Y.; et al. Activation of NRF2 blocks HIV replication and apoptosis in macrophages. Heliyon 2023, 9, e12575. [Google Scholar] [CrossRef]
- Staitieh, B.S.; Ding, L.; Neveu, W.A.; Spearman, P.; Guidot, D.M.; Fan, X. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. J. Leukoc. Biol. 2017, 102, 517–525. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G.; Denaro, F.; Calabrese, V.; Benedetti, F.; Krishnan, S.; Curreli, S.; Bryant, J.; Zella, D. Altered expression pattern of Nrf2/HO-1 axis during accelerated-senescence in HIV-1 transgenic rat. Biogerontology 2014, 15, 449–461. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Glu504Lys Single Nucleotide Polymorphism of Aldehyde Dehydrogenase 2 Gene and the Risk of Human Diseases. Biomed. Res. Int. 2015, 2015, 174050. [Google Scholar] [CrossRef]
- Calleja, L.F.; Yoval-Sanchez, B.; Hernandez-Esquivel, L.; Gallardo-Perez, J.C.; Sosa-Garrocho, M.; Marin-Hernandez, A.; Jasso-Chavez, R.; Macias-Silva, M.; Salud Rodriguez-Zavala, J. Activation of ALDH1A1 by omeprazole reduces cell oxidative stress damage. FEBS J. 2021, 288, 4064–4080. [Google Scholar] [CrossRef]
- Chiu, C.C.; Yeh, T.H.; Lai, S.C.; Wu-Chou, Y.H.; Chen, C.H.; Mochly-Rosen, D.; Huang, Y.C.; Chen, Y.J.; Chen, C.L.; Chang, Y.M.; et al. Neuroprotective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp. Neurol. 2015, 263, 244–253. [Google Scholar] [CrossRef]
- Pastel, E.; Pointud, J.C.; Volat, F.; Martinez, A.; Lefrancois-Martinez, A.M. Aldo-Keto Reductases 1B in Endocrinology and Metabolism. Front. Pharmacol. 2012, 3, 148. [Google Scholar] [CrossRef]
- Maccari, R.; Ottana, R. Targeting aldose reductase for the treatment of diabetes complications and inflammatory diseases: New insights and future directions. J. Med. Chem. 2015, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.S.; Bhardwaj, S.; Pandita, D.; Lather, V.; Sekhon, B.S. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini Rev. Med. Chem. 2016, 16, 120–162. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L.; Robinson, B.; Taylor, K.K.; Hunsaker, L.A. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J. Biol. Chem. 1992, 267, 4364–4369. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest. Ophthalmol. Vis. Sci. 2003, 44, 5287–5292. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J. Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence. Biochem. J. 2002, 364, 15–24. [Google Scholar] [CrossRef]
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar]
- Shuck, S.C.; Wuenschell, G.E.; Termini, J.S. Product Studies and Mechanistic Analysis of the Reaction of Methylglyoxal with Deoxyguanosine. Chem. Res. Toxicol. 2018, 31, 105–115. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.-A.; Kim, J.-H.; Kim, E.-H.; Bae, O.-N. Methylglyoxal-induced dysfunction in brain endothelial cells via the suppression of akt/HIF-1α pathway and activation of mitophagy associated with increased reactive oxygen species. Antioxidants 2020, 9, 820. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zheng, Y.; Jiang, J.; Wang, L.; Wu, J.; Zhang, C.; Luo, M. Glucose metabolite methylglyoxal induces vascular endothelial cell pyroptosis via NLRP3 inflammasome activation and oxidative stress in vitro and in vivo. Cell. Mol. Life Sci. 2024, 81, 401. