E5 Oncoprotein: A Key Player in Human Papillomavirus-Positive Head and Neck Cancer Pathogenesis and Therapy Resistance
Abstract
1. Introduction
2. HPV and the Carcinogenic Process
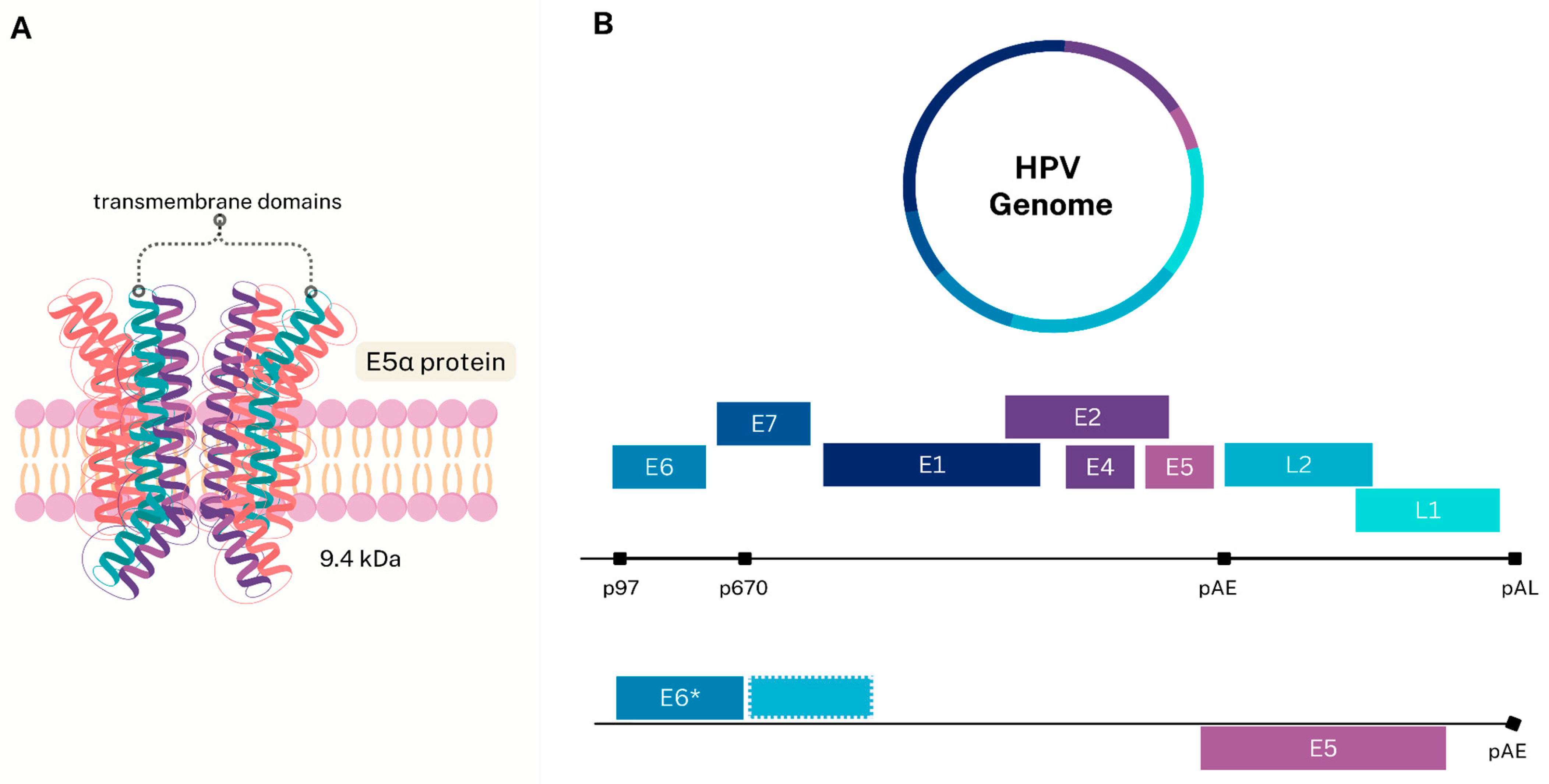
2.1. Structure and Viral Oncoproteins
2.2. E5 Oncoprotein Characteristics
3. Head and Neck Cancer: Epidemiology and General Characteristics
Human Papillomavirus as a Risk Factor for Head and Neck Cancer
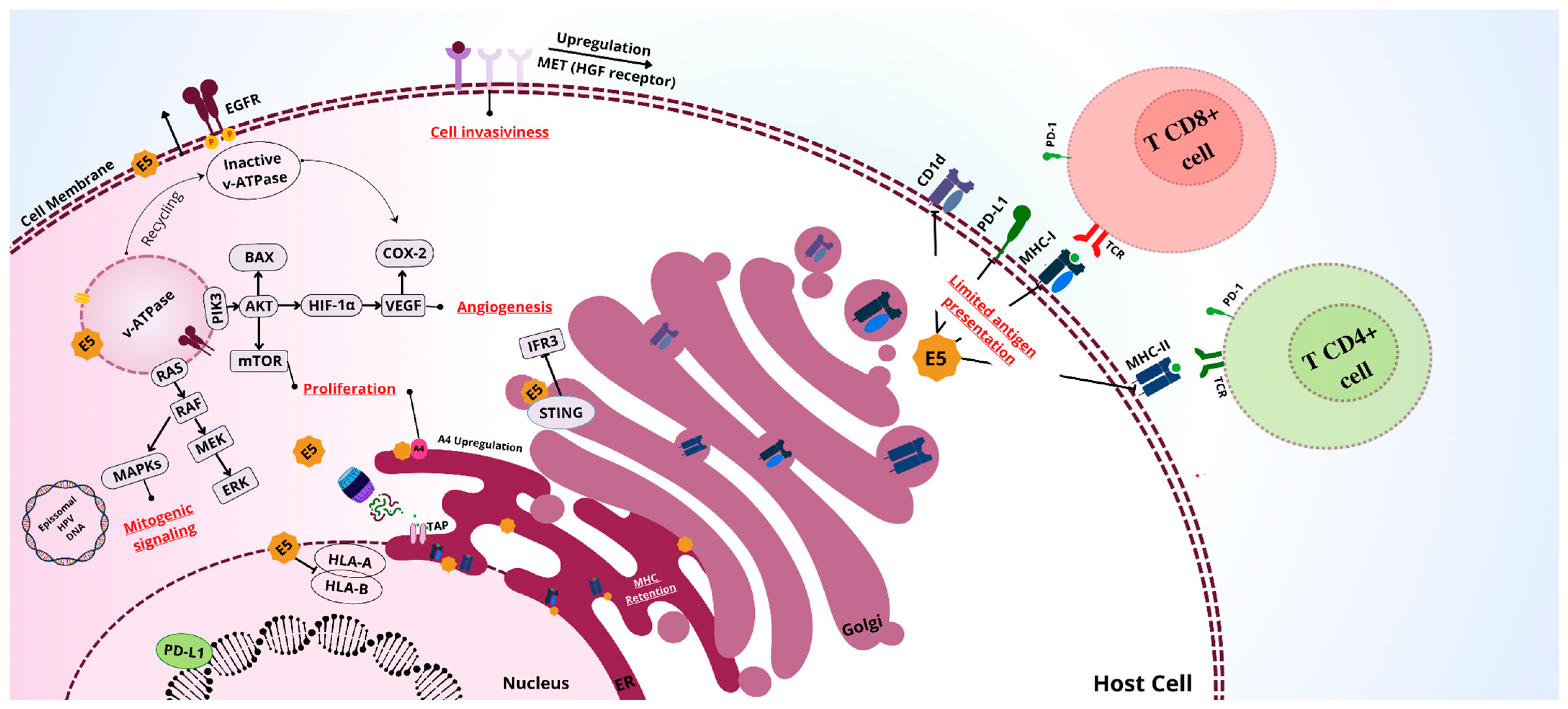
4. Activity of E5 in Head and Neck Cancer
4.1. Cellular Proliferation
4.2. Immune Evasion
4.3. Modulation of Apoptosis
4.4. Synergy with E6 and E7
5. E5 as a Therapeutic Target in Head and Neck Cancer Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kühn, J.P.; Schmid, W.; Körner, S.; Bochen, F.; Wemmert, S.; Rimbach, H.; Smola, S.; Radosa, J.C.; Wagner, M.; Morris, L.G.T.; et al. HPV Status as Prognostic Biomarker in Head and Neck Cancer—Which Method Fits the Best for Outcome Prediction? Cancers 2021, 13, 4730. [Google Scholar] [CrossRef]
- Makarewicz, J.; Kaźmierczak-Siedlecka, K.; Sobocki, B.K.; Dobrucki, I.T.; Kalinowski, L.; Stachowska, E. Anti-Cancer Management of Head and Neck Cancers and Oral Microbiome-What Can We Clinically Obtain? Front. Cell. Infect. Microbiol. 2024, 14, 1329057. [Google Scholar] [CrossRef]
- Muniz, I.D.A.F.; Araujo, M.; Bouassaly, J.; Farshadi, F.; Atique, M.; Esfahani, K.; Bonan, P.R.F.; Hier, M.; Mascarella, M.; Mlynarek, A.; et al. Therapeutic Advances and Challenges for the Management of HPV-Associated Oropharyngeal Cancer. Int. J. Mol. Sci. 2024, 25, 4009. [Google Scholar] [CrossRef]
- Park, J.C.; Bertaux, B.; Park, J.; Park, S. Current Status of Human Papillomavirus–Targeted Therapies Development in Head and Neck Cancer. JCO Precis. Oncol. 2023, 7, e2300098. [Google Scholar] [CrossRef]
- Roman, B.R.; Aragones, A. Epidemiology and Incidence of HPV-related Cancers of the Head and Neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Oyouni, A.A.A. Human Papillomavirus in Cancer: Infection, Disease Transmission, and Progress in Vaccines. J. Infect. Public Health 2023, 16, 626–631. [Google Scholar] [CrossRef]
- Perkins, R.B.; Wentzensen, N.; Guido, R.S.; Schiffman, M. Cervical Cancer Screening: A Review. JAMA 2023, 330, 547. [Google Scholar] [CrossRef]
- Roy, S.; Miyauchi, S.; Jones, R.; Kim, S.; Sharabi, A.B. Abstract 2875: Resistance to Conventional Chemo and Radiotherapy Associated with Increased DNA Repair and Stem Cell-like Phenotype in Head and Neck Squamous Cell Carcinoma Expressing HPV E5. Cancer Res. 2024, 84, 2875. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Qiu, S.; Wang, R. Therapeutic Strategies of Different HPV Status in Head and Neck Squamous Cell Carcinoma. Int. J. Biol. Sci. 2021, 17, 1104–1118. [Google Scholar] [CrossRef]
- Skelin, J.; Sabol, I.; Tomaić, V. Do or Die: HPV E5, E6 and E7 in Cell Death Evasion. Pathogens 2022, 11, 1027. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Kissick, H.T.; Patel, M.R.; Cardenas, M.A.; Prokhnevska, N.; Obeng, R.C.; Nasti, T.H.; Griffith, C.C.; Im, S.J.; Wang, X.; et al. Functional HPV-Specific PD-1+ Stem-like CD8 T Cells in Head and Neck Cancer. Nature 2021, 597, 279–284. [Google Scholar] [CrossRef]
- Williamson, A.-L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef]
- Del Prete, R.; Nesta, D.; Triggiano, F.; Lorusso, M.; Garzone, S.; Vitulano, L.; Denicolò, S.; Indraccolo, F.; Mastria, M.; Ronga, L.; et al. Human Papillomavirus Carcinogenicity and the Need of New Perspectives: Thoughts from a Retrospective Analysis on Human Papillomavirus Outcomes Conducted at the Hospital University of Bari, Apulia, Italy, between 2011 and 2022. Diagnostics 2024, 14, 968. [Google Scholar] [CrossRef]
