Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection
Abstract
1. Introduction
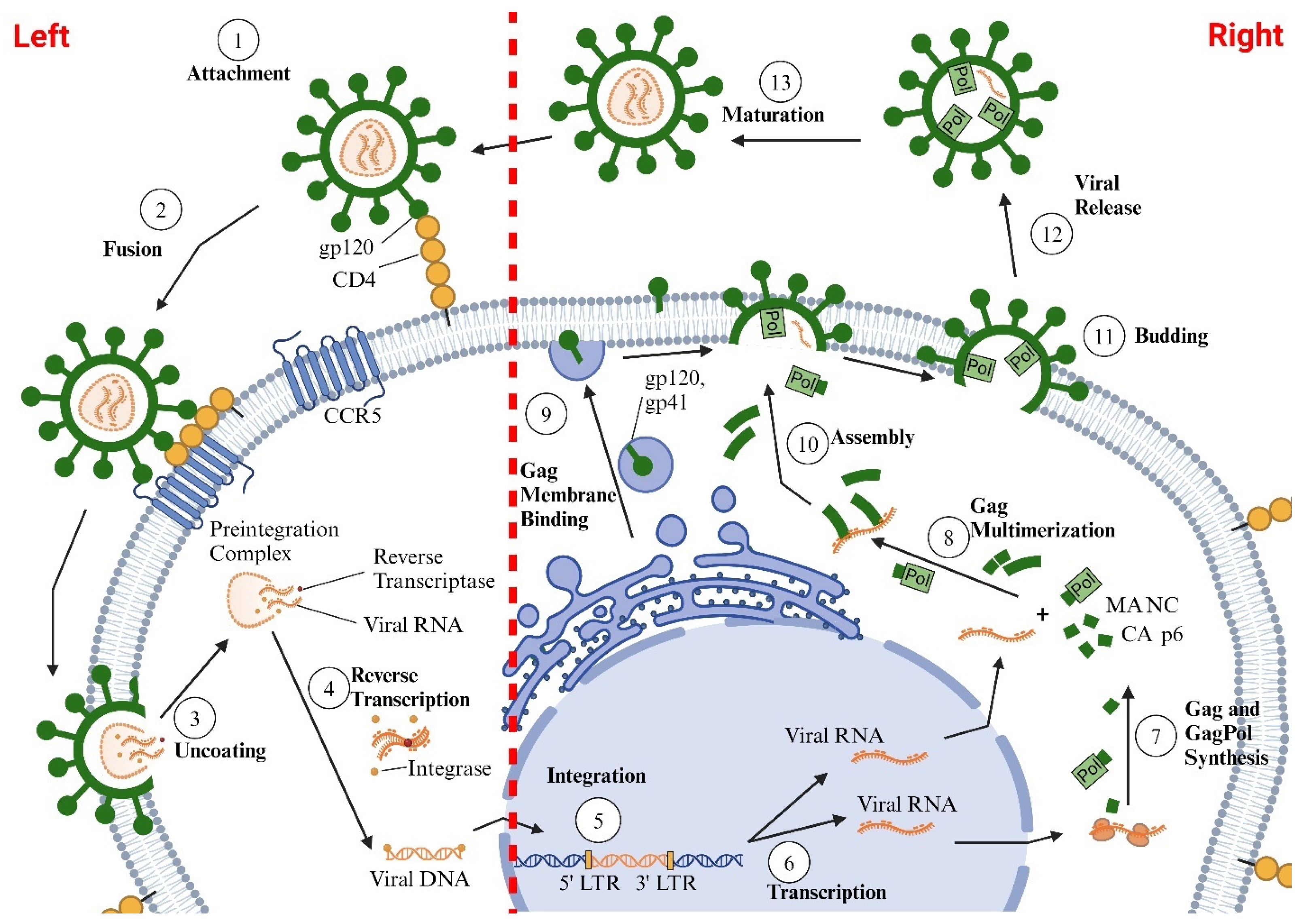
2. Life Cycle of HIV
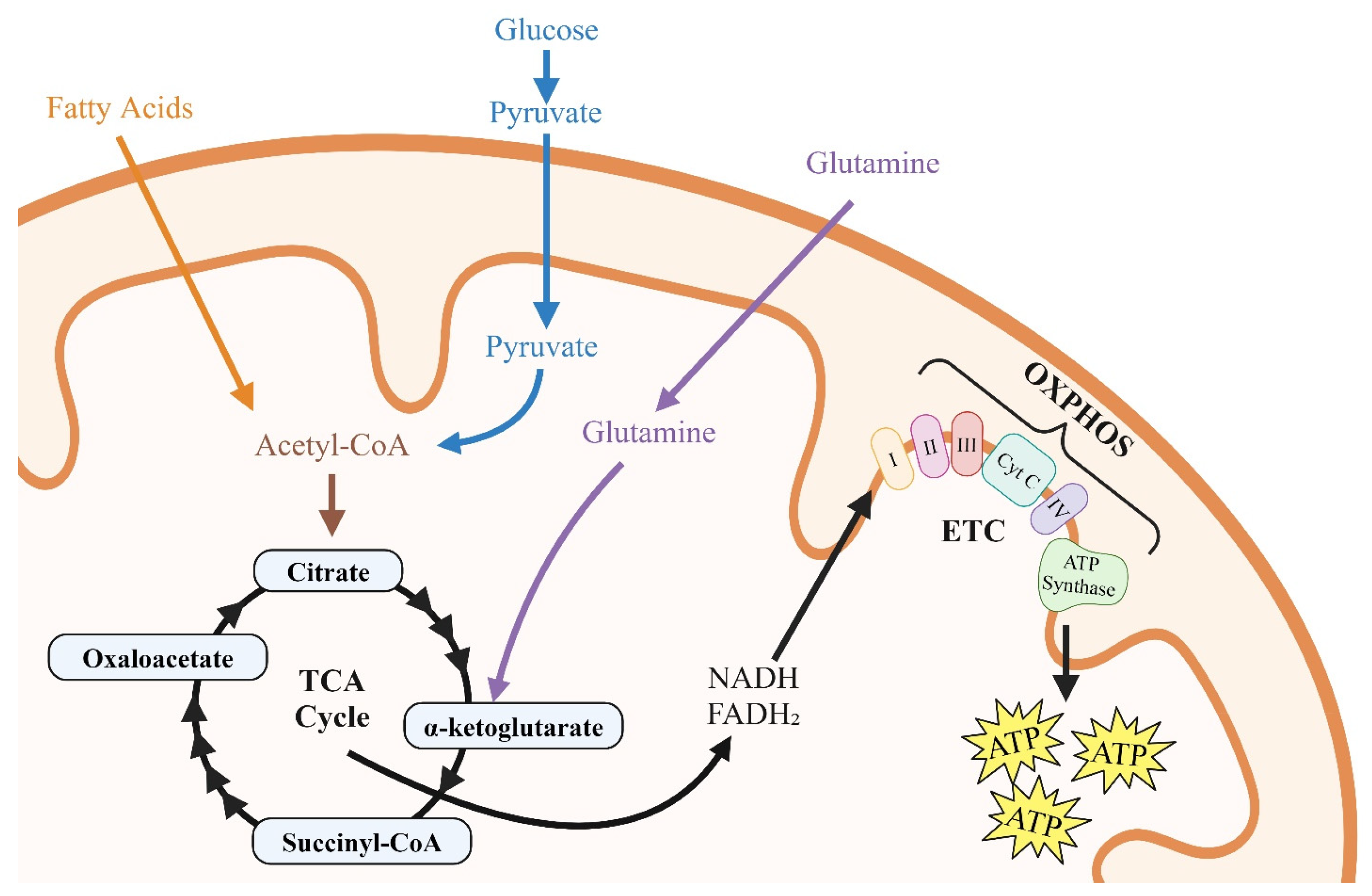
3. Energy Substrates
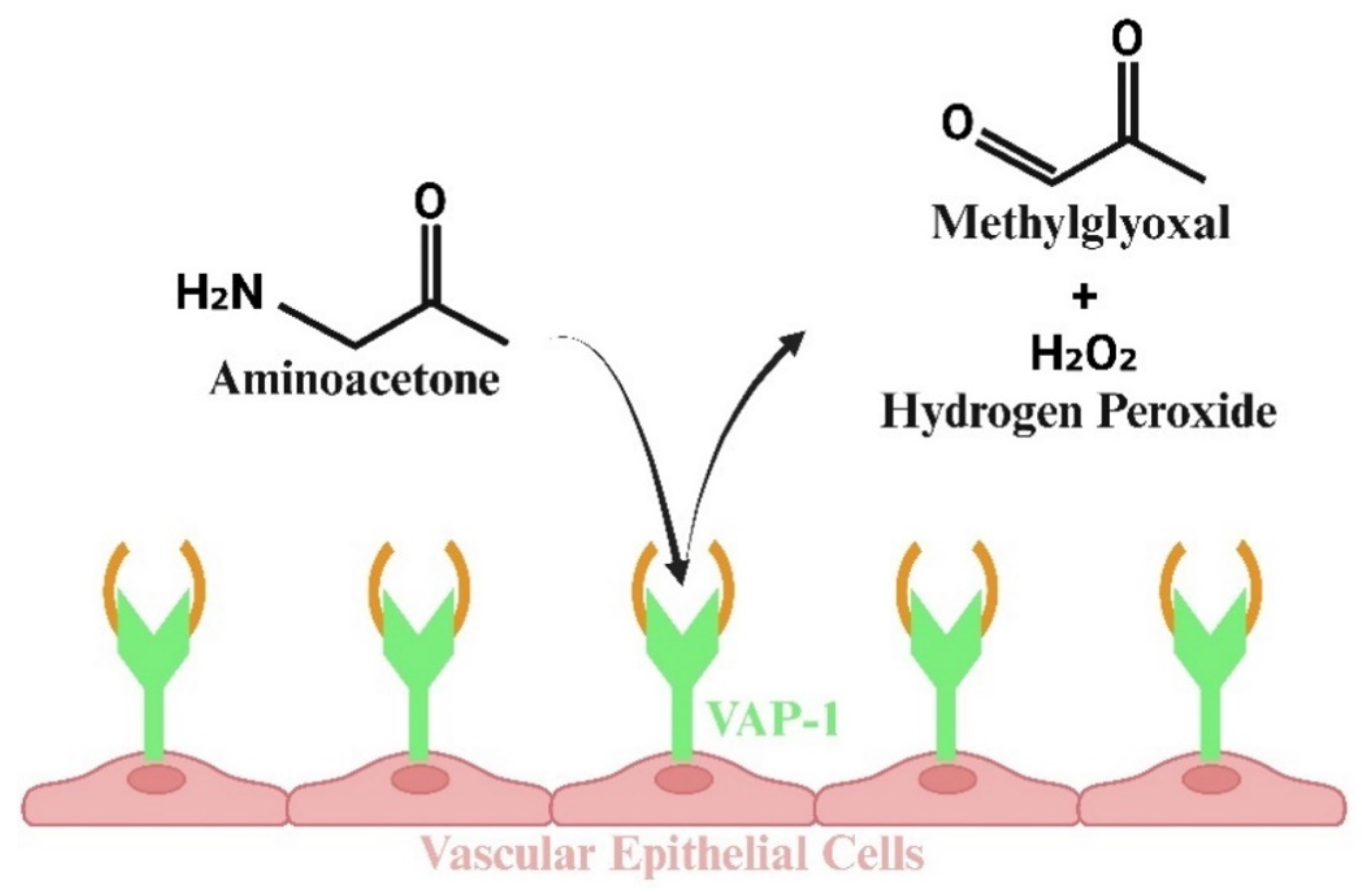
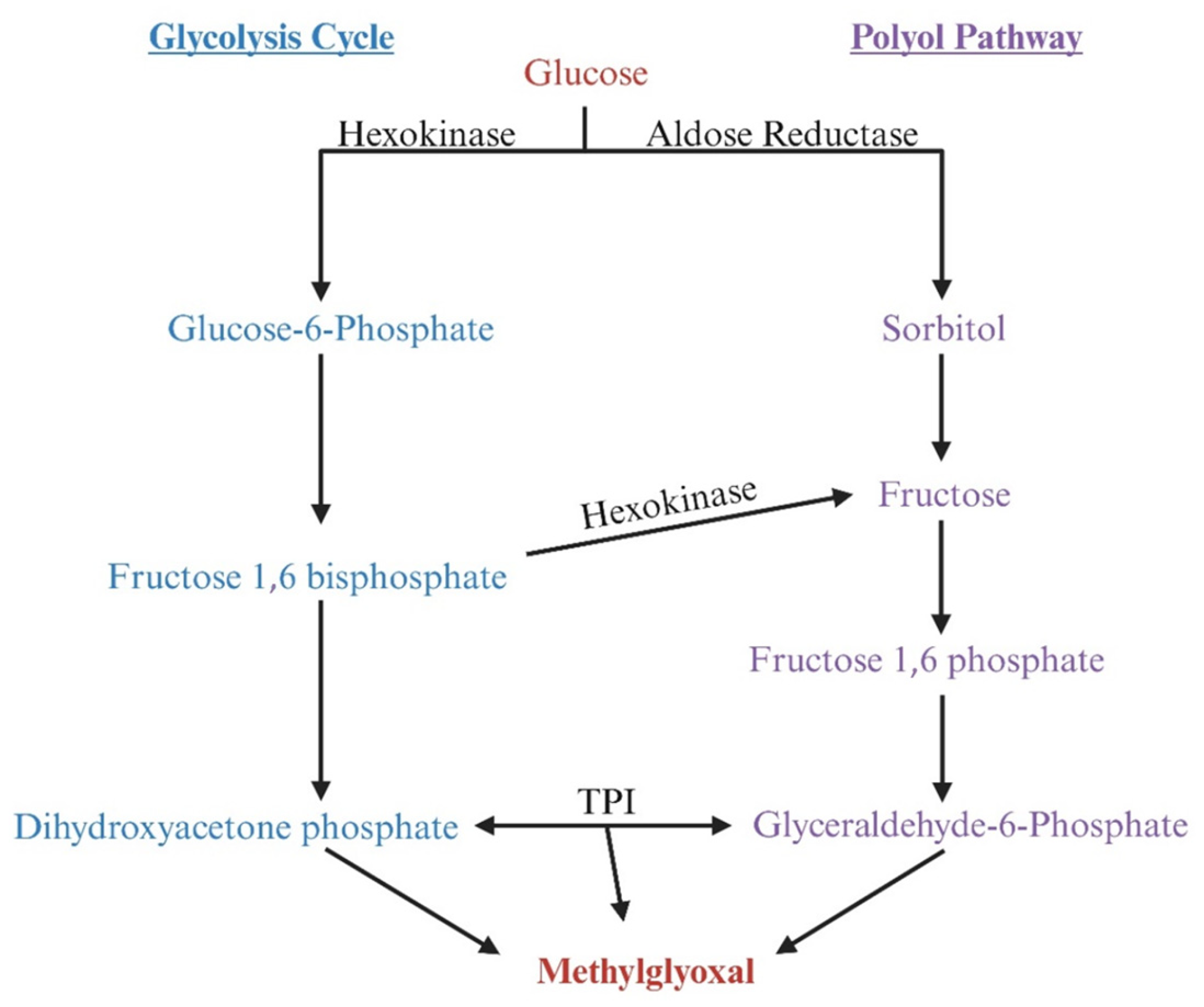
4. Methylglyoxal Production
5. Degradation of Methylglyoxal
5.1. MG Degradation via Glyoxalase System
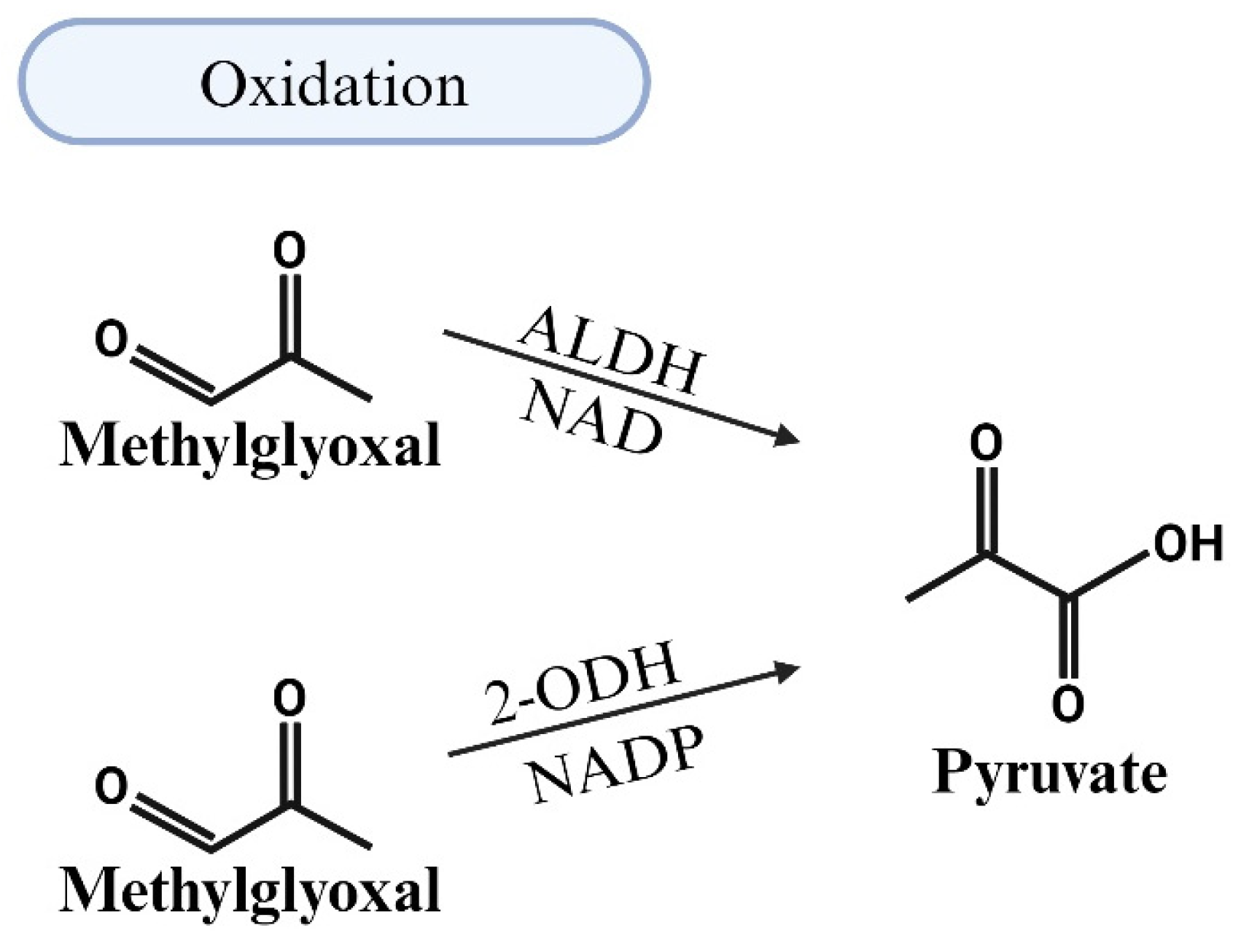
5.2. MG Degradation via Oxidation
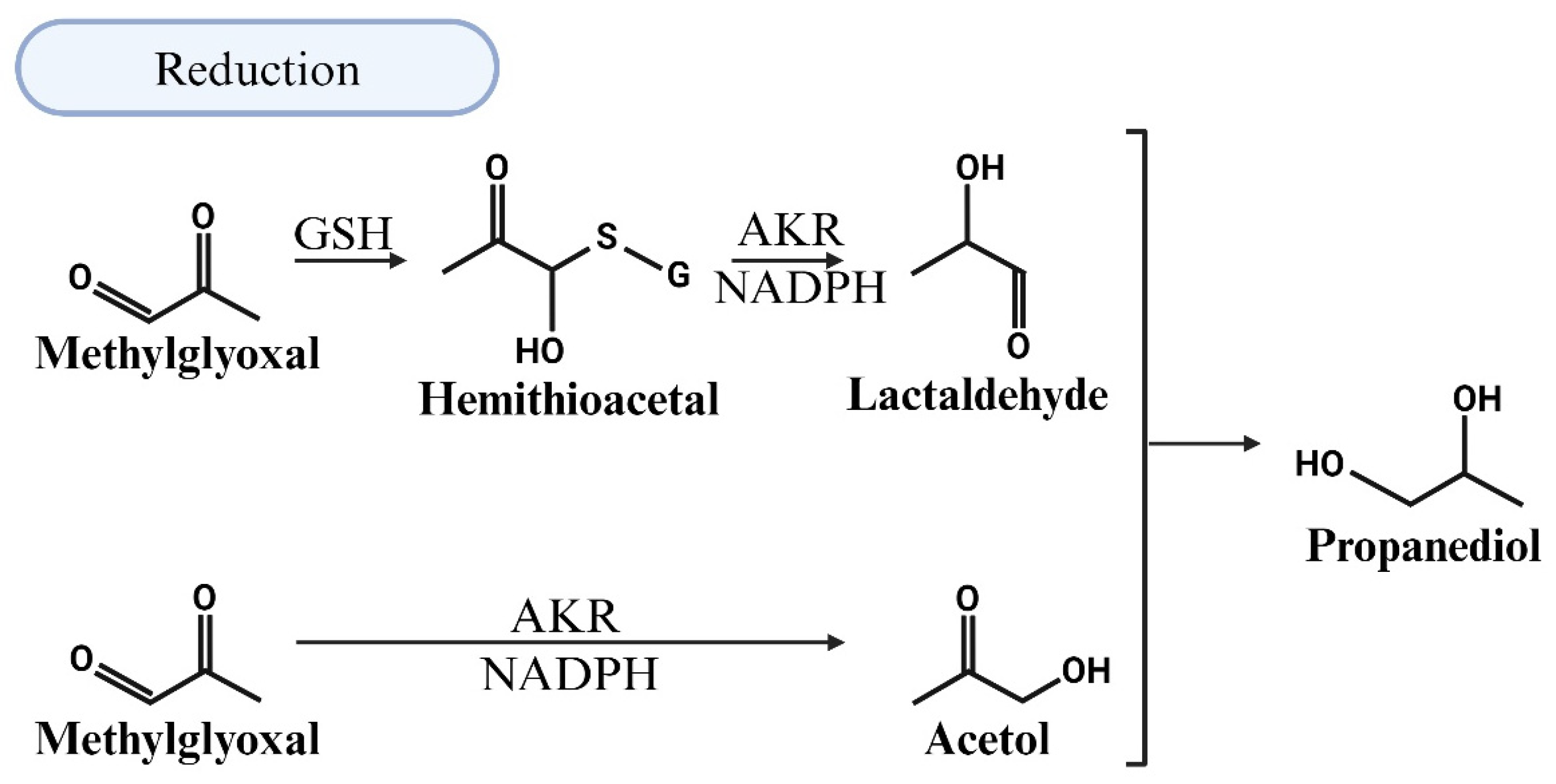
5.3. MG Degradation via Reduction
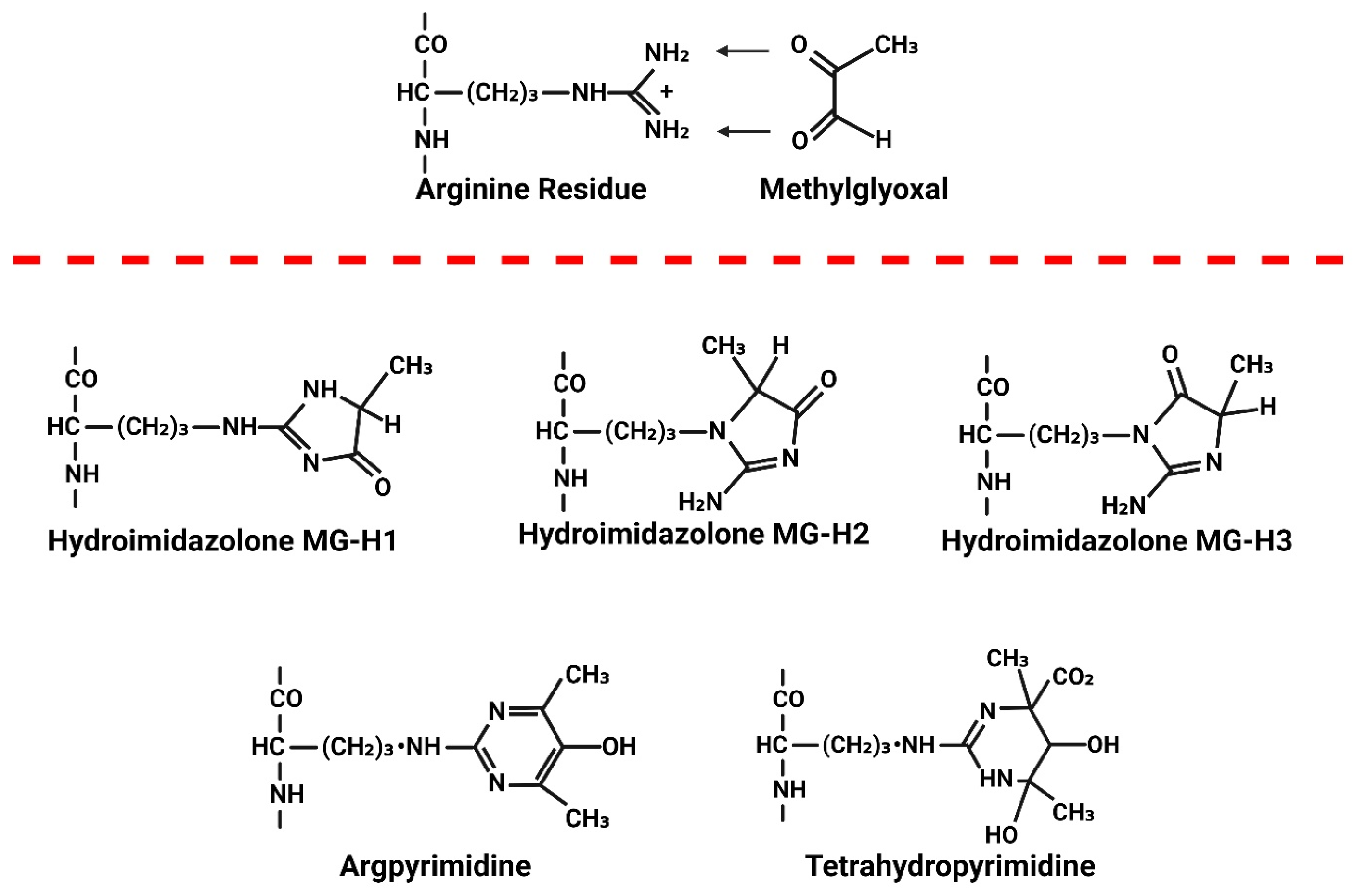
6. Consequences of Elevated MG
The Link Between Elevated MG and CVDs in HIV-1 Infection
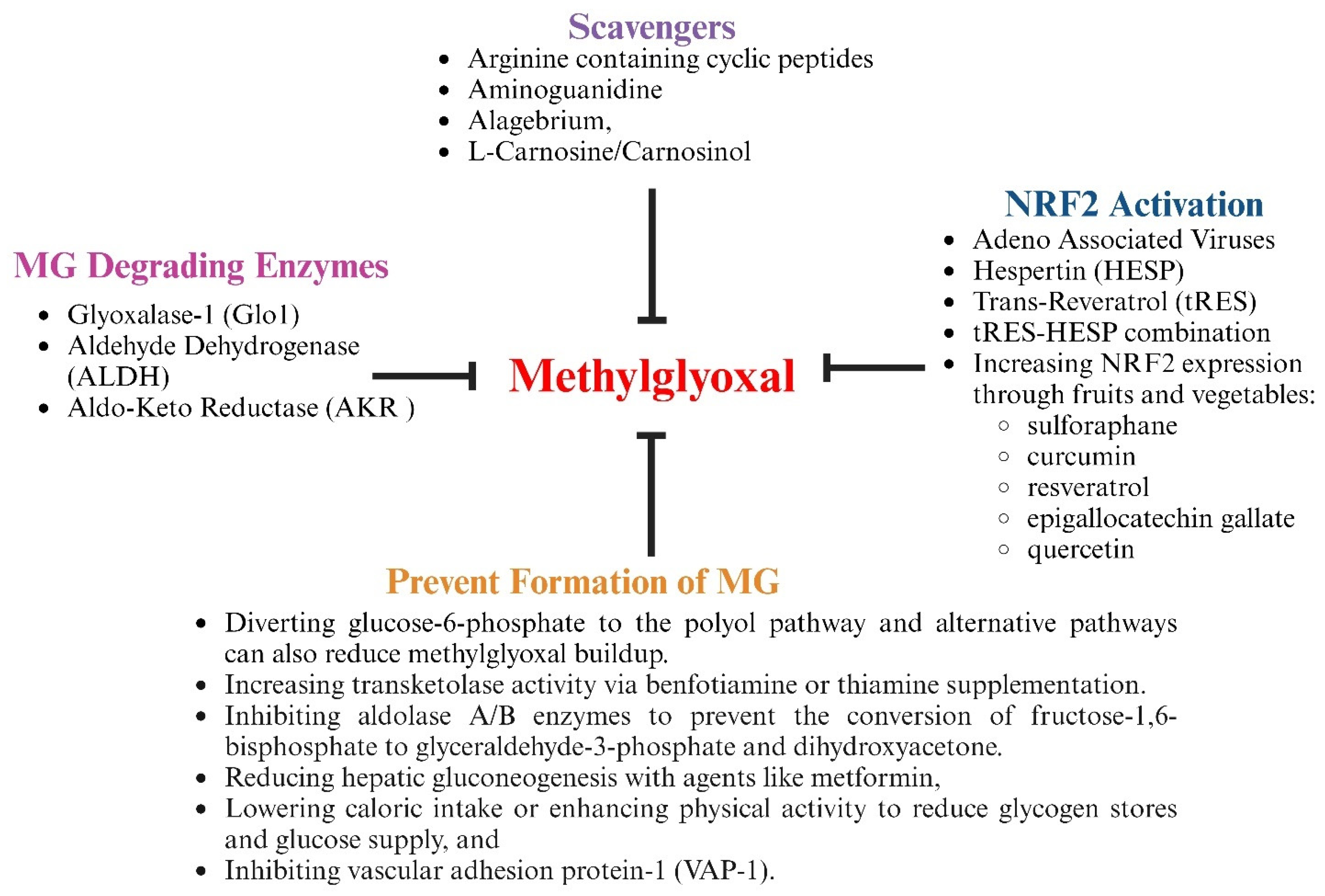
7. Therapeutic Strategies to Lower Methylglyoxal Levels
7.1. Treatment with Agents Containing Nucleophilic Groups to Scavenge MG
7.2. Treatment with Agents to Prevent or Reduce the Formation of MG
7.3. Treatment with Agents to Increase Expression of Nrf2 and MG-Degrading Enzymes
7.4. Treatment to Increase Co-Factors for MG-Degrading Enzymes
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frescura, L.; Godfrey-Faussett, P.; Feizzadeh, A.; El-Sadr, W.; Syarif, O.; Ghys, P.D.; on and behalf of the 2025 testing treatment target Working Group. Achieving the 95 95 95 targets for all: A pathway to ending AIDS. PLoS ONE 2022, 17, e0272405. [Google Scholar] [CrossRef] [PubMed]
- Raymond, H.F.; Scheer, S.; Santos, G.M.; McFarland, W. Examining progress toward the UNAIDS 90-90-90 framework among men who have sex with men, San Francisco, 2014. AIDS Care 2016, 28, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Granich, R.; Gupta, S.; Hall, I.; Aberle-Grasse, J.; Hader, S.; Mermin, J. Status and methodology of publicly available national HIV care continua and 90-90-90 targets: A systematic review. PLoS Med. 2017, 14, e1002253. [Google Scholar] [CrossRef] [PubMed]
- Grobler, A.; Cawood, C.; Khanyile, D.; Puren, A.; Kharsany, A.B. Progress of UNAIDS 90-90-90 targets in a district in KwaZulu-Natal, South Africa, with high HIV burden, in the HIPSS study: A household-based complex multilevel community survey. Lancet HIV 2017, 4, e505–e513. [Google Scholar] [CrossRef]
- Labhardt, N.; Ringera, I.; Lejone, T.; Cheleboi, M.; Wagner, S.; Muhairwe, J.; Klimkait, T. When patients fail UNAIDS’last 90-the. J. Int. AIDS Soc. 2017. [Google Scholar]
- Zhao, Y.; Han, M.; Ma, Y.; Li, D. Progress Towards the 90-90-90 Targets for Controlling HIV—China, 2018. China CDC Wkly 2019, 1, 5–7. [Google Scholar]
- Green, D.; Kharono, B.; Tordoff, D.M.; Akullian, A.; Bershteyn, A.; Morrison, M.; Garnett, G.; Duerr, A.; Drain, P. Demographic and risk group heterogeneity across the UNAIDS 90-90-90 targets: A systematic review and meta-analysis protocol. Syst. Rev. 2019, 8, 110. [Google Scholar]
- Ghazy, R.M.; Al Awaidy, S.; Taha, S.H.N. Trends of HIV indicators in Egypt from 1990 to 2021: Time-series analysis and forecast toward UNAIDS 90-90-90 targets. BMC Public Health 2023, 23, 625. [Google Scholar]
- de Bree, G.J.; van Sighem, A.; Zuilhof, W.; van Bergen, J.; Prins, M.; Heidenrijk, M.; van der Valk, M.; Brokx, P.; Reiss, P.; Initiative, H.I.V.T.E.A. Is reaching 90-90-90 enough to end AIDS? Lessons from Amsterdam. Curr. Opin. HIV AIDS 2019, 14, 455–463. [Google Scholar] [CrossRef]
- Suleman, M.; Khan, S.U.; Hussain, T.; Khan, M.U.; Shamsul Hassan, S.; Majid, M.; Khan, S.U.; Shehzad Khan, M.; Shan Ahmad, R.U.; Arif, M.; et al. Cardiovascular challenges in the era of antiretroviral therapy for AIDS/HIV: A comprehensive review of research advancements, pathophysiological insights, and future directions. Curr. Probl. Cardiol. 2024, 49, 102353. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, W.; He, J.; Duan, W.; Ma, X.; Guan, H.; Wu, Y.; Li, S.; Li, Y.; Tian, T. Higher cardiovascular disease risks in people living with HIV: A systematic review and meta-analysis. J. Glob. Health 2024, 14, 04078. [Google Scholar] [PubMed]
- Nazari, I.; Feinstein, M.J. Evolving mechanisms and presentations of cardiovascular disease in people with HIV: Implications for management. Clin. Microbiol. Rev. 2024, 37, e00098-22. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, M.J.; Hsue, P.Y.; Benjamin, L.A.; Bloomfield, G.S.; Currier, J.S.; Freiberg, M.S.; Grinspoon, S.K.; Levin, J.; Longenecker, C.T.; Post, W.S. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: A scientific statement from the American Heart Association. Circulation 2019, 140, e98–e124. [Google Scholar] [PubMed]
- Hsue, P.Y. Mechanisms of Cardiovascular Disease in the Setting of HIV Infection. Can. J. Cardiol. 2019, 35, 238–248. [Google Scholar] [CrossRef]
- Stone, L.; Looby, S.E.; Zanni, M.V. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr. Opin. HIV AIDS 2017, 12, 585–593. [Google Scholar] [CrossRef]
- Kovacs, L.; Kress, T.C.; Belin de Chantemèle, E.J. HIV, combination antiretroviral therapy, and vascular diseases in men and women. Basic. Transl. Sci. 2022, 7, 410–421. [Google Scholar]
- Raffe, S.; Sabin, C.; Gilleece, Y.; Women Against Viruses in Europe, E.A.C.S. Comorbidities in women living with HIV: A systematic review. HIV Med. 2022, 23, 331–361. [Google Scholar]
- Group, T.A.S. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N. Engl. J. Med. 2015, 373, 808–822. [Google Scholar]
- Lundgren, J.D.; Borges, A.H.; Neaton, J.D. Serious Non-AIDS Conditions in HIV: Benefit of Early ART. Curr. HIV/AIDS Rep. 2018, 15, 162–171. [Google Scholar] [CrossRef]