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Swami Vetha, B.S.; Chakraborty, R.; Wenegieme, T.Y.; Masenga, S.K.; Muthian, G.; Balasubramaniam, M.; Wanjalla, C.N.; Hinton, A.O., Jr.; Kirabo, A.; et al. HIV-Associated Hypertension: Risks, Mechanisms, and Knowledge Gaps. Circ. Res. 2024, 134, e150–e175. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Stuckless, J. Role of methylglyoxal in essential hypertension. Int. J. Angiol. 2010, 19, e58–e65. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Goodall, R.L.; Dunn, D.T.; Franzen, S.; Collaco-Moraes, Y.; Gazzard, B.G.; Williams, I.G.; Fisher, M.J.; Winston, A.; Fox, J.; et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. JAMA 2012, 308, 353–361. [Google Scholar] [CrossRef]
- Savarino, A.; Shytaj, I.L. Chloroquine and beyond: Exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology 2015, 12, 51. [Google Scholar] [CrossRef]
- Markowitz, M.; Vaida, F.; Hare, C.B.; Boden, D.; Mohri, H.; Hecht, F.M.; Kalayjian, R.C.; Conrad, A.; Mildvan, D.; Aberg, J.; et al. The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. J. Infect. Dis. 2010, 201, 1298–1302. [Google Scholar] [CrossRef]
- Donia, M.; McCubrey, J.A.; Bendtzen, K.; Nicoletti, F. Potential use of rapamycin in HIV infection. Br. J. Clin. Pharmacol. 2010, 70, 784–793. [Google Scholar] [CrossRef]
- Obare, L.M.; Temu, T.; Mallal, S.A.; Wanjalla, C.N. Inflammation in HIV and Its Impact on Atherosclerotic Cardiovascular Disease. Circ. Res. 2024, 134, 1515–1545. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Li, D.; Ma, Y.; Ishai, A.; Manion, M.; Nahrendorf, M.; Ganz, P.; Ridker, P.M.; Deeks, S.G.; Tawakol, A. IL-1beta Inhibition Reduces Atherosclerotic Inflammation in HIV Infection. J. Am. Coll. Cardiol. 2018, 72, 2809–2811. [Google Scholar] [CrossRef]
- Ting, P.T.; Koo, J.Y. Use of etanercept in human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) patients. Int. J. Dermatol. 2006, 45, 689–692. [Google Scholar] [CrossRef]
- Freeman, M.L.; Clagett, B.M.; Moisi, D.; Yeh, E.; Morris, C.D.; Ryu, A.; Rodriguez, B.; Stein, J.H.; Deeks, S.G.; Currier, J.S.; et al. Methotrexate Inhibits T Cell Proliferation but Not Inflammatory Cytokine Expression to Modulate Immunity in People Living With HIV. Front. Immunol. 2022, 13, 924718. [Google Scholar] [CrossRef]
- Marconi, V.C.; Moser, C.; Gavegnano, C.; Deeks, S.G.; Lederman, M.M.; Overton, E.T.; Tsibris, A.; Hunt, P.W.; Kantor, A.; Sekaly, R.P.; et al. Randomized Trial of Ruxolitinib in Antiretroviral-Treated Adults With Human Immunodeficiency Virus. Clin. Infect. Dis. 2022, 74, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Dhar, I.; Desai, K.M.; Wu, L. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br. J. Pharmacol. 2010, 161, 1843–1856. [Google Scholar] [PubMed]
- Nigro, C.; Leone, A.; Raciti, G.A.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef]
- Berends, E.; van Oostenbrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 2023, 20, 75. [Google Scholar]
- Schlotterer, A.; Kolibabka, M.; Lin, J.; Acunman, K.; Dietrichá, N.; Sticht, C.; Fleming, T.; Nawroth, P.; Hammes, H.-P. Methylglyoxal induces retinopathy-type lesions in the absence of hyperglycemia: Studies in a rat model. FASEB J. 2019, 33, 4141–4153. [Google Scholar]
- Knott, H.M.; Brown, B.E.; Davies, M.J.; Dean, R.T. Glycation and glycoxidation of low-density lipoproteins by glucose and low-molecular mass aldehydes. Formation of modified and oxidized particles. Eur. J. Biochem. 2003, 270, 3572–3582. [Google Scholar] [CrossRef]