- Tabatabaeian, H.; Bai, Y.; Huang, R.; Chaurasia, A.; Darido, C. Navigating Therapeutic Strategies: HPV Classification in Head and Neck Cancer. Br. J. Cancer 2024, 131, 220–230. [Google Scholar] [CrossRef]
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human Papilloma Virus: A Review Study of Epidemiology, Carcinogenesis, Diagnostic Methods, and Treatment of All HPV-Related Cancers. Med. J. Islam. Repub. Iran 2021, 35, 65. [Google Scholar] [CrossRef]
- Bzhalava, D.; Eklund, C.; Dillner, J. International Standardization and Classification of Human Papillomavirus Types. Virology 2015, 476, 341–344. [Google Scholar] [CrossRef]
- De Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; Zur Hausen, H. Classification of Papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Segondy, M. Classification des papillomavirus (HPV). Rev. Francoph. Lab. 2008, 2008, 23–25. [Google Scholar] [CrossRef]
- Shimizu, A.; Yamaguchi, R.; Kuriyama, Y. Recent Advances in Cutaneous HPV Infection. J. Dermatol. 2023, 50, 290–298. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; McBride, A.A. Molecular Archeological Evidence in Support of the Repeated Loss of a Papillomavirus Gene. Sci. Rep. 2016, 6, 33028. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Pol, S.V.; Podjarny, A.; et al. Structure of the E6/E6AP/P53 Complex Required for HPV-Mediated Degradation of P53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Y.Z.; Zhang, Z.Y.; Wang, J.Q.; Cui, J.; Qian, X.L. HPV16 E6 Promotes Breast Cancer Proliferation via Upregulation of COX-2 Expression. BioMed Res. Int. 2017, 2017, 2948467. [Google Scholar] [CrossRef]
- Boyer, S.N.; Wazer, D.E.; Band, V. E7 Protein of Human Papilloma Virus-16 Induces Degradation of Retinoblastoma Protein through the Ubiquitin-Proteasome Pathway. Cancer Res. 1996, 56, 4620–4624. [Google Scholar]
- White, E.A.; Münger, K.; Howley, P.M. High-Risk Human Papillomavirus E7 Proteins Target PTPN14 for Degradation. mBio 2016, 7, e01530-16. [Google Scholar] [CrossRef]
- Thakur, K.; Janjua, D.; Shishodia, G.; Chhokar, A.; Aggarwal, N.; Yadav, J.; Tripathi, T.; Chaudhary, A.; Senrung, A.; Bharti, A.C. Investigation of Molecular Mechanisms Underlying JAK/STAT Signaling Pathway in HPV-Induced Cervical Carcinogenesis Using ‘Omics’ Approach. Med. Oncol. 2022, 39, 255. [Google Scholar] [CrossRef]
- Xi, R.; Pan, S.; Chen, X.; Hui, B.; Zhang, L.; Fu, S.; Li, X.; Zhang, X.; Gong, T.; Guo, J.; et al. HPV16 E6-E7 Induces Cancer Stem-like Cells Phenotypes in Esophageal Squamous Cell Carcinoma through the Activation of PI3K/Akt Signaling Pathway In Vitro and In Vivo. Oncotarget 2016, 7, 57050–57065. [Google Scholar] [CrossRef]
- Ilahi, N.E.; Bhatti, A. Impact of HPV E5 on Viral Life Cycle via EGFR Signaling. Microb. Pathog. 2020, 139, 103923. [Google Scholar] [CrossRef]
- Miyauchi, S.; Sanders, P.D.; Guram, K.; Kim, S.S.; Paolini, F.; Venuti, A.; Cohen, E.E.W.; Gutkind, J.S.; Califano, J.A.; Sharabi, A.B. HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer. Cancer Res. 2020, 80, 732–746. [Google Scholar] [CrossRef]
- Van Doorslaer, K. Evolution of the Papillomaviridae. Virology 2013, 445, 11–20. [Google Scholar] [CrossRef]
- Bravo, I.G.; Alonso, A. Mucosal Human Papillomaviruses Encode Four Different E5 Proteins Whose Chemistry and Phylogeny Correlate with Malignant or Benign Growth. J. Virol. 2004, 78, 13613–13626. [Google Scholar] [CrossRef]