- Strategies for Management of Antiretroviral Therapy Study Group; Emery, S.; Neuhaus, J.A.; Phillips, A.N.; Babiker, A.; Cohen, C.J.; Gatell, J.M.; Girard, P.M.; Grund, B.; Law, M.; et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J. Infect. Dis. 2008, 197, 1133–1144. [Google Scholar] [CrossRef]
- Ruamtawee, W.; Tipayamongkholgul, M.; Aimyong, N.; Manosuthi, W. Prevalence and risk factors of cardiovascular disease among people living with HIV in the Asia-Pacific region: A systematic review. BMC Public Health 2023, 23, 477. [Google Scholar] [CrossRef] [PubMed]
- Pallipamu, N.; Taheri, S.; Thiagaraj, S.S.; Shukla, T.S.; Gutlapalli, S.D.; Farhat, H.; Irfan, H.; Muthiah, K.; Alfonso, M. A systematic review of how to reduce morbidity in hiv patients with cardiovascular diseases. Cureus 2023, 15, e34745. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, W.; Manuel, J.I.; Kariuki, N.; Tuchman, E.; O’Neal, J.; Lalanne, G.A. HIV and smoking: Associated risks and prevention strategies. HIV AIDS 2016, 8, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; de Boer, R.; Brul, S.; Budovskaya, Y.; van Spek, H. Premature and accelerated aging: HIV or HAART? Front. Genet. 2012, 3, 328. [Google Scholar] [CrossRef]
- Cohen, J.; D’Agostino, L.; Tuzer, F.; Torres, C. HIV antiretroviral therapy drugs induce premature senescence and altered physiology in HUVECs. Mech. Ageing Dev. 2018, 175, 74–82. [Google Scholar] [CrossRef]
- Grozdeva, R.; Ivanov, D.; Strashimirov, D.; Kapincheva, N.; Yordanova, R.; Mihailova, S.; Georgieva, A.; Alexiev, I.; Grigorova, L.; Partsuneva, A. Relationship between Modern ART Regimens and Immunosenescence Markers in Patients with Chronic HIV Infection. Viruses 2024, 16, 1205. [Google Scholar] [CrossRef]
- Owen, A.; Rannard, S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv. Drug Deliv. Rev. 2016, 103, 144–156. [Google Scholar] [CrossRef]
- Puri, A.; Sivaraman, A.; Zhang, W.; Clark, M.R.; Banga, A.K. Expanding the domain of drug delivery for HIV prevention: Exploration of the transdermal route. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 551–587. [Google Scholar] [CrossRef]
- Surve, D.H.; Jindal, A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J. Control Release 2020, 324, 379–404. [Google Scholar] [CrossRef]
- Nayan, M.U.; Sillman, B.; Hasan, M.; Deodhar, S.; Das, S.; Sultana, A.; Le, N.T.H.; Soriano, V.; Edagwa, B.; Gendelman, H.E. Advances in long-acting slow effective release antiretroviral therapies for treatment and prevention of HIV infection. Adv. Drug Deliv. Rev. 2023, 200, 115009. [Google Scholar] [CrossRef]
- Ntsekhe, M.; Baker, J.V. Cardiovascular disease among persons living with HIV: New insights into pathogenesis and clinical manifestations in a global context. Circulation 2023, 147, 83–100. [Google Scholar] [PubMed]
- El Kamari, V.; Rodriguez, K.; Moser, C.; Currier, J.S.; Kelesidis, T.; Stein, J.H.; Brown, T.T.; Howell, S.K.; Beisswenger, P.J.; McComsey, G.A. Advanced Glycation End Products Associated With Cardiometabolic Biomarkers in Treated Human Immunodeficiency Virus Infection. Open Forum Infect. Dis. 2021, 8, ofab423. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Alomar, F.A.; Cox, J.L.; McMillan, J.; Hackfort, B.T.; Makarov, E.; Morsey, B.; Fox, H.S.; Gendelman, H.E.; Gorantla, S.; et al. A Link Between Methylglyoxal and Heart Failure During HIV-1 Infection. Front. Cardiovasc. Med. 2021, 8, 792180. [Google Scholar] [CrossRef]
- Douek, D.C.; Brenchley, J.M.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Okamoto, Y.; Casazza, J.P.; Kuruppu, J.; Kunstman, K.; Wolinsky, S.; et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002, 417, 95–98. [Google Scholar] [CrossRef]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef]
- Zink, W.E.; Zheng, J.; Persidsky, Y.; Poluektova, L.; Gendelman, H.E. The neuropathogenesis of HIV-1 infection. FEMS Immunol. Med. Microbiol. 1999, 26, 233–241. [Google Scholar] [CrossRef]
- Naranjo, O.; Torices, S.; Clifford, P.R.; Daftari, M.T.; Osborne, O.M.; Fattakhov, N.; Toborek, M. Pericyte infection by HIV-1: A fatal attraction. Retrovirology 2022, 19, 27. [Google Scholar]
- Li, G.H.; Henderson, L.; Nath, A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr. HIV Res. 2016, 14, 373–381. [Google Scholar] [CrossRef]
- Nottet, H.S. Interactions between macrophages and brain microvascular endothelial cells: Role in pathogenesis of HIV-1 infection and blood—Brain barrier function. J. Neurovirol 1999, 5, 659–669. [Google Scholar] [CrossRef]
- Rodriguez, E.R.; Nasim, S.; Hsia, J.; Sandin, R.L.; Ferreira, A.; Hilliard, B.A.; Ross, A.M.; Garrett, C.T. Cardiac myocytes and dendritic cells harbor human immunodeficiency virus in infected patients with and without cardiac dysfunction: Detection by multiplex, nested, polymerase chain reaction in individually microdissected cells from right ventricular endomyocardial biopsy tissue. Am. J. Cardiol. 1991, 68, 1511–1520. [Google Scholar] [CrossRef]
- Moris, A.; Pajot, A.; Blanchet, F.; Guivel-Benhassine, F.; Salcedo, M.; Schwartz, O. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 2006, 108, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Valle-Casuso, J.C.; Angin, M.; Volant, S.; Passaes, C.; Monceaux, V.; Mikhailova, A.; Bourdic, K.; Avettand-Fenoel, V.; Boufassa, F.; Sitbon, M.; et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4+ T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2019, 29, 611–626.e5. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, A.; Kavanagh Williamson, M.; Huthoff, H. HIV-1 pathogenicity and virion production are dependent on the metabolic phenotype of activated CD4+ T cells. Retrovirology 2014, 11, 98. [Google Scholar] [CrossRef]