- Kirca, M. Methylglyoxal enhances the proliferation of vascular smooth muscle cells via Akt phosphorylation. J. Recept. Signal Transduct. Res. 2022, 42, 567–572. [Google Scholar] [CrossRef]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 2010, 56, 471–476. [Google Scholar] [CrossRef]
- van der Bruggen, M.M.; Spronck, B.; Delhaas, T.; Reesink, K.D.; Schalkwijk, C.G. The Putative Role of Methylglyoxal in Arterial Stiffening: A Review. Heart Lung Circ. 2021, 30, 1681–1693. [Google Scholar] [CrossRef]
- Hadas, K.; Randriamboavonjy, V.; Elgheznawy, A.; Mann, A.; Fleming, I. Methylglyoxal induces platelet hyperaggregation and reduces thrombus stability by activating PKC and inhibiting PI3K/Akt pathway. PLoS ONE 2013, 8, e74401. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-J.; Yang, H.-Y.; Pai, M.-H.; Wu, C.-H.; Chen, J.-R. Long-term administration of advanced glycation end-product stimulates the activation of NLRP3 inflammasome and sparking the development of renal injury. J. Nutr. Biochem. 2017, 39, 68–76. [Google Scholar] [CrossRef]
- Chu, P.; Han, G.; Ahsan, A.; Sun, Z.; Liu, S.; Zhang, Z.; Sun, B.; Song, Y.; Lin, Y.; Peng, J.; et al. Phosphocreatine protects endothelial cells from Methylglyoxal induced oxidative stress and apoptosis via the regulation of PI3K/Akt/eNOS and NF-kappaB pathway. Vasc. Pharmacol. 2017, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, O.S.; Kim, C.-S.; Kim, N.H.; Kim, J.S. Cytotoxic role of methylglyoxal in rat retinal pericytes: Involvement of a nuclear factor-kappaB and inducible nitric oxide synthase pathway. Chem. Biol. Interact. 2010, 188, 86–93. [Google Scholar] [PubMed]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef]
- Fleming, T.H.; Humpert, P.M.; Nawroth, P.P.; Bierhaus, A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process—A mini-review. Gerontology 2011, 57, 435–443. [Google Scholar]
- Hollenbach, M. The Role of Glyoxalase-I (Glo-I), Advanced Glycation Endproducts (AGEs), and Their Receptor (RAGE) in Chronic Liver Disease and Hepatocellular Carcinoma (HCC). Int. J. Mol. Sci. 2017, 18, 2466. [Google Scholar] [CrossRef]
- Ahmad, S.; Akhter, F.; Shahab, U.; Rafi, Z.; Khan, M.S.; Nabi, R.; Khan, M.S.; Ahmad, K.; Ashraf, J.M.; Moinuddin. Do all roads lead to the Rome? The glycation perspective! Semin. Cancer Biol. 2018, 49, 9–19. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Huang, Y.P.; Hsu, S.H.; Kang, L.Y.; Shen, C.M.; Lin, W.W. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J. Cell Mol. Med. 2016, 20, 1749–1760. [Google Scholar] [CrossRef]
- Shao, C.H.; Tian, C.; Ouyang, S.; Moore, C.J.; Alomar, F.; Nemet, I.; D’Souza, A.; Nagai, R.; Kutty, S.; Rozanski, G.J.; et al. Carbonylation induces heterogeneity in cardiac ryanodine receptor function in diabetes mellitus. Mol. Pharmacol. 2012, 82, 383–399. [Google Scholar] [CrossRef]