- DiMaio, D.; Petti, L.M. The E5 Proteins. Virology 2013, 445, 99–114. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Galvão De Araújo, J.M.; Allyrio Araújo De Medeiros Fernandes, T. Biology and Natural History of Human Papillomavirus Infection. Open Access J. Clin. Trials 2013, 5, 1–12. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human Papillomavirus Molecular Biology and Disease Association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef]
- García, A.; Maldonado, G.; Hernández, G. Translational Control of Papillomavirus mRNAs in the Spotlight. Trends Cell Biol. 2024, 34, 703–706. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Qu, W.; Gong, Y.; Wang, Y.; Chen, L.; Zhou, Q.; Mo, J.; Zhang, H.; Lin, L.; et al. Alternative Splicing in the Genome of HPV and Its Regulation. Front. Cell. Infect. Microbiol. 2024, 14, 1443868. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Müller, M.; Prescott, E.L.; Wasson, C.W.; Macdonald, A. Human Papillomavirus E5 Oncoprotein: Function and Potential Target for Antiviral Therapeutics. Future Virol. 2015, 10, 27–39. [Google Scholar] [CrossRef]
- Nilsson, K.; Norberg, C.; Mossberg, A.-K.; Schwartz, S. HPV16 E5 Is Produced from an HPV16 Early mRNA Spliced from SD226 to SA3358. Virus Res. 2018, 244, 128–136. [Google Scholar] [CrossRef]
- Schwartz, S. Papillomavirus Transcripts and Posttranscriptional Regulation. Virology 2013, 445, 187–196. [Google Scholar] [CrossRef]
- Chen, S.M.Y.; Krinsky, A.L.; Woolaver, R.A.; Wang, X.; Chen, Z.; Wang, J.H. Tumor Immune Microenvironment in Head and Neck Cancers. Mol. Carcinog. 2020, 59, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Kounatidis, D.; Panagopoulos, F.; Geladari, E.; Karampela, I.; Stratigou, T.; Dalamaga, M. Immunotherapy in Head and Neck Cancer: Where Do We Stand? Curr. Oncol. Rep. 2023, 25, 897–912. [Google Scholar] [CrossRef]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the Epidemiology of Head and Neck Cancer: Definitions, Trends and Risk Factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The Changing Therapeutic Landscape of Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-Associated Oropharyngeal Cancer: Epidemiology, Molecular Biology and Clinical Management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Saba, N.F.; Pamulapati, S.; Patel, B.; Mody, M.; Strojan, P.; Takes, R.; Mäkitie, A.A.; Cohen, O.; Pace-Asciak, P.; Vermorken, J.B.; et al. Novel Immunotherapeutic Approaches to Treating HPV-Related Head and Neck Cancer. Cancers 2023, 15, 1959. [Google Scholar] [CrossRef]
- Sarwar, S.; Mulla, M.; Mulla, M.; Tanveer, R.; Sabir, M.; Sultan, A.; Malik, S.A. Human Papillomavirus, Tobacco, and Poor Oral Hygiene Can Act Synergetically, Modulate the Expression of the Nuclear Factor Kappa B Signaling Pathway for the Development and Progression of Head and Neck Cancer in the Pakistani Population. Chin. Med. J. 2022, 135, 1829–1836. [Google Scholar] [CrossRef]
- Ndon, S.; Singh, A.; Ha, P.K.; Aswani, J.; Chan, J.Y.-K.; Xu, M.J. Human Papillomavirus-Associated Oropharyngeal Cancer: Global Epidemiology and Public Policy Implications. Cancers 2023, 15, 4080. [Google Scholar] [CrossRef] [PubMed]
