Equine Rotavirus A Outbreaks in Ireland (2023–2024): An Epidemiological Investigation and Virus Genotyping
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Epidemiological Investigation
2.3. Extraction of RNA and Real-Time RT-PCR
2.4. RVA Genotyping
2.5. Sequence Analysis and Phylogenetic Analysis
3. Results
3.1. Samples
3.2. Epidemiology
3.3. RVA Genotyping
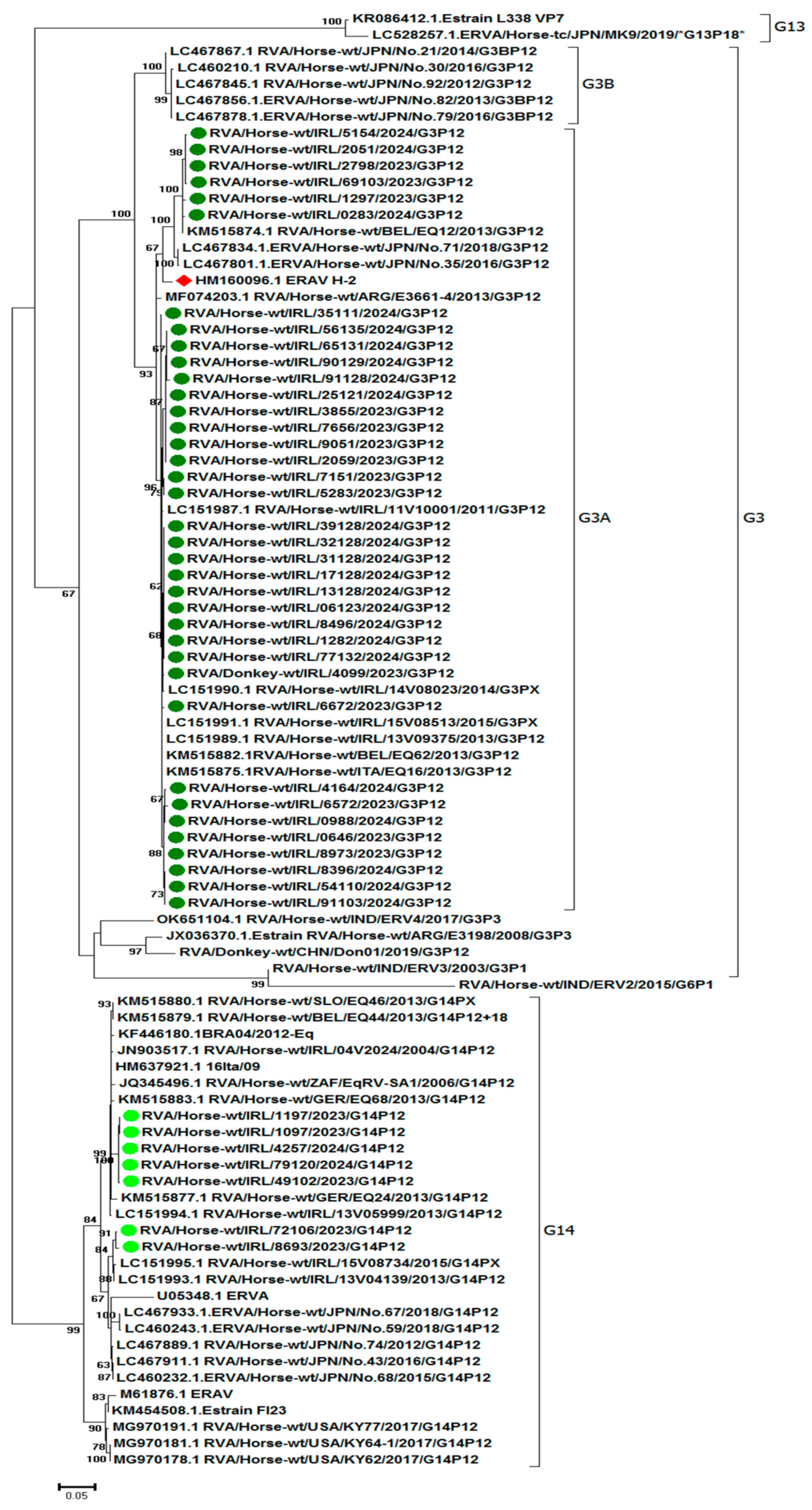
3.4. RVA Phylogenetic Analysis
3.5. Accession Numbers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RVA | Equine group A rotavirus |
References
- Newman, J.F.; Brown, F.; Bridger, J.C.; Woode, G.N. Characterisation of a rotavirus.20b. Nature 1975, 258, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Miño, S.; Papp, H.; Potgieter, C.; Novo, L.; Heylen, E.; Zeller, M.; Garaicoechea, L.; Badaracco, A.; Lengyel, G.; et al. Complete molecular genome analyses of equine rotavirus A strains from different continents reveal several novel genotypes and a largely conserved genotype constellation. J. Gen. Virol. 2012, 93, 866–875. [Google Scholar] [CrossRef]
- Flewett, T.H.; Bryden, A.S.; Davies, H. Letter: Virus diarrhoea in foals and other animals. Vet. Rec. 1975, 96, 477. [Google Scholar] [PubMed]
- Strickland, K.L.; Lenihan, P.; O’Connor, M.G.; Condon, J.C. Diarrhoea in foals associated with rotavirus. Vet. Rec. 1982, 111, 421. [Google Scholar] [CrossRef]
- Browning, G.F.; Chalmers, R.M.; Snodgrass, D.R.; Batt, R.M.; Hart, C.A.; Ormarod, S.E.; Leadon, D.; Stoneham, S.J.; Rossdale, P.D. The prevalence of enteric pathogens in diarrhoeic thoroughbred foals in Britain and Ireland. Equine Vet. J. 1991, 23, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Tsunemitsu, H.; Imagawa, H.; Hata, H.; Higuchi, T.; Sato, S.; Orita, Y.; Sugita, S.; Bannai, H.; Tsujimura, K.; et al. Molecular characterization and analysis of equine rotavirus circulating in Japan from 2003 to 2008. Vet. Microbiol. 2011, 152, 67–73. [Google Scholar] [CrossRef]
- Ntafis, V.; Fragkiadaki, E.; Xylouri, E.; Omirou, A.; Lavazza, A.; Martella, V. Rotavirus-associated diarrhoea in foals in Greece. Vet. Microbiol. 2010, 144, 461–465. [Google Scholar] [CrossRef]
- Magdesian, K.; Dwyer, R.; Arguedas, M. Viral Diarrhea. In Equine Infectious Diseases, 2nd ed.; Saunders: Philadelphia, PA, USA, 2013; pp. 198–203.e2. [Google Scholar] [CrossRef]