- Shao, C.H.; Rozanski, G.J.; Nagai, R.; Stockdale, F.E.; Patel, K.P.; Wang, M.; Singh, J.; Mayhan, W.G.; Bidasee, K.R. Carbonylation of myosin heavy chains in rat heart during diabetes. Biochem. Pharmacol. 2010, 80, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.H.; Capek, H.L.; Patel, K.P.; Wang, M.; Tang, K.; DeSouza, C.; Nagai, R.; Mayhan, W.; Periasamy, M.; Bidasee, K.R. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes 2011, 60, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Bovo, E.; Seflova, J.; Robia, S.L.; Zima, A.V. Protein carbonylation causes sarcoplasmic reticulum Ca2+ overload by increasing intracellular Na+ level in ventricular myocytes. Pflügers Arch. Eur. J. Physiol. 2024, 476, 1077–1086. [Google Scholar]
- Arsov, S.; Graaff, R.; van Oeveren, W.; Stegmayr, B.; Sikole, A.; Rakhorst, G.; Smit, A.J. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: A review. Clin. Chem. Lab. Med. 2014, 52, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal stress, the glyoxalase system, and diabetic chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Dimitropoulos, A.; Rosado, C.J.; Thomas, M.C. Dicarbonyl-mediated AGEing and diabetic kidney disease. J. Nephrol. 2020, 33, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Schmoch, T.; Uhle, F.; Siegler, B.H.; Fleming, T.; Morgenstern, J.; Nawroth, P.P.; Weigand, M.A.; Brenner, T. The Glyoxalase System and Methylglyoxal-Derived Carbonyl Stress in Sepsis: Glycotoxic Aspects of Sepsis Pathophysiology. Int. J. Mol. Sci. 2017, 18, 657. [Google Scholar] [CrossRef]
- van Bussel, B.C.; van de Poll, M.C.; Schalkwijk, C.G.; Bergmans, D.C. Increased Dicarbonyl Stress as a Novel Mechanism of Multi-Organ Failure in Critical Illness. Int. J. Mol. Sci. 2017, 18, 346. [Google Scholar] [CrossRef]
- Kold-Christensen, R.; Johannsen, M. Methylglyoxal metabolism and aging-related disease: Moving from correlation toward causation. Trends Endocrinol. Metab. 2020, 31, 81–92. [Google Scholar]
- Kim, J.Y.; Jung, J.H.; Lee, S.J.; Han, S.S.; Hong, S.H. Glyoxalase 1 as a Therapeutic Target in Cancer and Cancer Stem Cells. Mol. Cells 2022, 45, 869–876. [Google Scholar] [CrossRef]
- Papadaki, M.; Holewinski, R.J.; Previs, S.B.; Martin, T.G.; Stachowski, M.J.; Li, A.; Blair, C.A.; Moravec, C.S.; Van Eyk, J.E.; Campbell, K.S.; et al. Diabetes with heart failure increases methylglyoxal modifications in the sarcomere, which inhibit function. JCI Insight 2018, 3, e121264. [Google Scholar] [CrossRef] [PubMed]
- Bellier, J.; Nokin, M.J.; Larde, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcene, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Prisco, S.Z.; Hartweck, L.; Keen, J.L.; Vogel, N.; Kazmirczak, F.; Eklund, M.; Hemnes, A.R.; Brittain, E.L.; Prins, K.W. Glyoxylase-1 combats dicarbonyl stress and right ventricular dysfunction in rodent pulmonary arterial hypertension. Front. Cardiovasc. Med. 2022, 9, 940932. [Google Scholar] [CrossRef]
- Alomar, F.A.; Al-Rubaish, A.; Al-Muhanna, F.; Al-Ali, A.K.; McMillan, J.; Singh, J.; Bidasee, K.R. Adeno-Associated Viral Transfer of Glyoxalase-1 Blunts Carbonyl and Oxidative Stresses in Hearts of Type 1 Diabetic Rats. Antioxidants 2020, 9, 592. [Google Scholar] [CrossRef]
- Lewis, E.J.; Greene, T.; Spitalewiz, S.; Blumenthal, S.; Berl, T.; Hunsicker, L.G.; Pohl, M.A.; Rohde, R.D.; Raz, I.; Yerushalmy, Y. Pyridorin in type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 2012, 23, 131–136. [Google Scholar]
- Abouzed, T.K.; Munesue, S.; Harashima, A.; Masuo, Y.; Kato, Y.; Khailo, K.; Yamamoto, H.; Yamamoto, Y. Preventive effect of salicylate and pyridoxamine on diabetic nephropathy. J. Diabetes Res. 2016, 2016, 1786789. [Google Scholar]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar]