- Fonsêca, T.C.; Jural, L.A.; Marañón-Vásquez, G.A.; Magno, M.B.; Roza, A.L.O.C.; Ferreira, D.M.T.P.; Maia, L.C.; Romañach, M.J.; Agostini, M.; Abrahão, A.C. Global Prevalence of Human Papillomavirus-Related Oral and Oropharyngeal Squamous Cell Carcinomas: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2023, 28, 62. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S.; Strati, K.; Shin, M.K.; Pitot, H.C.; Lambert, P.F. Human Papillomavirus Type 16 E6 and E7 Oncoproteins Act Synergistically to Cause Head and Neck Cancer in Mice. Virology 2010, 407, 60–67. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Ren, S.; Gaykalova, D.A.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Tamayo, P.; Callejas-Valera, J.L.; Allevato, M.; Gilardi, M.; Santos, J.; et al. HPV E2, E4, E5 Drive Alternative Carcinogenic Pathways in HPV Positive Cancers. Oncogene 2020, 39, 6327–6339. [Google Scholar] [CrossRef]
- Shigeishi, H. Association between Human Papillomavirus and Oral Cancer: A Literature Review. Int. J. Clin. Oncol. 2023, 28, 982–989. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, B.; Ma, F.; Tong, F.; Yan, B.; Liu, T.; Xie, H.; Song, L.; Yu, S.; Wei, L. Characteristics of B Lymphocyte Infiltration in HPV + Head and Neck Squamous Cell Carcinoma. Cancer Sci. 2021, 112, 1402–1416. [Google Scholar] [CrossRef]
- Medda, A.; Duca, D.; Chiocca, S. Human Papillomavirus and Cellular Pathways: Hits and Targets. Pathogens 2021, 10, 262. [Google Scholar] [CrossRef]
- Um, S.H.; Mundi, N.; Yoo, J.; Palma, D.A.; Fung, K.; MacNeil, D.; Wehrli, B.; Mymryk, J.S.; Barrett, J.W.; Nichols, A.C. Variable Expression of the Forgotten Oncogene E5 in HPV-Positive Oropharyngeal Cancer. J. Clin. Virol. 2014, 61, 94–100. [Google Scholar] [CrossRef]
- Mahmood, H.A.; Tomas Bort, E.; Walker, A.J.; Grose, R.P.; Chioni, A. FGF Signalling Facilitates Cervical Cancer Progression. FEBS J. 2022, 289, 3440–3456. [Google Scholar] [CrossRef]
- Wasson, C.W.; Morgan, E.L.; Müller, M.; Ross, R.L.; Hartley, M.; Roberts, S.; Macdonald, A. Human Papillomavirus Type 18 E5 Oncogene Supports Cell Cycle Progression and Impairs Epithelial Differentiation by Modulating Growth Factor Receptor Signalling during the Virus Life Cycle. Oncotarget 2017, 8, 103581–103600. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, D.; Belleudi, F.; Magenta, A.; Torrisi, M.R. HPV16 E5 Expression Induces Switching from FGFR2b to FGFR2c and Epithelial-mesenchymal Transition. Int. J. Cancer 2015, 137, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, W.; Zhang, Y.; Hong, S.; Kang, S.; Yan, Y.; Chen, N.; Zhan, J.; He, X.; Qin, T.; et al. The Association between PD-L1 and EGFR Status and the Prognostic Value of PD-L1 in Advanced Non-Small Cell Lung Cancer Patients Treated with EGFR-TKIs. Oncotarget 2015, 6, 14209–14219. [Google Scholar] [CrossRef]
- Allouch, S.; Malki, A.; Allouch, A.; Gupta, I.; Vranic, S.; Al Moustafa, A.-E. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front. Oncol. 2020, 10, 914. [Google Scholar] [CrossRef]
- Lee, B.S.; Park, D.I.; Lee, D.H.; Lee, J.E.; Yeo, M.; Park, Y.H.; Lim, D.S.; Choi, W.; Lee, D.H.; Yoo, G.; et al. Hippo Effector YAP Directly Regulates the Expression of PD-L1 Transcripts in EGFR-TKI-Resistant Lung Adenocarcinoma. Biochem. Biophys. Res. Commun. 2017, 491, 493–499. [Google Scholar] [CrossRef]
- Yang, W.; Song, Y.; Lu, Y.; Sun, J.; Wang, H. Increased Expression of Programmed Death (PD)-1 and Its Ligand PD -L1 Correlates with Impaired Cell-mediated Immunity in High-risk Human Papillomavirus-related Cervical Intraepithelial Neoplasia. Immunology 2013, 139, 513–522. [Google Scholar] [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.R.; Marchetti, B.; O’Brien, P.M.; Campo, M.S. E5 Protein of Human Papillomavirus Type 16 Selectively Downregulates Surface HLA Class I. Int. J. Cancer 2005, 113, 276–283. [Google Scholar] [CrossRef]
- Campo, M.S.; Graham, S.V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-Regulates Expression of Surface HLA Class I and Reduces Recognition by CD8 T Cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef]