- Imagawa, H.; Sekiguchi, K.; Anzai, T.; Fukunaga, Y.; Kanemaru, T.; Ohishi, H.; Higuchi, T.; Kamada, M. Epidemiology of equine rotavirus infection among foals in the breeding region. J. Vet. Med. Sci. 1991, 53, 1079–1080. [Google Scholar] [CrossRef]
- Slovis, N.M.; Elam, J.; Estrada, M.; Leutenegger, C.M. Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: A comprehensive molecular study. Equine Vet. J. 2014, 46, 311–316. [Google Scholar] [CrossRef]
- Frederick, J.; Giguère, S.; Sanchez, L.C. Infectious agents detected in the feces of diarrheic foals: A retrospective study of 233 cases (2003–2008). J. Vet. Intern. Med. 2009, 23, 1254–1260. [Google Scholar] [CrossRef]
- Bailey, K.E.; Gilkerson, J.R.; Browning, G.F. Equine rotaviruses--current understanding and continuing challenges. Vet. Microbiol. 2013, 167, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Uprety, T.; Sreenivasan, C.C.; Hause, B.M.; Li, G.; Odemuyiwa, S.O.; Locke, S.; Morgan, J.; Zeng, L.; Gilsenan, W.F.; Slovis, N.; et al. Identification of a Ruminant Origin Group B Rotavirus Associated with Diarrhea Outbreaks in Foals. Viruses 2021, 13, 1330. [Google Scholar] [CrossRef]
- Otto, P.H.; Rosenhain, S.; Elschner, M.C.; Hotzel, H.; Machnowska, P.; Trojnar, E.; Hoffmann, K.; Johne, R. Detection of rotavirus species A, B and C in domestic mammalian animals with diarrhoea and genotyping of bovine species A rotavirus strains. Vet. Microbiol. 2015, 179, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Adam, E. Equine rotaviruses—An update from Kentucky. Vet. Rec. 2023, 192, e3139. [Google Scholar] [CrossRef]
- Dickson, J.; Smith, V.W.; Coackley, W.; McKean, P.; Adams, P.S. Rotavirus infection of foals. Aust. Vet. J. 1979, 55, 207–208. [Google Scholar] [CrossRef]
- Higgins, W.P.; Gillespie, J.H.; Schiff, E.I.; Pennow, N.N.; Tanneberger, M.J. Infectivity and immunity studies in foals with cell culture-propagated equine rotaviruses. In Equine Infectious Diseases V: Proceedings of the Fifth International Conference, Lexington, KY, USA, 7–10 October 1987; University of Kentucky Press: Lexington, KY, USA, 1987; pp. 241–247. [Google Scholar]
- Slovis, N.M. Rotavirus. In Infectious Disease of the Horse; Mair, T.S., Hutchinson, R.E., Eds.; Equine Veterinary Journal: Ely, UK, 2009; pp. 144–151. [Google Scholar]