- Bonaccorso, A.; Privitera, A.; Grasso, M.; Salamone, S.; Carbone, C.; Pignatello, R.; Musumeci, T.; Caraci, F.; Caruso, G. The therapeutic potential of novel carnosine formulations: Perspectives for drug development. Pharmaceuticals 2023, 16, 778. [Google Scholar] [CrossRef]
- Brings, S.; Fleming, T.; De Buhr, S.; Beijer, B.; Lindner, T.; Wischnjow, A.; Kender, Z.; Peters, V.; Kopf, S.; Haberkorn, U. A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim. Et Biophys. Acta Mol. Basis Dis. 2017, 1863, 654–662. [Google Scholar]
- Hammes, H.-P.; Du, X.; Edelstein, D.; Taguchi, T.; Matsumura, T.; Ju, Q.; Lin, J.; Bierhaus, A.; Nawroth, P.; Hannak, D. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003, 9, 294–299. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Nizheradze, K.; Berrone, E.; Tarallo, S.; Porta, M. Thiamine and benfotiamine prevent apoptosis induced by high glucose-conditioned extracellular matrix in human retinal pericytes. Diabetes/Metab. Res. Rev. 2009, 25, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Clifton, P.M. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Delbin, M.A.; Davel, A.P.; Couto, G.K.; de Araujo, G.G.; Rossoni, L.V.; Antunes, E.; Zanesco, A. Interaction between advanced glycation end products formation and vascular responses in femoral and coronary arteries from exercised diabetic rats. PLoS ONE 2012, 7, e53318. [Google Scholar] [CrossRef]
- Tanaka, M.; Kanazashi, M.; Kondo, H.; Fujino, H. Methylglyoxal reduces resistance exercise-induced protein synthesis and anabolic signaling in rat tibialis anterior muscle. J. Muscle Res. Cell Motil. 2024, 45, 263–273. [Google Scholar] [CrossRef]
- Noda, K.; Miyahara, S.; Nakazawa, T.; Almulki, L.; Nakao, S.; Hisatomi, T.; She, H.; Thomas, K.L.; Garland, R.C.; Miller, J.W. Inhibition of vascular adhesion protein-1 suppresses endotoxin-induced uveitis. FASEB J. 2008, 22, 1094–1103. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Renfurm, R.W.; Bakris, G.; Rossing, P.; Perkovic, V.; Hou, F.F.; Nangaku, M.; Sharma, K.; Heerspink, H.J.; Garcia-Hernandez, A. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): A randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2018, 6, 925–933. [Google Scholar] [CrossRef]
- Li, H.; Du, S.; Niu, P.; Gu, X.; Wang, J.; Zhao, Y. Vascular adhesion Protein-1 (VAP-1)/Semicarbazide-sensitive amine oxidase (SSAO): A potential therapeutic target for atherosclerotic cardiovascular diseases. Front. Pharmacol. 2021, 12, 679707. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Sheng, Z.; Lin, H.; Weng, Z.; Song, W.; Cao, B.; Zhao, Y.; Gao, Y.; Ni, S. The first selective VAP-1 inhibitor in China, TT-01025-CL: Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-and multiple-ascending doses. Front. Pharmacol. 2024, 15, 1327008. [Google Scholar] [CrossRef]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals–just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [PubMed]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s expectation be matched by the reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar]
- Rosenbaugh, E.G.; Savalia, K.K.; Manickam, D.S.; Zimmerman, M.C. Antioxidant-based therapies for angiotensin II-associated cardiovascular diseases. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2013, 304, R917–R928. [Google Scholar] [PubMed]