- De Freitas, A.C.; De Oliveira, T.H.A.; Barros, M.R.; Venuti, A. hrHPV E5 Oncoprotein: Immune Evasion and Related Immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36, 71. [Google Scholar] [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.; Marchetti, B.; Campo, M.S. E5 Protein of Human Papillomavirus 16 Downregulates HLA Class I and Interacts with the Heavy Chain via Its First Hydrophobic Domain. Int. J. Cancer 2006, 119, 2105–2112. [Google Scholar] [CrossRef]
- Cortese, M.S.; Ashrafi, G.H.; Campo, M.S. All 4 Di-leucine Motifs in the First Hydrophobic Domain of the E5 Oncoprotein of Human Papillomavirus Type 16 Are Essential for Surface MHC Class I Downregulation Activity and E5 Endomembrane Localization. Int. J. Cancer 2010, 126, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Schapiro, F.; Sparkowski, J.; Adduci, A.; Suprynowicz, F.; Schlegel, R.; Grinstein, S. Golgi Alkalinization by the Papillomavirus E5 Oncoprotein. J. Cell Biol. 2000, 148, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Straight, S.W.; Herman, B.; McCance, D.J. The E5 Oncoprotein of Human Papillomavirus Type 16 Inhibits the Acidification of Endosomes in Human Keratinocytes. J. Virol. 1995, 69, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Kotnik Halavaty, K.; Regan, J.; Mehta, K.; Laimins, L. Human Papillomavirus E5 Oncoproteins Bind the A4 Endoplasmic Reticulum Protein to Regulate Proliferative Ability upon Differentiation. Virology 2014, 452–453, 223–230. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Kronenberg, M. Going Both Ways: Immune Regulation via CD1d-Dependent NKT Cells. J. Clin. Investig. 2004, 114, 1379–1388. [Google Scholar] [CrossRef]
- Chaudhry, M.S.; Karadimitris, A. Role and Regulation of CD1d in Normal and Pathological B Cells. J. Immunol. 2014, 193, 4761–4768. [Google Scholar] [CrossRef]
- Miura, S.; Kawana, K.; Schust, D.J.; Fujii, T.; Yokoyama, T.; Iwasawa, Y.; Nagamatsu, T.; Adachi, K.; Tomio, A.; Tomio, K.; et al. CD1d, a Sentinel Molecule Bridging Innate and Adaptive Immunity, Is Downregulated by the Human Papillomavirus (HPV) E5 Protein: A Possible Mechanism for Immune Evasion by HPV. J. Virol. 2010, 84, 11614–11623. [Google Scholar] [CrossRef]
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K.; Bodily, J.M. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Jones, R.N.; Zhang, L.; Guram, K.; Sharma, S.; Schoenberger, S.P.; Cohen, E.E.W.; Califano, J.A.; Sharabi, A.B. Human Papillomavirus E5 Suppresses Immunity via Inhibition of the Immunoproteasome and STING Pathway. Cell Rep. 2023, 42, 112508. [Google Scholar] [CrossRef]
- Hemmat, N.; Bannazadeh Baghi, H. Association of Human Papillomavirus Infection and Inflammation in Cervical Cancer. Pathog. Dis. 2019, 77, ftz048. [Google Scholar] [CrossRef]
- Scott, M.L.; Coleman, D.T.; Kelly, K.C.; Carroll, J.L.; Woodby, B.; Songock, W.K.; Cardelli, J.A.; Bodily, J.M. Human Papillomavirus Type 16 E5-Mediated Upregulation of Met in Human Keratinocytes. Virology 2018, 519, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oelze, I.; Kartenbeck, J.; Crusius, K.; Alonso, A. Human Papillomavirus Type 16 E5 Protein Affects Cell-Cell Communication in an Epithelial Cell Line. J. Virol. 1995, 69, 4489–4494. [Google Scholar] [CrossRef] [PubMed]
- Stöppler, M.C.; Straight, S.W.; Tsao, G.; Schlegel, R.; Mccance, D.J. The E5 Gene of HPV-16 Enhances Keratinocyte Immortalization by Full-Length DNA. Virology 1996, 223, 251–254. [Google Scholar] [CrossRef]