- Joshi, A.; Garg, H.; Ablan, S.; Freed, E.O.; Nagashima, K.; Manjunath, N.; Shankar, P. Targeting the HIV entry, assembly and release pathways for anti-HIV gene therapy. Virology 2011, 415, 95–106. [Google Scholar] [CrossRef]
- Church, J.D.; Huang, W.; Mwatha, A.; Toma, J.; Stawiski, E.; Donnell, D.; Guay, L.A.; Mmiro, F.; Musoke, P.; Jackson, J.B.; et al. HIV-1 tropism and survival in vertically infected Ugandan infants. J. Infect. Dis. 2008, 197, 1382–1388. [Google Scholar] [CrossRef]
- Poli, G.; Buonaguro, L. Introducing the issue on “differential use of CCR5 versus CXCR4 by HIV-1. Pathogenic, translational and clinical open questions”. J. Transl. Med. 2011, 9 (Suppl. S1), I1. [Google Scholar] [CrossRef][Green Version]
- Barmania, F.; Pepper, M.S. C-C chemokine receptor type five (CCR5): An emerging target for the control of HIV infection. Appl. Transl. Genom. 2013, 2, 3–16. [Google Scholar] [CrossRef]
- Graumann, U.; Ritz, M.F.; Rivero, B.G.; Hausmann, O. CD133 expressing pericytes and relationship to SDF-1 and CXCR4 in spinal cord injury. Curr. Neurovasc Res. 2010, 7, 144–154. [Google Scholar] [CrossRef]
- Pyo, R.T.; Sui, J.; Dhume, A.; Palomeque, J.; Blaxall, B.C.; Diaz, G.; Tunstead, J.; Logothetis, D.E.; Hajjar, R.J.; Schecter, A.D. CXCR4 modulates contractility in adult cardiac myocytes. J. Mol. Cell Cardiol. 2006, 41, 834–844. [Google Scholar] [CrossRef]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic substrate utilization in stress-induced immune cells. Intensive Care Med. Exp. 2020, 8, 28. [Google Scholar] [CrossRef]
- Kang, S.; Tang, H. HIV-1 Infection and Glucose Metabolism Reprogramming of T Cells: Another Approach Toward Functional Cure and Reservoir Eradication. Front. Immunol. 2020, 11, 572677. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, G.J.; Pearce, E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012, 249, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, L.R.; Maldarelli, F.; Chamoun, G.; Schilling, B.; Chokekijcahi, S.; Staudt, L.; Mitsuya, H.; Simpson, I.A.; Zeichner, S.L. Human immunodeficiency virus type 1 infection of H9 cells induces increased glucose transporter expression. J. Virol. 1996, 70, 7275–7279. [Google Scholar] [CrossRef]
- Kishimoto, N.; Yamamoto, K.; Abe, T.; Yasuoka, N.; Takamune, N.; Misumi, S. Glucose-dependent aerobic glycolysis contributes to recruiting viral components into HIV-1 particles to maintain infectivity. Biochem. Biophys. Res. Commun. 2021, 549, 187–193. [Google Scholar] [CrossRef]
- Palmer, C.S.; Ostrowski, M.; Gouillou, M.; Tsai, L.; Yu, D.; Zhou, J.; Henstridge, D.C.; Maisa, A.; Hearps, A.C.; Lewin, S.R.; et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS 2014, 28, 297–309. [Google Scholar] [CrossRef]
- Palmer, C.S.; Crowe, S.M. The role of glucose and lipid metabolism in the pathogenesis of HIV-1 infection. Immunology 2012, 13, 37–50. [Google Scholar]
- Scholz, E.M.B.; Kashuba, A.D.M. The Lymph Node Reservoir: Physiology, HIV Infection, and Antiretroviral Therapy. Clin. Pharmacol. Ther. 2021, 109, 918–927. [Google Scholar] [CrossRef]
- Simonetti, F.R.; Sobolewski, M.D.; Fyne, E.; Shao, W.; Spindler, J.; Hattori, J.; Anderson, E.M.; Watters, S.A.; Hill, S.; Wu, X.; et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 1883–1888. [Google Scholar] [CrossRef]
- Richard, J.P. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans. 1993, 21, 549–553. [Google Scholar] [CrossRef]
- Phillips, S.A.; Thornalley, P.J. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 1993, 212, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem. J. 1988, 254, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Tajes, M.; Guivernau, B.; Ramos-Fernandez, E.; Bosch-Morato, M.; Palomer, E.; Guix, F.X.; Munoz, F.J. The pathophysiology of triose phosphate isomerase dysfunction in Alzheimer’s disease. Histol. Histopathol. 2013, 28, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, S.; Ibrahim, A.; Rahman, M.D.M.; Eid, A.H.; Munusamy, S. Role of Methylglyoxal in Diabetic Cardiovascular and Kidney Diseases: Insights from Basic Science for Application into Clinical Practice. Curr. Pharm. Des. 2018, 24, 3072–3083. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. VAP-1: An adhesin and an enzyme. Trends Immunol. 2001, 22, 211–216. [Google Scholar]
- Yu, P.H.; Wright, S.; Fan, E.H.; Lun, Z.R.; Gubisne-Harberle, D. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim. Biophys. Acta 2003, 1647, 193–199. [Google Scholar] [CrossRef]
- Jaakkola, K.; Kaunismaki, K.; Tohka, S.; Yegutkin, G.; Vanttinen, E.; Havia, T.; Pelliniemi, L.J.; Virolainen, M.; Jalkanen, S.; Salmi, M. Human vascular adhesion protein-1 in smooth muscle cells. Am. J. Pathol. 1999, 155, 1953–1965. [Google Scholar] [CrossRef]
- Alomar, F.; Singh, J.; Jang, H.S.; Rozanzki, G.J.; Shao, C.H.; Padanilam, B.J.; Mayhan, W.G.; Bidasee, K.R. Smooth muscle-generated methylglyoxal impairs endothelial cell-mediated vasodilatation of cerebral microvessels in type 1 diabetic rats. Br. J. Pharmacol. 2016, 173, 3307–3326. [Google Scholar]