- Ortiz, M.C.; Manriquez, M.C.; Romero, J.C.; Juncos, L.A. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 2001, 38, 655–659. [Google Scholar]
- Kumar, P.; Liu, C.; Suliburk, J.W.; Minard, C.G.; Muthupillai, R.; Chacko, S.; Hsu, J.W.; Jahoor, F.; Sekhar, R.V. Supplementing glycine and N-acetylcysteine (GlyNAC) in aging HIV patients improves oxidative stress, mitochondrial dysfunction, inflammation, endothelial dysfunction, insulin resistance, genotoxicity, strength, and cognition: Results of an open-label clinical trial. Biomedicines 2020, 8, 390. [Google Scholar] [CrossRef]
- Magdaleno, F.; Blajszczak, C.C.; Charles-Niño, C.L.; Guadrón-Llanos, A.M.; Vázquez-Álvarez, A.O.; Miranda-Díaz, A.G.; Nieto, N.; Islas-Carbajal, M.C.; Rincón-Sánchez, A.R. Aminoguanidine reduces diabetes-associated cardiac fibrosis. Exp. Ther. Med. 2019, 18, 3125–3138. [Google Scholar] [CrossRef]
- Matthews, J.J.; Turner, M.D.; Santos, L.; Elliott-Sale, K.J.; Sale, C. Carnosine increases insulin-stimulated glucose uptake and reduces methylglyoxal-modified proteins in type-2 diabetic human skeletal muscle cells. Amino Acids 2023, 55, 413–420. [Google Scholar]
- Kamei, J.; Ohsawa, M.; Miyata, S.; Tanaka, S.-i. Preventive effect of L-carnosine on changes in the thermal nociceptive threshold in streptozotocin-induced diabetic mice. Eur. J. Pharmacol. 2008, 600, 83–86. [Google Scholar]
- Skrypnyk, N.I.; Voziyan, P.; Yang, H.; de Caestecker, C.R.; Theberge, M.-C.; Drouin, M.; Hudson, B.; Harris, R.C.; de Caestecker, M.P. Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury. Am. J. Physiol. Ren. Physiol. 2016, 311, F268–F277. [Google Scholar]
- Kinsky, O.R.; Hargraves, T.L.; Anumol, T.; Jacobsen, N.E.; Dai, J.; Snyder, S.A.; Monks, T.J.; Lau, S.S. Metformin scavenges methylglyoxal to form a novel imidazolinone metabolite in humans. Chem. Res. Toxicol. 2016, 29, 227–234. [Google Scholar]
- Rabbani, N.; Thornalley, P.J. Emerging role of thiamine therapy for prevention and treatment of early-stage diabetic nephropathy. Diabetes Obes. Metab. 2011, 13, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Desai, K.; Wang, R.; Wu, L. Up-regulation of aldolase A and methylglyoxal production in adipocytes. Br. J. Pharmacol. 2013, 168, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Arndtz, K.; Chen, Y.-Y.; Rowe, A.; Homer, V.; Kirkham, A.; Douglas-Pugh, J.; Slade, D.; Thorburn, D.; Barnes, E.; Aithal, G. Monoclonal antibody BTT1023 targeting vascular adhesion protein 1 for treating primary sclerosing cholangitis: BUTEO single-arm Phase II trial. Effic. Mech. Eval. 2022, 9, 1–54. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Devaraj, S.; Bellur, P.; Lakkanna, S.; Vicini, J.; Boddupalli, S. Novel concepts of broccoli sulforaphanes and disease: Induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutr. Rev. 2012, 70, 654–665. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Bustamante Munguira, E.; Juan, C.A.; Plou, F.J.; Pérez Lebeña, E. Electrophilic Compounds in the Human Diet and Their Role in the Induction of the Transcription Factor NRF2. Int. J. Mol. Sci. 2024, 25, 3521. [Google Scholar] [CrossRef]