- Oh, J.-M.; Kim, S.-H.; Cho, E.-A.; Song, Y.-S.; Kim, W.-H.; Juhnn, Y.-S. Human Papillomavirus Type 16 E5 Protein Inhibits Hydrogen Peroxide-Induced Apoptosis by Stimulating Ubiquitin-Proteasome-Mediated Degradation of Bax in Human Cervical Cancer Cells. Carcinogenesis 2010, 31, 402–410. [Google Scholar] [CrossRef]
- Raudenská, M.; Balvan, J.; Masařík, M. Cell Death in Head and Neck Cancer Pathogenesis and Treatment. Cell Death Dis. 2021, 12, 192. [Google Scholar] [CrossRef]
- Zhang, B.; Spandau, D.F.; Roman, A. E5 Protein of Human Papillomavirus Type 16 Protects Human Foreskin Keratinocytes from UV B-Irradiation-Induced Apoptosis. J. Virol. 2002, 76, 220–231. [Google Scholar] [CrossRef]
- Sudarshan, S.R.; Schlegel, R.; Liu, X. The HPV-16 E5 Protein Represses Expression of Stress Pathway Genes XBP-1 and COX-2 in Genital Keratinocytes. Biochem. Biophys. Res. Commun. 2010, 399, 617–622. [Google Scholar] [CrossRef]
- Parida, S.; Mandal, M. Inflammation Induced by Human Papillomavirus in Cervical Cancer and Its Implication in Prevention. Eur. J. Cancer Prev. 2014, 23, 432–448. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, L.; Yang, R.; Xu, T. Advances in Molecular Mechanism of HPV16 E5 Oncoprotein Carcinogenesis. Arch. Biochem. Biophys. 2023, 745, 109716. [Google Scholar] [CrossRef]
- Bouvard, V.; Matlashewski, G.; Gu, Z.-M.; Storey, A.; Banks, L. The Human Papillomavirus Type 16 E5 Gene Cooperates with the E7 Gene to Stimulate Proliferation of Primary Cells and Increases Viral Gene Expression. Virology 1994, 203, 73–80. [Google Scholar] [CrossRef]
- Hochmann, J.; Millán, M.; Hernández, P.; Lafon-Hughes, L.; Aiuto, N.D.; Silva, A.; Llaguno, J.; Alonso, J.; Fernández, A.; Pereira-Prado, V.; et al. Contributions of Viral Oncogenes of HPV-18 and Hypoxia to Oxidative Stress and Genetic Damage in Human Keratinocytes. Sci. Rep. 2023, 13, 17734. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.E.; Chiocca, S. Human Papillomavirus as a Driver of Head and Neck Cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.R.; de Oliveira, T.H.A.; de Melo, C.M.L.; Venuti, A.; de Freitas, A.C. Viral Modulation of TLRs and Cytokines and the Related Immunotherapies for HPV-Associated Cancers. J. Immunol. Res. 2018, 2018, 2912671. [Google Scholar] [CrossRef] [PubMed]
- Boilesen, D.R.; Nielsen, K.N.; Holst, P.J. Novel Antigenic Targets of HPV Therapeutic Vaccines. Vaccines 2021, 9, 1262. [Google Scholar] [CrossRef]
- Badillo-Godinez, O.; Pedroza-Saavedra, A.; Valverde-Garduño, V.; Bermudez-Morales, V.; Maldonado-Gama, M.; Leon-Letelier, R.; Bonifaz, L.C.; Esquivel-Guadarrama, F.; Gutierrez-Xicotencatl, L. Induction of Therapeutic Protection in an HPV16-Associated Mouse Tumor Model Through Targeting the Human Papillomavirus-16 E5 Protein to Dendritic Cells. Front. Immunol. 2021, 12, 593161. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lin, C.-W.; Tsao, Y.-P.; Chen, S.-L. Cytotoxic-T-Lymphocyte Human Papillomavirus Type 16 E5 Peptide with CpG-Oligodeoxynucleotide Can Eliminate Tumor Growth in C57BL/6 Mice. J. Virol. 2004, 78, 1333–1343. [Google Scholar] [CrossRef]
- Liao, S.; Deng, D.; Zeng, D.; Zhang, L.; Hu, X.; Zhang, W.; Li, L.; Jiang, X.; Wang, C.; Zhou, J.; et al. HPV16 E5 Peptide Vaccine in Treatment of Cervical Cancer In Vitro and In Vivo. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2013, 33, 735–742. [Google Scholar] [CrossRef]
- Namvar, A.; Panahi, H.A.; Agi, E.; Bolhassani, A. Development of HPV16,18,31,45 E5 and E7 Peptides-Based Vaccines Predicted by Immunoinformatics Tools. Biotechnol. Lett. 2020, 42, 403–418. [Google Scholar] [CrossRef]