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Vander Jagt, D.L. Methylglyoxal, diabetes mellitus and diabetic complications. Drug Metabol. Drug Interact. 2008, 23, 93–124. [Google Scholar] [CrossRef]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, T.; Yabe-Nishimura, C. Transcription factor Nrf2 regulates promoter activity of mouse aldose reductase (AKR1B3) gene. J. Pharmacol. Sci. 2005, 97, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Abordo, E.A.; Minhas, H.S.; Thornalley, P.J. Accumulation of alpha-oxoaldehydes during oxidative stress: A role in cytotoxicity. Biochem. Pharmacol. 1999, 58, 641–648. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, D.M.; Kwon, K.; Park, C. Generation and characterization of mouse knockout for glyoxalase 1. Biochem. Biophys. Res. Commun. 2017, 490, 460–465. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Lee, Y.S.; Liu, Y.L.; Hsu, W.C.; Ho, W.M.; Huang, Y.H.; Tsai, S.J.; Kuo, P.H.; Chen, Y.C. Association Study of Alcohol Dehydrogenase and Aldehyde Dehydrogenase Polymorphism With Alzheimer Disease in the Taiwanese Population. Front. Neurosci. 2021, 15, 625885. [Google Scholar] [CrossRef]
- Giacco, F.; Du, X.; D’Agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Dobariya, P.; Xie, W.; Rao, S.P.; Xie, J.; Seelig, D.M.; Vince, R.; Lee, M.K.; More, S.S. Deletion of Glyoxalase 1 exacerbates acetaminophen-induced hepatotoxicity in mice. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nigro, C.; Leone, A.; Longo, M.; Prevenzano, I.; Fleming, T.H.; Nicolo, A.; Parrillo, L.; Spinelli, R.; Formisano, P.; Nawroth, P.P.; et al. Methylglyoxal accumulation de-regulates HoxA5 expression, thereby impairing angiogenesis in glyoxalase 1 knock-down mouse aortic endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 73–85. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Geoffrion, M.; Kuraitis, D.; McBane, J.E.; Lochhead, M.; Vanderhyden, B.C.; Korbutt, G.S.; Milne, R.W.; Suuronen, E.J. Glyoxalase-1 overexpression in bone marrow cells reverses defective neovascularization in STZ-induced diabetic mice. Cardiovasc. Res. 2014, 101, 306–316. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.; Miyata, T.; Ostergaard, J.A.; Flyvbjerg, A.; Peutz-Kootstra, C.J.; Sieber, J.; Mundel, P.H.; Brownlee, M.; Janssen, B.J.; et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 2014, 57, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, D.P.; Lou, B.; Middel, C.S.; Morgenstern, J.; Fleming, T.; Sticht, C.; Hausser, I.; Hell, R.; Hammes, H.P.; Szendrodi, J.; et al. Accumulation of acetaldehyde in aldh2.1−/− zebrafish causes increased retinal angiogenesis and impaired glucose metabolism. Redox Biol. 2022, 50, 102249. [Google Scholar] [CrossRef] [PubMed]
- Jana, G.A.; Yaish, M.W. Functional characterization of the Glyoxalase-I (PdGLX1) gene family in date palm under abiotic stresses. Plant Signal Behav. 2020, 15, 1811527. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Staitieh, B.S.; Jensen, J.S.; Mould, K.J.; Greenberg, J.A.; Joshi, P.C.; Koval, M.; Guidot, D.M. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L267–L277. [Google Scholar] [CrossRef]
- Han, D.; Lu, X.; Yin, W.; Fu, H.; Zhang, X.; Cheng, L.; Liu, F.; Jin, C.; Tian, X.; Xie, Y.; et al. Activation of NRF2 blocks HIV replication and apoptosis in macrophages. Heliyon 2023, 9, e12575. [Google Scholar] [CrossRef]
- Staitieh, B.S.; Ding, L.; Neveu, W.A.; Spearman, P.; Guidot, D.M.; Fan, X. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. J. Leukoc. Biol. 2017, 102, 517–525. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G.; Denaro, F.; Calabrese, V.; Benedetti, F.; Krishnan, S.; Curreli, S.; Bryant, J.; Zella, D. Altered expression pattern of Nrf2/HO-1 axis during accelerated-senescence in HIV-1 transgenic rat. Biogerontology 2014, 15, 449–461. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Glu504Lys Single Nucleotide Polymorphism of Aldehyde Dehydrogenase 2 Gene and the Risk of Human Diseases. Biomed. Res. Int. 2015, 2015, 174050. [Google Scholar] [CrossRef]
- Calleja, L.F.; Yoval-Sanchez, B.; Hernandez-Esquivel, L.; Gallardo-Perez, J.C.; Sosa-Garrocho, M.; Marin-Hernandez, A.; Jasso-Chavez, R.; Macias-Silva, M.; Salud Rodriguez-Zavala, J. Activation of ALDH1A1 by omeprazole reduces cell oxidative stress damage. FEBS J. 2021, 288, 4064–4080. [Google Scholar] [CrossRef]
- Chiu, C.C.; Yeh, T.H.; Lai, S.C.; Wu-Chou, Y.H.; Chen, C.H.; Mochly-Rosen, D.; Huang, Y.C.; Chen, Y.J.; Chen, C.L.; Chang, Y.M.; et al. Neuroprotective effects of aldehyde dehydrogenase 2 activation in rotenone-induced cellular and animal models of parkinsonism. Exp. Neurol. 2015, 263, 244–253. [Google Scholar] [CrossRef]
- Pastel, E.; Pointud, J.C.; Volat, F.; Martinez, A.; Lefrancois-Martinez, A.M. Aldo-Keto Reductases 1B in Endocrinology and Metabolism. Front. Pharmacol. 2012, 3, 148. [Google Scholar] [CrossRef]
- Maccari, R.; Ottana, R. Targeting aldose reductase for the treatment of diabetes complications and inflammatory diseases: New insights and future directions. J. Med. Chem. 2015, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.S.; Bhardwaj, S.; Pandita, D.; Lather, V.; Sekhon, B.S. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini Rev. Med. Chem. 2016, 16, 120–162. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L.; Robinson, B.; Taylor, K.K.; Hunsaker, L.A. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. J. Biol. Chem. 1992, 267, 4364–4369. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest. Ophthalmol. Vis. Sci. 2003, 44, 5287–5292. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J. Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence. Biochem. J. 2002, 364, 15–24. [Google Scholar] [CrossRef]
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar]