- Nemoto, M.; Hata, H.; Higuchi, T.; Imagawa, H.; Yamanaka, T.; Niwa, H.; Bannai, H.; Tsujimura, K.; Kondo, T.; Matsumura, T. Evaluation of rapid antigen detection kits for diagnosis of equine rotavirus infection. J. Vet. Med. Sci. 2010, 72, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Nelly, M.; Dayot, L.; Lukaseviciute, G.; Garvey, M.; Healy, J.; Gallagher, R. Diagnostic Performance of Rapid Antigen Tests to Detect Equine Rotavirus A. Viruses 2025, 17, 413. [Google Scholar] [CrossRef]
- Nemoto, M.; Ryan, E.; Lyons, P.; Cullinane, A. Molecular characterisation of equine group A rotaviruses in Ireland (2011–2015). Vet. J. 2017, 226, 12–14. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Cullinane, A.; Martella, V.; O’Shea, H. Molecular characterization of equine rotavirus in Ireland. J. Clin. Microbiol. 2008, 46, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ons, E.; De Coster, S.; Conceição-Neto, N.; Gryspeerdt, A.; Van Ranst, M.; Raue, R. Molecular characterization of equine rotaviruses isolated in Europe in 2013: Implications for vaccination. Vet. Microbiol. 2015, 176, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Niwa, H.; Kida, H.; Higuchi, T.; Orita, Y.; Sato, S.; Bannai, H.; Tsujimura, K.; Ohta, M. Isolation and characterization of a rare group A rotavirus G13P[18] strain from a diarrhoeic foal in Japan. J. Gen. Virol. 2020, 101, 800–805. [Google Scholar] [CrossRef]
- Carossino, M.; Barrandeguy, M.E.; Li, Y.; Parreño, V.; Janes, J.; Loynachan, A.T.; Balasuriya, U.B.R. Detection, molecular characterization and phylogenetic analysis of G3P[12] and G14P[12] equine rotavirus strains co-circulating in central Kentucky. Virus Res. 2018, 255, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Browning, G.F.; Chalmers, R.M.; Fitzgerald, T.A.; Snodgrass, D.R. Evidence for two serotype G3 subtypes among equine rotaviruses. J. Clin. Microbiol. 1992, 30, 485–491. [Google Scholar] [CrossRef]
- Nemoto, M.; Tsunemitsu, H.; Murase, H.; Nambo, Y.; Sato, S.; Orita, Y.; Imagawa, H.; Bannai, H.; Tsujimura, K.; Yamanaka, T.; et al. Antibody response in vaccinated pregnant mares to recent G3BP[12] and G14P[12] equine rotaviruses. Acta Vet. Scand. 2012, 54, 63. [Google Scholar] [CrossRef]
- Stoneham, S.J. Practical aspects of diarrhoea in the foal with particular reference to rotavirus and gastroduodenal ulceration. Equine Vet. Educ. 1996, 8, 84–90. [Google Scholar] [CrossRef]
- METEireann. Climate Statement for March 2024. Available online: https://www.met.ie/climate-statement-for-march-2024 (accessed on 1 March 2025).