- Paolini, F.; Curzio, G.; Cordeiro, M.N.; Massa, S.; Mariani, L.; Pimpinelli, F.; De Freitas, A.C.; Franconi, R.; Venuti, A. HPV 16 E5 Oncoprotein Is Expressed in Early Stage Carcinogenesis and Can Be a Target of Immunotherapy. Hum. Vaccines Immunother. 2017, 13, 291–297. [Google Scholar] [CrossRef]
- Cordeiro, M.N.; Paolini, F.; Massa, S.; Curzio, G.; Illiano, E.; Duarte Silva, A.J.; Franconi, R.; Bissa, M.; Morghen, C.D.G.; De Freitas, A.C.; et al. Anti-Tumor Effects of Genetic Vaccines against HPV Major Oncogenes. Hum. Vaccines Immunother. 2015, 11, 45–52. [Google Scholar] [CrossRef]
- Gao, P.; Zheng, J. High-Risk HPV E5-Induced Cell Fusion: A Critical Initiating Event in the Early Stage of HPV-Associated Cervical Cancer. Virol. J. 2010, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Sahab, Z.; Sudarshan, S.R.; Liu, X.; Zhang, Y.; Kirilyuk, A.; Kamonjoh, C.M.; Simic, V.; Dai, Y.; Byers, S.W.; Doorbar, J.; et al. Quantitative Measurement of Human Papillomavirus Type 16 E5 Oncoprotein Levels in Epithelial Cell Lines by Mass Spectrometry. J. Virol. 2012, 86, 9465–9473. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-L.; Tsao, Y.-P.; Liu, D.-W.; Huang, S.-J.; Lee, W.H.; Chen, S.-L. The Expression of HPV-16 E5 Protein in Squamous Neoplastic Changes in the Uterine Cervix. J. Biomed. Sci. 2001, 8, 206–213. [Google Scholar] [CrossRef]
- Shirasawa, H.; Tomita, Y.; Sekiya, S.; Takamizawa, H.; Simizu, B. Integration and Transcription of Human Papillomavirus Type 16 and 18 Sequences in Cell Lines Derived from Cervical Carcinomas. J. Gen. Virol. 1987, 68, 583–591. [Google Scholar] [CrossRef]
- Taberna, M.; Torres, M.; Alejo, M.; Mena, M.; Tous, S.; Marquez, S.; Pavón, M.A.; León, X.; García, J.; Guix, M.; et al. The Use of HPV16-E5, EGFR, and pEGFR as Prognostic Biomarkers for Oropharyngeal Cancer Patients. Front. Oncol. 2018, 8, 589. [Google Scholar] [CrossRef]
- Oweida, A.; Lennon, S.; Calame, D.; Korpela, S.; Bhatia, S.; Sharma, J.; Graham, C.; Binder, D.; Serkova, N.; Raben, D.; et al. Ionizing Radiation Sensitizes Tumors to PD-L1 Immune Checkpoint Blockade in Orthotopic Murine Head and Neck Squamous Cell Carcinoma. OncoImmunology 2017, 6, e1356153. [Google Scholar] [CrossRef]
- Patsoukis, N.; Wang, Q.; Strauss, L.; Boussiotis, V.A. Revisiting the PD-1 Pathway. Sci. Adv. 2020, 6, eabd2712. [Google Scholar] [CrossRef]
- Rebucci-Peixoto, M.; Vienot, A.; Adotevi, O.; Jacquin, M.; Ghiringhelli, F.; De La Fouchardière, C.; You, B.; Maurina, T.; Kalbacher, E.; Bazan, F.; et al. A Phase II Study Evaluating the Interest to Combine UCPVax, a Telomerase CD4 TH1-Inducer Cancer Vaccine, and Atezolizumab for the Treatment of HPV Positive Cancers: VolATIL Study. Front. Oncol. 2022, 12, 957580. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Algazi, A.P.; Jimeno, A.; Good, J.S.; Fayette, J.; Bouganim, N.; Ready, N.E.; Clement, P.M.; Even, C.; Jang, R.W.; et al. Durvalumab for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Results from a Single-Arm, Phase II Study in Patients with ≥25% Tumour Cell PD-L1 Expression Who Have Progressed on Platinum-Based Chemotherapy. Eur. J. Cancer 2019, 107, 142–152. [Google Scholar] [CrossRef]
- Yarbrough, W.G.; Schrank, T.P.; Burtness, B.A.; Issaeva, N. De-Escalated Therapy and Early Treatment of Recurrences in HPV-Associated Head and Neck Cancer: The Potential for Biomarkers to Revolutionize Personalized Therapy. Viruses 2024, 16, 536. [Google Scholar] [CrossRef]
- Jefferson, T.; Demicheli, V.; Di Pietrantonj, C.; Rivetti, D. Amantadine and Rimantadine for Influenza A in Adults. Cochrane Database Syst. Rev. 2006, 2012, 1996. [Google Scholar] [CrossRef]