- Shuck, S.C.; Wuenschell, G.E.; Termini, J.S. Product Studies and Mechanistic Analysis of the Reaction of Methylglyoxal with Deoxyguanosine. Chem. Res. Toxicol. 2018, 31, 105–115. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.-A.; Kim, J.-H.; Kim, E.-H.; Bae, O.-N. Methylglyoxal-induced dysfunction in brain endothelial cells via the suppression of akt/HIF-1α pathway and activation of mitophagy associated with increased reactive oxygen species. Antioxidants 2020, 9, 820. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zheng, Y.; Jiang, J.; Wang, L.; Wu, J.; Zhang, C.; Luo, M. Glucose metabolite methylglyoxal induces vascular endothelial cell pyroptosis via NLRP3 inflammasome activation and oxidative stress in vitro and in vivo. Cell. Mol. Life Sci. 2024, 81, 401. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Swami Vetha, B.S.; Chakraborty, R.; Wenegieme, T.Y.; Masenga, S.K.; Muthian, G.; Balasubramaniam, M.; Wanjalla, C.N.; Hinton, A.O., Jr.; Kirabo, A.; et al. HIV-Associated Hypertension: Risks, Mechanisms, and Knowledge Gaps. Circ. Res. 2024, 134, e150–e175. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Stuckless, J. Role of methylglyoxal in essential hypertension. Int. J. Angiol. 2010, 19, e58–e65. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Goodall, R.L.; Dunn, D.T.; Franzen, S.; Collaco-Moraes, Y.; Gazzard, B.G.; Williams, I.G.; Fisher, M.J.; Winston, A.; Fox, J.; et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. JAMA 2012, 308, 353–361. [Google Scholar] [CrossRef]
- Savarino, A.; Shytaj, I.L. Chloroquine and beyond: Exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology 2015, 12, 51. [Google Scholar] [CrossRef]
- Markowitz, M.; Vaida, F.; Hare, C.B.; Boden, D.; Mohri, H.; Hecht, F.M.; Kalayjian, R.C.; Conrad, A.; Mildvan, D.; Aberg, J.; et al. The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. J. Infect. Dis. 2010, 201, 1298–1302. [Google Scholar] [CrossRef]
- Donia, M.; McCubrey, J.A.; Bendtzen, K.; Nicoletti, F. Potential use of rapamycin in HIV infection. Br. J. Clin. Pharmacol. 2010, 70, 784–793. [Google Scholar] [CrossRef]
- Obare, L.M.; Temu, T.; Mallal, S.A.; Wanjalla, C.N. Inflammation in HIV and Its Impact on Atherosclerotic Cardiovascular Disease. Circ. Res. 2024, 134, 1515–1545. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Li, D.; Ma, Y.; Ishai, A.; Manion, M.; Nahrendorf, M.; Ganz, P.; Ridker, P.M.; Deeks, S.G.; Tawakol, A. IL-1beta Inhibition Reduces Atherosclerotic Inflammation in HIV Infection. J. Am. Coll. Cardiol. 2018, 72, 2809–2811. [Google Scholar] [CrossRef]
- Ting, P.T.; Koo, J.Y. Use of etanercept in human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) patients. Int. J. Dermatol. 2006, 45, 689–692. [Google Scholar] [CrossRef]
- Freeman, M.L.; Clagett, B.M.; Moisi, D.; Yeh, E.; Morris, C.D.; Ryu, A.; Rodriguez, B.; Stein, J.H.; Deeks, S.G.; Currier, J.S.; et al. Methotrexate Inhibits T Cell Proliferation but Not Inflammatory Cytokine Expression to Modulate Immunity in People Living With HIV. Front. Immunol. 2022, 13, 924718. [Google Scholar] [CrossRef]
- Marconi, V.C.; Moser, C.; Gavegnano, C.; Deeks, S.G.; Lederman, M.M.; Overton, E.T.; Tsibris, A.; Hunt, P.W.; Kantor, A.; Sekaly, R.P.; et al. Randomized Trial of Ruxolitinib in Antiretroviral-Treated Adults With Human Immunodeficiency Virus. Clin. Infect. Dis. 2022, 74, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Dhar, I.; Desai, K.M.; Wu, L. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br. J. Pharmacol. 2010, 161, 1843–1856. [Google Scholar] [PubMed]
- Nigro, C.; Leone, A.; Raciti, G.A.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef]
- Berends, E.; van Oostenbrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 2023, 20, 75. [Google Scholar]
- Schlotterer, A.; Kolibabka, M.; Lin, J.; Acunman, K.; Dietrichá, N.; Sticht, C.; Fleming, T.; Nawroth, P.; Hammes, H.-P. Methylglyoxal induces retinopathy-type lesions in the absence of hyperglycemia: Studies in a rat model. FASEB J. 2019, 33, 4141–4153. [Google Scholar]
- Knott, H.M.; Brown, B.E.; Davies, M.J.; Dean, R.T. Glycation and glycoxidation of low-density lipoproteins by glucose and low-molecular mass aldehydes. Formation of modified and oxidized particles. Eur. J. Biochem. 2003, 270, 3572–3582. [Google Scholar] [CrossRef]
- Kirca, M. Methylglyoxal enhances the proliferation of vascular smooth muscle cells via Akt phosphorylation. J. Recept. Signal Transduct. Res. 2022, 42, 567–572. [Google Scholar] [CrossRef]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 2010, 56, 471–476. [Google Scholar] [CrossRef]
- van der Bruggen, M.M.; Spronck, B.; Delhaas, T.; Reesink, K.D.; Schalkwijk, C.G. The Putative Role of Methylglyoxal in Arterial Stiffening: A Review. Heart Lung Circ. 2021, 30, 1681–1693. [Google Scholar] [CrossRef]
- Hadas, K.; Randriamboavonjy, V.; Elgheznawy, A.; Mann, A.; Fleming, I. Methylglyoxal induces platelet hyperaggregation and reduces thrombus stability by activating PKC and inhibiting PI3K/Akt pathway. PLoS ONE 2013, 8, e74401. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-J.; Yang, H.-Y.; Pai, M.-H.; Wu, C.-H.; Chen, J.-R. Long-term administration of advanced glycation end-product stimulates the activation of NLRP3 inflammasome and sparking the development of renal injury. J. Nutr. Biochem. 2017, 39, 68–76. [Google Scholar] [CrossRef]
- Chu, P.; Han, G.; Ahsan, A.; Sun, Z.; Liu, S.; Zhang, Z.; Sun, B.; Song, Y.; Lin, Y.; Peng, J.; et al. Phosphocreatine protects endothelial cells from Methylglyoxal induced oxidative stress and apoptosis via the regulation of PI3K/Akt/eNOS and NF-kappaB pathway. Vasc. Pharmacol. 2017, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, O.S.; Kim, C.-S.; Kim, N.H.; Kim, J.S. Cytotoxic role of methylglyoxal in rat retinal pericytes: Involvement of a nuclear factor-kappaB and inducible nitric oxide synthase pathway. Chem. Biol. Interact. 2010, 188, 86–93. [Google Scholar] [PubMed]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef]