- Powell, D.G.; Dwyer, R.M.; Traub-Dargatz, J.L.; Fulker, R.H.; Whalen, J.W., Jr.; Srinivasappa, J.; Acree, W.M.; Chu, H.J. Field study of the safety, immunogenicity, and efficacy of an inactivated equine rotavirus vaccine. J. Am. Vet. Med. Assoc. 1997, 211, 193–198. [Google Scholar] [CrossRef]
- Barrandeguy, M.; Parreño, V.; Lagos Mármol, M.; Pont Lezica, F.; Rivas, C.; Valle, C.; Fernandez, F. Prevention of rotavirus diarrhoea in foals by parenteral vaccination of the mares: Field trial. Dev. Biol. Stand. 1998, 92, 253–257. [Google Scholar]
- Imagawa, H.; Kato, T.; Tsunemitsu, H.; Tanaka, H.; Sato, S.; Higuchi, T. Field Study of Inactivated Equine Rotavirus Vaccine. J. Equine Sci. 2005, 16, 35–44. [Google Scholar] [CrossRef]
- Imagawa, H.; Wada, R.; Sugita, S.; Fukunaga, Y. Passive immunity in foals of mares immunised with inactivated equine rotavirus vaccine. In Proceedings of the Equine Infectious Disease VIII: Proceedings of the Eighth International Conference, Dubai, United Arab Emirates, 23–26 March 1998; pp. 201–205. [Google Scholar]
- Erdoğan, H.M.Ç.; Çitil, M.; Güneş, V.; Saatci, M. Dairy Cattle Farming in Kars District, Turkey: I. Characteristics and Production. Turk. J. Vet. Anim. Sci. 2004, 28, 16. [Google Scholar]
- Platt, H. Septicaemia in the Foal. A Review of 61 Cases. Br. Vet. J. 1973, 129, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Platt, H. Acute infections in young foals. In Pract. 1983, 5, 41–49. [Google Scholar] [CrossRef]
- McGuire, T.C.; Crawford, T.B.; Hallowell, A.L.; Macomber, L.E. Failure of colostral immunoglobulin transfer as an explanation for most infections and deaths of neonatal foals. J. Am. Vet. Med. Assoc. 1977, 170, 1302–1304. [Google Scholar]
- McSloy, A. Clinical: Hypoxic ischaemic encephalopathy: Recognising and treating the dummy foal. Companion Anim. 2008, 13, 4–8. [Google Scholar] [CrossRef]
- Jeffcott, L.B. Studies on passive immunity in the foal: I. γ-Globulin and antibody variations associated with the maternal transfer of immunity and the onset of active immunity. J. Comp. Pathol. 1974, 84, 93–101. [Google Scholar] [CrossRef]
- Back, H.; Berndtsson, L.T.; Gröndahl, G.; Ståhl, K.; Pringle, J.; Zohari, S. The first reported Florida clade 1 virus in the Nordic countries, isolated from a Swedish outbreak of equine influenza in 2011. Vet. Microbiol. 2016, 184, 1–6. [Google Scholar] [CrossRef]
- Cullinane, A. Equine influenza and air transport. Equine Vet. Educ. 2014, 26, 456–457. [Google Scholar] [CrossRef]
- Dwyer, R.M.; Powell, D.G.; Roberts, W.; Donahue, M.; Lyons, E.T.; Osborne, M.; Wood, G. A study of the etiology and control of infectious diarrhea among foals in central Kentucky. In Proceedings of the 36th Annual Convention of the American Association of Equine Practitioners, Lexington, KY, USA, 2–5 December 1990; pp. 337–355. [Google Scholar]
- Tzipori, S.; Makin, T.; Smith, M.; Krautil, F. Enteritis in foals induced by rotavirus and enterotoxigenic Escherichia coli. Aust. Vet. J. 1982, 58, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Conner, Μ.; Darlington, R. Rotavirus infection in foals. Am. J. Vet. Res. 1980, 41, 1699–1703. [Google Scholar] [PubMed]

| VP7 | VP4 | |||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis by RT-PCR | G3 | G14 | P12 | |||||
| Year | No. of Positive Samples | Number of Positive Foals | Number of Positive Premises | G3 Number of Samples | G3 Number of Premises | G14 Number of Samples | G14 Number of Premises | |
| 2023 | 22/140 (15.7%) | 22 | 16 | 14 | 11 | 5 | 4 | 20 |
| 2024 | 28/237 (11.8%) | 26 | 20 | 23 | 16 | 2 | 2 | 27 |
| Total | 50/377 (13.3%) | 48 | 36 | 37 | 27 1 | 7 | 6 | 47 |
| Farm | Date | Age | Hygiene | Vaccination | Overstocking | Other Possible Risk Factors | Genotype | Virus Name |
|---|---|---|---|---|---|---|---|---|
| 1/24 | March | Not provided | Poor | No | Yes | The foal had joint ill. Maiden mare. | G3P[12] | IRL/2051/2024/G3P[12] |