- Wetherill, L.F.; Holmes, K.K.; Verow, M.; Müller, M.; Howell, G.; Harris, M.; Fishwick, C.; Stonehouse, N.; Foster, R.; Blair, G.E.; et al. High-Risk Human Papillomavirus E5 Oncoprotein Displays Channel-Forming Activity Sensitive to Small-Molecule Inhibitors. J. Virol. 2012, 86, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, L.F.; Wasson, C.W.; Swinscoe, G.; Kealy, D.; Foster, R.; Griffin, S.; Macdonald, A. Alkyl-Imino Sugars Inhibit the pro-Oncogenic Ion Channel Function of Human Papillomavirus (HPV) E5. Antivir. Res. 2018, 158, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Villa-Ruano, N.; Marin-Cevada, V.; Sanchez-Esgua, G.; Villafaña-Diaz, L.; Perez-Santos, M. Drug Repurposing of Rimantadine for Treatment of Cancer. Pharm. Pat. Anal. 2023, 12, 231–236. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, W.; Hou, F.; Feng, X. Precision Therapeutic Targets for HPV-Positive Cancers: An Overview and New Insights. Infect. Agent. Cancer 2025, 20, 17. [Google Scholar] [CrossRef]
- Nizard, M.; Sandoval, F.; Badoual, C.; Pere, H.; Terme, M.; Hans, S.; Benhamouda, N.; Granier, C.; Brasnu, D.; Tartour, E. Immunotherapy of HPV-Associated Head and Neck Cancer: Critical Parameters. OncoImmunology 2013, 2, e24534. [Google Scholar] [CrossRef]
- Kumar, V.; McNerney, M.E. A New Self: MHC-Class-I-Independent Natural-Killer-Cell Self-Tolerance. Nat. Rev. Immunol. 2005, 5, 363–374. [Google Scholar] [CrossRef]
- Rasizadeh, R.; Shiri Aghbash, P.; Mokhtarzadeh, A.; Poortahmasebi, V.; Ahangar Oskouee, M.; Sadri Nahand, J.; Amini, M.; Zahra Bahojb Mahdavi, S.; Hossein Yari, A.; Bannazadeh Baghi, H. Novel Strategies in HPV-16-related Cervical Cancer Treatment: An In Vitro Study of Combined siRNA-E5 with Oxaliplatin and Ifosfamide Chemotherapy. Gene 2025, 932, 148904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira Santos, V.E.; de França São Marcos, B.; Fontes, P.H.B.; Silva, M.E.d.S.; Leão, S.L.; da Silva, G.R.P.; Ribeiro, D.E.; da Gama, M.A.T.M.; de Oliveira Isídio, B.E.; de Moura, I.A.; et al. E5 Oncoprotein: A Key Player in Human Papillomavirus-Positive Head and Neck Cancer Pathogenesis and Therapy Resistance. Viruses 2025, 17, 512. https://doi.org/10.3390/v17040512
Pereira Santos VE, de França São Marcos B, Fontes PHB, Silva MEdS, Leão SL, da Silva GRP, Ribeiro DE, da Gama MATM, de Oliveira Isídio BE, de Moura IA, et al. E5 Oncoprotein: A Key Player in Human Papillomavirus-Positive Head and Neck Cancer Pathogenesis and Therapy Resistance. Viruses. 2025; 17(4):512. https://doi.org/10.3390/v17040512
Chicago/Turabian StylePereira Santos, Vanessa Emanuelle, Bianca de França São Marcos, Pedro Henrique Bezerra Fontes, Micaela Evellin dos Santos Silva, Stephanie Loureiro Leão, Gabriel Rômulo Parente da Silva, Davi Emanuel Ribeiro, Marco Antonio Turiah Machado da Gama, Beatriz Eda de Oliveira Isídio, Ingrid Andrêssa de Moura, and et al. 2025. "E5 Oncoprotein: A Key Player in Human Papillomavirus-Positive Head and Neck Cancer Pathogenesis and Therapy Resistance" Viruses 17, no. 4: 512. https://doi.org/10.3390/v17040512
APA StylePereira Santos, V. E., de França São Marcos, B., Fontes, P. H. B., Silva, M. E. d. S., Leão, S. L., da Silva, G. R. P., Ribeiro, D. E., da Gama, M. A. T. M., de Oliveira Isídio, B. E., de Moura, I. A., Lussón, D. B., Leal, L. R. S., Venuti, A., & Freitas, A. C. d. (2025). E5 Oncoprotein: A Key Player in Human Papillomavirus-Positive Head and Neck Cancer Pathogenesis and Therapy Resistance. Viruses, 17(4), 512. https://doi.org/10.3390/v17040512