- Fleming, T.H.; Humpert, P.M.; Nawroth, P.P.; Bierhaus, A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process—A mini-review. Gerontology 2011, 57, 435–443. [Google Scholar]
- Hollenbach, M. The Role of Glyoxalase-I (Glo-I), Advanced Glycation Endproducts (AGEs), and Their Receptor (RAGE) in Chronic Liver Disease and Hepatocellular Carcinoma (HCC). Int. J. Mol. Sci. 2017, 18, 2466. [Google Scholar] [CrossRef]
- Ahmad, S.; Akhter, F.; Shahab, U.; Rafi, Z.; Khan, M.S.; Nabi, R.; Khan, M.S.; Ahmad, K.; Ashraf, J.M.; Moinuddin. Do all roads lead to the Rome? The glycation perspective! Semin. Cancer Biol. 2018, 49, 9–19. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Huang, Y.P.; Hsu, S.H.; Kang, L.Y.; Shen, C.M.; Lin, W.W. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J. Cell Mol. Med. 2016, 20, 1749–1760. [Google Scholar] [CrossRef]
- Shao, C.H.; Tian, C.; Ouyang, S.; Moore, C.J.; Alomar, F.; Nemet, I.; D’Souza, A.; Nagai, R.; Kutty, S.; Rozanski, G.J.; et al. Carbonylation induces heterogeneity in cardiac ryanodine receptor function in diabetes mellitus. Mol. Pharmacol. 2012, 82, 383–399. [Google Scholar] [CrossRef]
- Shao, C.H.; Rozanski, G.J.; Nagai, R.; Stockdale, F.E.; Patel, K.P.; Wang, M.; Singh, J.; Mayhan, W.G.; Bidasee, K.R. Carbonylation of myosin heavy chains in rat heart during diabetes. Biochem. Pharmacol. 2010, 80, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.H.; Capek, H.L.; Patel, K.P.; Wang, M.; Tang, K.; DeSouza, C.; Nagai, R.; Mayhan, W.; Periasamy, M.; Bidasee, K.R. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes 2011, 60, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Bovo, E.; Seflova, J.; Robia, S.L.; Zima, A.V. Protein carbonylation causes sarcoplasmic reticulum Ca2+ overload by increasing intracellular Na+ level in ventricular myocytes. Pflügers Arch. Eur. J. Physiol. 2024, 476, 1077–1086. [Google Scholar]
- Arsov, S.; Graaff, R.; van Oeveren, W.; Stegmayr, B.; Sikole, A.; Rakhorst, G.; Smit, A.J. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: A review. Clin. Chem. Lab. Med. 2014, 52, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal stress, the glyoxalase system, and diabetic chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Dimitropoulos, A.; Rosado, C.J.; Thomas, M.C. Dicarbonyl-mediated AGEing and diabetic kidney disease. J. Nephrol. 2020, 33, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Schmoch, T.; Uhle, F.; Siegler, B.H.; Fleming, T.; Morgenstern, J.; Nawroth, P.P.; Weigand, M.A.; Brenner, T. The Glyoxalase System and Methylglyoxal-Derived Carbonyl Stress in Sepsis: Glycotoxic Aspects of Sepsis Pathophysiology. Int. J. Mol. Sci. 2017, 18, 657. [Google Scholar] [CrossRef]
- van Bussel, B.C.; van de Poll, M.C.; Schalkwijk, C.G.; Bergmans, D.C. Increased Dicarbonyl Stress as a Novel Mechanism of Multi-Organ Failure in Critical Illness. Int. J. Mol. Sci. 2017, 18, 346. [Google Scholar] [CrossRef]
- Kold-Christensen, R.; Johannsen, M. Methylglyoxal metabolism and aging-related disease: Moving from correlation toward causation. Trends Endocrinol. Metab. 2020, 31, 81–92. [Google Scholar]
- Kim, J.Y.; Jung, J.H.; Lee, S.J.; Han, S.S.; Hong, S.H. Glyoxalase 1 as a Therapeutic Target in Cancer and Cancer Stem Cells. Mol. Cells 2022, 45, 869–876. [Google Scholar] [CrossRef]
- Papadaki, M.; Holewinski, R.J.; Previs, S.B.; Martin, T.G.; Stachowski, M.J.; Li, A.; Blair, C.A.; Moravec, C.S.; Van Eyk, J.E.; Campbell, K.S.; et al. Diabetes with heart failure increases methylglyoxal modifications in the sarcomere, which inhibit function. JCI Insight 2018, 3, e121264. [Google Scholar] [CrossRef] [PubMed]
- Bellier, J.; Nokin, M.J.; Larde, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcene, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Prisco, S.Z.; Hartweck, L.; Keen, J.L.; Vogel, N.; Kazmirczak, F.; Eklund, M.; Hemnes, A.R.; Brittain, E.L.; Prins, K.W. Glyoxylase-1 combats dicarbonyl stress and right ventricular dysfunction in rodent pulmonary arterial hypertension. Front. Cardiovasc. Med. 2022, 9, 940932. [Google Scholar] [CrossRef]
- Alomar, F.A.; Al-Rubaish, A.; Al-Muhanna, F.; Al-Ali, A.K.; McMillan, J.; Singh, J.; Bidasee, K.R. Adeno-Associated Viral Transfer of Glyoxalase-1 Blunts Carbonyl and Oxidative Stresses in Hearts of Type 1 Diabetic Rats. Antioxidants 2020, 9, 592. [Google Scholar] [CrossRef]
- Lewis, E.J.; Greene, T.; Spitalewiz, S.; Blumenthal, S.; Berl, T.; Hunsicker, L.G.; Pohl, M.A.; Rohde, R.D.; Raz, I.; Yerushalmy, Y. Pyridorin in type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 2012, 23, 131–136. [Google Scholar]
- Abouzed, T.K.; Munesue, S.; Harashima, A.; Masuo, Y.; Kato, Y.; Khailo, K.; Yamamoto, H.; Yamamoto, Y. Preventive effect of salicylate and pyridoxamine on diabetic nephropathy. J. Diabetes Res. 2016, 2016, 1786789. [Google Scholar]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar]
- Bonaccorso, A.; Privitera, A.; Grasso, M.; Salamone, S.; Carbone, C.; Pignatello, R.; Musumeci, T.; Caraci, F.; Caruso, G. The therapeutic potential of novel carnosine formulations: Perspectives for drug development. Pharmaceuticals 2023, 16, 778. [Google Scholar] [CrossRef]
- Brings, S.; Fleming, T.; De Buhr, S.; Beijer, B.; Lindner, T.; Wischnjow, A.; Kender, Z.; Peters, V.; Kopf, S.; Haberkorn, U. A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim. Et Biophys. Acta Mol. Basis Dis. 2017, 1863, 654–662. [Google Scholar]