| 2/24 | March | 1 month | Moderate | No | No | G3P[12] | IRL/5154/2024/G3P[12] | |
| 3/24 | March | 1 week | Very Good | No | No | Maiden mare | G14P[12] | IRL/4257/2024/G14P[12] |
| 4/24 | March | 1 week | Poor | No | No | Boarding farm 3, visiting mare | G3P[12] | IRL/4164/2024/G3P[12] |
| 5/24 | April | 2 months | Very good | No | Moderate | Boarding farm, visiting mare | P[12] | IRL/3581/2024/P[12] |
| 5/24 | June | 2 months | Very Good | No | Moderate | G14P[12] | IRL/79120/2024/G14P[12] | |
| 6/24 | April | 2 weeks | Poor | No | Yes | G3P[12] | IRL/1282/2024/G3P[12] | |
| 7/24 | April | 1 week | Very Good | Yes 1 | Yes | G3P[12] | IRL/0283/2024/G3P[12] | |
| 8/24 | April | 2 weeks | Poor | No | Yes | G3P[12] | IRL/0988/2024/G3P[12] | |
| 9/24 | April | 2 months | Very Good | Unknown | No | Boarding farm, visiting mare | G3P[12] | IRL/8396/2024/G3P[12] |
| 9/24 | May | 3 weeks | Very Good 2 | Unknown | No | Visiting mare–transported from another country | G3P[12] | IRL/91103/2024/G3P[12] |
| 10/24 | April | 2 months | Very Good | Yes | No | Neonatal maladjustment syndrome | G3P[12] | IRL/8496/2024/G3P[12] |
| 10/24 | July | 3.5 months | Very Good | Yes | No | G3P[12] | IRL/65131/2024/G3P[12] | |
| 11/24 | May | 2 months | Very Good | No | No | G3P[12] | IRL/54110/2024/G3P[12] | |
| 12/24 | May | 1.5 months | Very Good | No | No | G3P[12] | IRL/35111/2024/G3P[12] | |
| 13/24 | June | 2 months | Moderate | Yes | Moderate | Boarding farm | G3P[12] | IRL/25121/2024/G3P[12] |
| 14/24 | June | Not provided | Very Good 2 | Yes | No | Transported: four foals travelled together in their own box and all developed diarrhoea | G3P[12] | IRL/06123/2024/G3P[12] |
| 14/24 | July | Not provided | Very Good 2 | Yes | No | G3P[12] | IRL/13128/2024/G3P[12] | |
| 14/24 | July | Not provided | Very Good 2 | Yes | No | G3P[12] | IRL/17128/2024/G3P[12] IRL/32128/2024/G3P[12] IRL/39128/2024/G3P[12] | |
| 14/24 | July | Not provided | Very Good 2 | Yes | No | G3P[12] | IRL/31128/2024/G3P[12] | |
| 15/24 | July | 2 months | Moderate | No | No | G3P[12] | IRL/91128/2024/G3P[12] | |
| 16/24 | July | 3 months | Very Good | No | No | G3P[12] | IRL/90129/2024/G3P[12] | |
| 17/24 | July | 3.5 months | Unknown | No | No | P[12] | IRL/08132/2024/GXP[12] | |
| 18/24 | July | 2 months | Unknown | No | No | ND | ND | |
| 19/24 | August | 5 months | Moderate | Yes | Moderate | Boarding farm | G3P12 | IRL/77132/2024/G3P[12] |
| 20/24 | August | 2 months | Very Good | Yes | No | G3P12 | IRL/56135/2024/G3P[12] |
| Antigenic Site | Site A | Site B | Site C | Site F | ||||||||||||||
| Virus | 90 | 91 | 92 | 94 | 96 | 145 | 147 | 209 | 211 | 212 | 213 | 215 | 217 | 218 | 221 | 237 | 238 | 242 |
| HM160096.1 VP7 ERAV_H2 Vaccine | A | T | E | N | N | N | T | T | D | V | A | F | T | I | A | L | D | A |
| L49043.1 ERV316/Aus/G3A | A | T | E | N | N | N | T | T | D | V | A | I | E | I | A | L | D | T |
| RVA/IRL/69103/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | N |
| RVA/IRL/2798/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | N |
| RVA/IRL/1297/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | N |
| RVA/IRL/2051/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | N |
| RVA/IRL/5154/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | N |
| RVA/IRL/0283/2024/G3P12 | A | T | E | N | N | N | T | T | N | V | A | F | E | I | A | L | D | N |
| RVA/IRL/4099/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/6572/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | T | F | E | I | A | L | D | A |