- Shilovsky, G.A.; Dibrova, D.V. Regulation of cell proliferation and nrf2-mediated antioxidant defense: Conservation of keap1 cysteines and nrf2 binding site in the context of the evolution of KLHL family. Life 2023, 13, 1045. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-kappaB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 809952. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 1998, 17, 3145–3156. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L.; Hunsaker, L.A. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem. Biol. Interact. 2003, 143, 341–351. [Google Scholar] [PubMed]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Reversal of insulin resistance in overweight and obese subjects by trans-resveratrol and hesperetin combination—Link to dysglycemia, blood pressure, dyslipidemia, and low-grade inflammation. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef]
- Pace, G.W.; Leaf, C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995, 19, 523–528. [Google Scholar]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Jaruga, P.; Jaruga, B.; Gackowski, D.; Olczak, A.; Halota, W.; Pawlowska, M.; Olinski, R. Supplementation with antioxidant vitamins prevents oxidative modification of DNA in lymphocytes of HIV-infected patients. Free Radic. Biol. Med. 2002, 32, 414–420. [Google Scholar]
- Silva, T.A.L.; Medeiros, D.C.; Medeiros, G.C.B.S.; Medeiros, R.C.S.C.; de Souza Araújo, J.; Medeiros, J.A.; Ururahy, M.A.G.; Santos, R.V.T.; Medeiros, R.M.V.; Leite-Lais, L. Influence of curcumin supplementation on metabolic and lipid parameters of people living with HIV/AIDS: A randomized controlled trial. BMC Complement. Altern. Med. 2019, 19, 202. [Google Scholar]
- Erdos, T.; Masuda, M.; Venketaraman, V. Glutathione in HIV-Associated Neurocognitive Disorders. Curr. Issues Mol. Biol. 2024, 46, 5530–5549. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Huey, S.L.; Mehta, S. Antioxidants and HIV/AIDS: Zinc, Selenium, and Vitamins C and E. In Nutrition and HIV; CRC Press: Boca Raton, FL, USA, 2018; pp. 191–205. [Google Scholar]
- Zazzo, J.; Rouveix, B.; Rajagopalon, P.; Levacher, M.; Girard, P. Effect of zinc on the immune status of zinc-depleted AIDS related complex patients. Clin. Nutr. 1989, 8, 259–261. [Google Scholar]
- Isa, L.; Lucchini, A.; Lodi, S.; Giachetti, M. Blood zinc status and zinc treatment in human immunodeficiency virus-infected patients. Int. J. Clin. Lab. Res. 1992, 22, 45–47. [Google Scholar] [PubMed]
- Bobat, R.; Coovadia, H.; Stephen, C.; Naidoo, K.L.; McKerrow, N.; Black, R.E.; Moss, W.J. Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: A randomised double-blind placebo-controlled trial. Lancet 2005, 366, 1862–1867. [Google Scholar]
- Shor-Posner, G.; Lecusay, R.; Miguez, M.-J.; Moreno-Black, G.; Zhang, G.; Rodriguez, N.; Burbano, X.; Baum, M.; Wilkie, F. Psychological burden in the era of HAART: Impact of selenium therapy. Int. J. Psychiatry Med. 2003, 33, 55–69. [Google Scholar]
- Hurwitz, B.E.; Klaus, J.R.; Llabre, M.M.; Gonzalez, A.; Lawrence, P.J.; Maher, K.J.; Greeson, J.M.; Baum, M.K.; Shor-Posner, G.; Skyler, J.S. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 148–154. [Google Scholar]
- Kupka, R.; Mugusi, F.; Aboud, S.; Hertzmark, E.; Spiegelman, D.; Fawzi, W.W. Effect of selenium supplements on hemoglobin concentration and morbidity among HIV-1–Infected Tanzanian Women. Clin. Infect. Dis. 2009, 48, 1475–1478. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).