- Hammes, H.-P.; Du, X.; Edelstein, D.; Taguchi, T.; Matsumura, T.; Ju, Q.; Lin, J.; Bierhaus, A.; Nawroth, P.; Hannak, D. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003, 9, 294–299. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Nizheradze, K.; Berrone, E.; Tarallo, S.; Porta, M. Thiamine and benfotiamine prevent apoptosis induced by high glucose-conditioned extracellular matrix in human retinal pericytes. Diabetes/Metab. Res. Rev. 2009, 25, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Clifton, P.M. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Delbin, M.A.; Davel, A.P.; Couto, G.K.; de Araujo, G.G.; Rossoni, L.V.; Antunes, E.; Zanesco, A. Interaction between advanced glycation end products formation and vascular responses in femoral and coronary arteries from exercised diabetic rats. PLoS ONE 2012, 7, e53318. [Google Scholar] [CrossRef]
- Tanaka, M.; Kanazashi, M.; Kondo, H.; Fujino, H. Methylglyoxal reduces resistance exercise-induced protein synthesis and anabolic signaling in rat tibialis anterior muscle. J. Muscle Res. Cell Motil. 2024, 45, 263–273. [Google Scholar] [CrossRef]
- Noda, K.; Miyahara, S.; Nakazawa, T.; Almulki, L.; Nakao, S.; Hisatomi, T.; She, H.; Thomas, K.L.; Garland, R.C.; Miller, J.W. Inhibition of vascular adhesion protein-1 suppresses endotoxin-induced uveitis. FASEB J. 2008, 22, 1094–1103. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Renfurm, R.W.; Bakris, G.; Rossing, P.; Perkovic, V.; Hou, F.F.; Nangaku, M.; Sharma, K.; Heerspink, H.J.; Garcia-Hernandez, A. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): A randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2018, 6, 925–933. [Google Scholar] [CrossRef]
- Li, H.; Du, S.; Niu, P.; Gu, X.; Wang, J.; Zhao, Y. Vascular adhesion Protein-1 (VAP-1)/Semicarbazide-sensitive amine oxidase (SSAO): A potential therapeutic target for atherosclerotic cardiovascular diseases. Front. Pharmacol. 2021, 12, 679707. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Sheng, Z.; Lin, H.; Weng, Z.; Song, W.; Cao, B.; Zhao, Y.; Gao, Y.; Ni, S. The first selective VAP-1 inhibitor in China, TT-01025-CL: Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-and multiple-ascending doses. Front. Pharmacol. 2024, 15, 1327008. [Google Scholar] [CrossRef]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals–just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [PubMed]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s expectation be matched by the reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar]
- Rosenbaugh, E.G.; Savalia, K.K.; Manickam, D.S.; Zimmerman, M.C. Antioxidant-based therapies for angiotensin II-associated cardiovascular diseases. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2013, 304, R917–R928. [Google Scholar] [PubMed]
- Ortiz, M.C.; Manriquez, M.C.; Romero, J.C.; Juncos, L.A. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 2001, 38, 655–659. [Google Scholar]
- Kumar, P.; Liu, C.; Suliburk, J.W.; Minard, C.G.; Muthupillai, R.; Chacko, S.; Hsu, J.W.; Jahoor, F.; Sekhar, R.V. Supplementing glycine and N-acetylcysteine (GlyNAC) in aging HIV patients improves oxidative stress, mitochondrial dysfunction, inflammation, endothelial dysfunction, insulin resistance, genotoxicity, strength, and cognition: Results of an open-label clinical trial. Biomedicines 2020, 8, 390. [Google Scholar] [CrossRef]
- Magdaleno, F.; Blajszczak, C.C.; Charles-Niño, C.L.; Guadrón-Llanos, A.M.; Vázquez-Álvarez, A.O.; Miranda-Díaz, A.G.; Nieto, N.; Islas-Carbajal, M.C.; Rincón-Sánchez, A.R. Aminoguanidine reduces diabetes-associated cardiac fibrosis. Exp. Ther. Med. 2019, 18, 3125–3138. [Google Scholar] [CrossRef]
- Matthews, J.J.; Turner, M.D.; Santos, L.; Elliott-Sale, K.J.; Sale, C. Carnosine increases insulin-stimulated glucose uptake and reduces methylglyoxal-modified proteins in type-2 diabetic human skeletal muscle cells. Amino Acids 2023, 55, 413–420. [Google Scholar]
- Kamei, J.; Ohsawa, M.; Miyata, S.; Tanaka, S.-i. Preventive effect of L-carnosine on changes in the thermal nociceptive threshold in streptozotocin-induced diabetic mice. Eur. J. Pharmacol. 2008, 600, 83–86. [Google Scholar]
- Skrypnyk, N.I.; Voziyan, P.; Yang, H.; de Caestecker, C.R.; Theberge, M.-C.; Drouin, M.; Hudson, B.; Harris, R.C.; de Caestecker, M.P. Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury. Am. J. Physiol. Ren. Physiol. 2016, 311, F268–F277. [Google Scholar]
- Kinsky, O.R.; Hargraves, T.L.; Anumol, T.; Jacobsen, N.E.; Dai, J.; Snyder, S.A.; Monks, T.J.; Lau, S.S. Metformin scavenges methylglyoxal to form a novel imidazolinone metabolite in humans. Chem. Res. Toxicol. 2016, 29, 227–234. [Google Scholar]
- Rabbani, N.; Thornalley, P.J. Emerging role of thiamine therapy for prevention and treatment of early-stage diabetic nephropathy. Diabetes Obes. Metab. 2011, 13, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Desai, K.; Wang, R.; Wu, L. Up-regulation of aldolase A and methylglyoxal production in adipocytes. Br. J. Pharmacol. 2013, 168, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Arndtz, K.; Chen, Y.-Y.; Rowe, A.; Homer, V.; Kirkham, A.; Douglas-Pugh, J.; Slade, D.; Thorburn, D.; Barnes, E.; Aithal, G. Monoclonal antibody BTT1023 targeting vascular adhesion protein 1 for treating primary sclerosing cholangitis: BUTEO single-arm Phase II trial. Effic. Mech. Eval. 2022, 9, 1–54. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Devaraj, S.; Bellur, P.; Lakkanna, S.; Vicini, J.; Boddupalli, S. Novel concepts of broccoli sulforaphanes and disease: Induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutr. Rev. 2012, 70, 654–665. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Bustamante Munguira, E.; Juan, C.A.; Plou, F.J.; Pérez Lebeña, E. Electrophilic Compounds in the Human Diet and Their Role in the Induction of the Transcription Factor NRF2. Int. J. Mol. Sci. 2024, 25, 3521. [Google Scholar] [CrossRef]