| RVA/IRL/6672/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/8973/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/5283/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/0646/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/7151/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/4164/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/1282/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/0988/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/8396/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/8496/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/91103/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/54110/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/35111/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/06123/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/13128/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/17128/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/31128/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/32128/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/77132/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/39128/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | I | A | L | D | A |
| RVA/IRL/9051/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/25121/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/3855/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/7656/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/2059/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/56135/2023/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/90129/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/65131/2024/G3P12 | A | T | E | N | N | N | T | T | D | V | A | F | E | M | A | L | D | A |
| RVA/IRL/91128/2024/G3P12 | A | T | E | N | N | N | T | A | D | V | A | F | E | M | A | L | D | A |
| LC467867.1_RVA/JPN/2014/G3B | V | A | E | N | N | N | T | T | D | T | T | F | E | V | A | L | D | S |
| LC467856.1.ERVA/JPN/2013/G3B | V | A | E | N | N | N | T | T | D | T | T | F | E | V | A | L | D | S |
| LC467878.1.ERVA/JPN/2016/G3B | V | A | E | N | N | N | T | T | D | T | T | F | E | V | A | L | D | S |
| RVA/IRL/1097/2023/G14P12 | A | A | Q | A | S | D | A | T | N | V | D | F | E | V | S | I | N | T |
| RVA/IRL/1197/2023/G14P12 | A | A | Q | A | S | D | A | T | N | V | D | F | E | V | S | I | N | T |
| RVA/IRL/49102/2023/G14P12 | A | A | Q | A | S | D | A | T | N | V | D | F | E | V | S | I | N | T |
| RVA/IRL/4257/2024/G14P12 | A | A | Q | A | S | D | A | T | N | V | D | F | E | V | S | I | N | T |
| RVA/IRL/79120/2024/G14P12 | A | A | Q | A | S | D | A | T | N | V | D | F | E | V | S | I | N | T |
| RVA/IRL/8693/2023/G14P12 | A | T | Q | D | S | D | A | T | N | V | E | F | E | V | S | I | N | T |
| RVA/IRL/72106/2023/G14P12 | A | T | Q | D | S | D | A | T | N | V | E | F | E | V | S | I | N | T |
| LC467889.1_RVA/JPN/2012/G14P12 | A | T | Q | D | S | D | A | T | N | V | E | F | E | V | S | I | N | T |
| LC460232.1.ERVA/JPN/2015/G14P12 | A | T | Q | D | S | D | A | T | N | V | E | F | E | V | S | I | N | T |
| LC467911.1_RVA/JPN/2016/G14P12 | A | T | Q | D | S | D | A | T | N | V | E | F | E | V | S | I | N | T |
| LC460243.1.ERVA/JPN/2018/G14P12 | A | T | Q | D | S | D | I | T | N | V | E | F | E | V | S | I | N | T |
| LC467933.1.ERVA/JPN/2018/G14P12 | A | T | Q | D | S | D | I | T | N | V | E | F | E | V | S | I | N | T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cullinane, A.; Garvey, M.; Dayot, L.; Lukaseviciute, G. Equine Rotavirus A Outbreaks in Ireland (2023–2024): An Epidemiological Investigation and Virus Genotyping. Viruses 2025, 17, 511. https://doi.org/10.3390/v17040511
Cullinane A, Garvey M, Dayot L, Lukaseviciute G. Equine Rotavirus A Outbreaks in Ireland (2023–2024): An Epidemiological Investigation and Virus Genotyping. Viruses. 2025; 17(4):511. https://doi.org/10.3390/v17040511
Chicago/Turabian StyleCullinane, Ann, Marie Garvey, Laura Dayot, and Gabija Lukaseviciute. 2025. "Equine Rotavirus A Outbreaks in Ireland (2023–2024): An Epidemiological Investigation and Virus Genotyping" Viruses 17, no. 4: 511. https://doi.org/10.3390/v17040511
APA StyleCullinane, A., Garvey, M., Dayot, L., & Lukaseviciute, G. (2025). Equine Rotavirus A Outbreaks in Ireland (2023–2024): An Epidemiological Investigation and Virus Genotyping. Viruses, 17(4), 511. https://doi.org/10.3390/v17040511