- Shilovsky, G.A.; Dibrova, D.V. Regulation of cell proliferation and nrf2-mediated antioxidant defense: Conservation of keap1 cysteines and nrf2 binding site in the context of the evolution of KLHL family. Life 2023, 13, 1045. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-kappaB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 809952. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 1998, 17, 3145–3156. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L.; Hunsaker, L.A. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem. Biol. Interact. 2003, 143, 341–351. [Google Scholar] [PubMed]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Reversal of insulin resistance in overweight and obese subjects by trans-resveratrol and hesperetin combination—Link to dysglycemia, blood pressure, dyslipidemia, and low-grade inflammation. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef]
- Pace, G.W.; Leaf, C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995, 19, 523–528. [Google Scholar]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Jaruga, P.; Jaruga, B.; Gackowski, D.; Olczak, A.; Halota, W.; Pawlowska, M.; Olinski, R. Supplementation with antioxidant vitamins prevents oxidative modification of DNA in lymphocytes of HIV-infected patients. Free Radic. Biol. Med. 2002, 32, 414–420. [Google Scholar]
- Silva, T.A.L.; Medeiros, D.C.; Medeiros, G.C.B.S.; Medeiros, R.C.S.C.; de Souza Araújo, J.; Medeiros, J.A.; Ururahy, M.A.G.; Santos, R.V.T.; Medeiros, R.M.V.; Leite-Lais, L. Influence of curcumin supplementation on metabolic and lipid parameters of people living with HIV/AIDS: A randomized controlled trial. BMC Complement. Altern. Med. 2019, 19, 202. [Google Scholar]
- Erdos, T.; Masuda, M.; Venketaraman, V. Glutathione in HIV-Associated Neurocognitive Disorders. Curr. Issues Mol. Biol. 2024, 46, 5530–5549. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Huey, S.L.; Mehta, S. Antioxidants and HIV/AIDS: Zinc, Selenium, and Vitamins C and E. In Nutrition and HIV; CRC Press: Boca Raton, FL, USA, 2018; pp. 191–205. [Google Scholar]
- Zazzo, J.; Rouveix, B.; Rajagopalon, P.; Levacher, M.; Girard, P. Effect of zinc on the immune status of zinc-depleted AIDS related complex patients. Clin. Nutr. 1989, 8, 259–261. [Google Scholar]
- Isa, L.; Lucchini, A.; Lodi, S.; Giachetti, M. Blood zinc status and zinc treatment in human immunodeficiency virus-infected patients. Int. J. Clin. Lab. Res. 1992, 22, 45–47. [Google Scholar] [PubMed]
- Bobat, R.; Coovadia, H.; Stephen, C.; Naidoo, K.L.; McKerrow, N.; Black, R.E.; Moss, W.J. Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: A randomised double-blind placebo-controlled trial. Lancet 2005, 366, 1862–1867. [Google Scholar]
- Shor-Posner, G.; Lecusay, R.; Miguez, M.-J.; Moreno-Black, G.; Zhang, G.; Rodriguez, N.; Burbano, X.; Baum, M.; Wilkie, F. Psychological burden in the era of HAART: Impact of selenium therapy. Int. J. Psychiatry Med. 2003, 33, 55–69. [Google Scholar]
- Hurwitz, B.E.; Klaus, J.R.; Llabre, M.M.; Gonzalez, A.; Lawrence, P.J.; Maher, K.J.; Greeson, J.M.; Baum, M.K.; Shor-Posner, G.; Skyler, J.S. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 148–154. [Google Scholar]
- Kupka, R.; Mugusi, F.; Aboud, S.; Hertzmark, E.; Spiegelman, D.; Fawzi, W.W. Effect of selenium supplements on hemoglobin concentration and morbidity among HIV-1–Infected Tanzanian Women. Clin. Infect. Dis. 2009, 48, 1475–1478. [Google Scholar]

| Strategy | Agents and Mechanism of Action | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Agents containing nucleophilic groups to scavenge MG | Aminoguanidine, pyridoxamine, L-carnosinol, and arginine-containing peptides to scavenge MG by forming adducts with nucleophilic moieties. |
|
| [145,146,147,148,149] |
| Agents to prevent or reduce MG formation |
|
|
| [150,151,152,153,154,155] |
| VAP-1 inhibitors |
|
|
| [156,157,158,159] |
| Nrf2 activators and MG-degrading enzyme upregulation |
|
|
| [33,68,144,160,161,162] |
| Increasing co-factors for MG-degrading enzymes | Supplementation with antioxidants like glutathione and vitamins C and E and co-factors such as NAD(P)H. |
|
| [163,164,165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasamy, M.; Venn, Z.L.; Alomar, F.A.; Namvaran, A.; Edagwa, B.; Gorantla, S.; Bidasee, K.R. Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection. Viruses 2025, 17, 547. https://doi.org/10.3390/v17040547
Ramasamy M, Venn ZL, Alomar FA, Namvaran A, Edagwa B, Gorantla S, Bidasee KR. Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection. Viruses. 2025; 17(4):547. https://doi.org/10.3390/v17040547
Chicago/Turabian StyleRamasamy, Mahendran, Zachary L. Venn, Fadhel A. Alomar, Ali Namvaran, Benson Edagwa, Santhi Gorantla, and Keshore R. Bidasee. 2025. "Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection" Viruses 17, no. 4: 547. https://doi.org/10.3390/v17040547
APA StyleRamasamy, M., Venn, Z. L., Alomar, F. A., Namvaran, A., Edagwa, B., Gorantla, S., & Bidasee, K. R. (2025). Elevated Methylglyoxal: An Elusive Risk Factor Responsible for Early-Onset Cardiovascular Diseases in People Living with HIV-1 Infection. Viruses, 17(4), 547. https://doi.org/10.3390/v17040547







