Concurrent Circulation of Viral Agents in Pediatric Patients Presenting with Respiratory Illness and Diarrheal Symptoms in Metropolitan Region of São Paulo, Brazil, 2021
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Viral Screening
2.3. HAdV, HBoV, NoV, EV, and PeV-A Typing
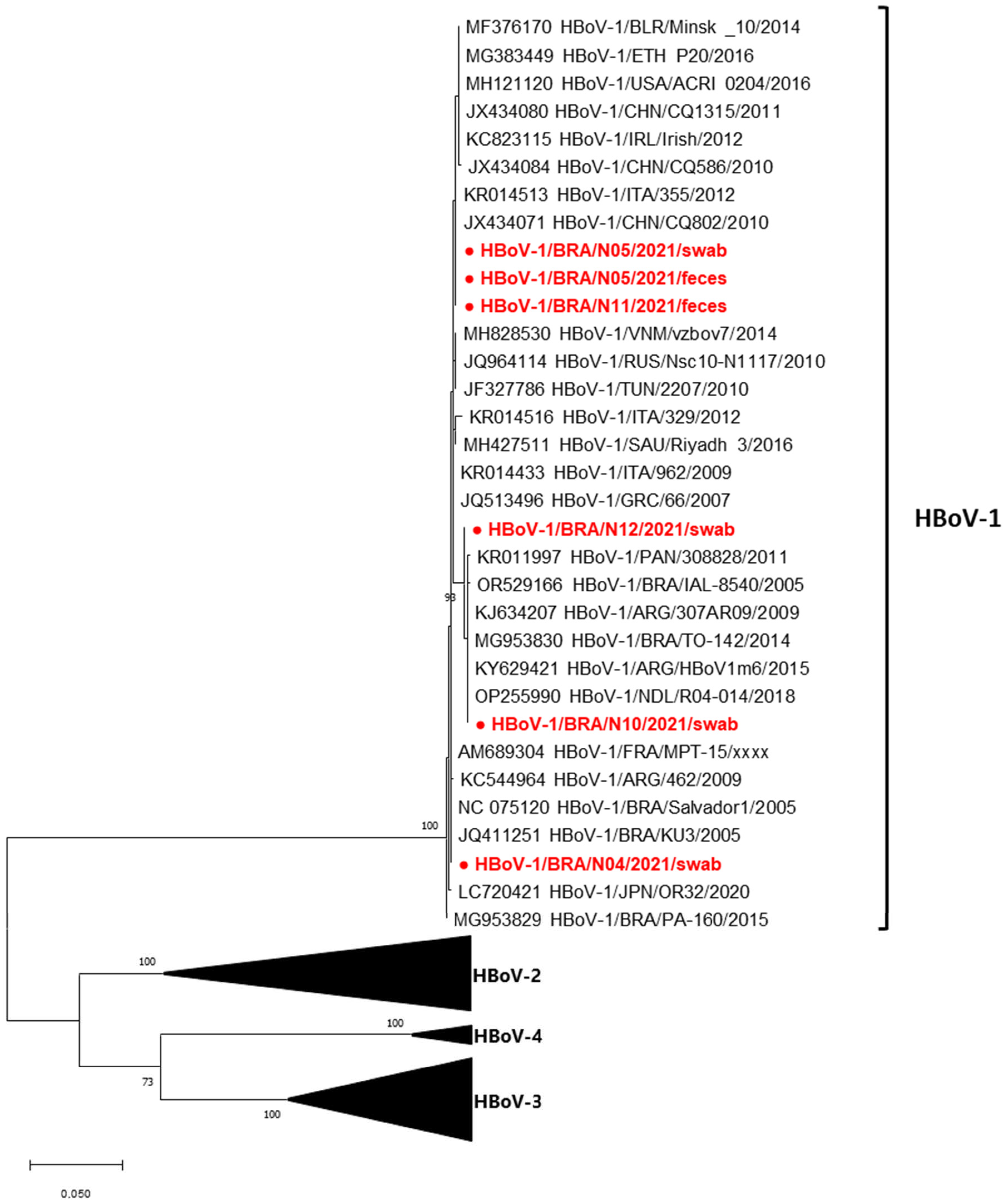
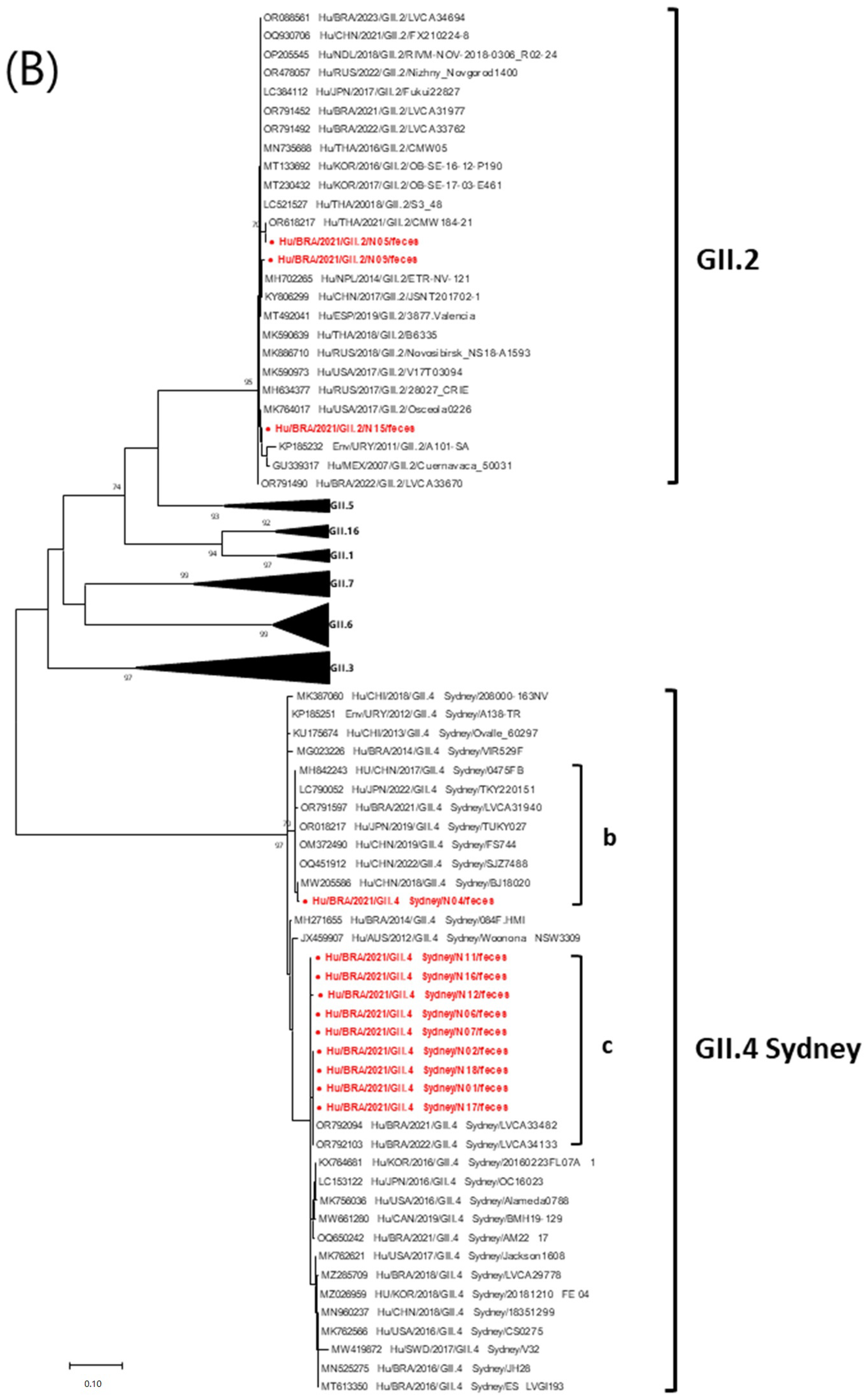
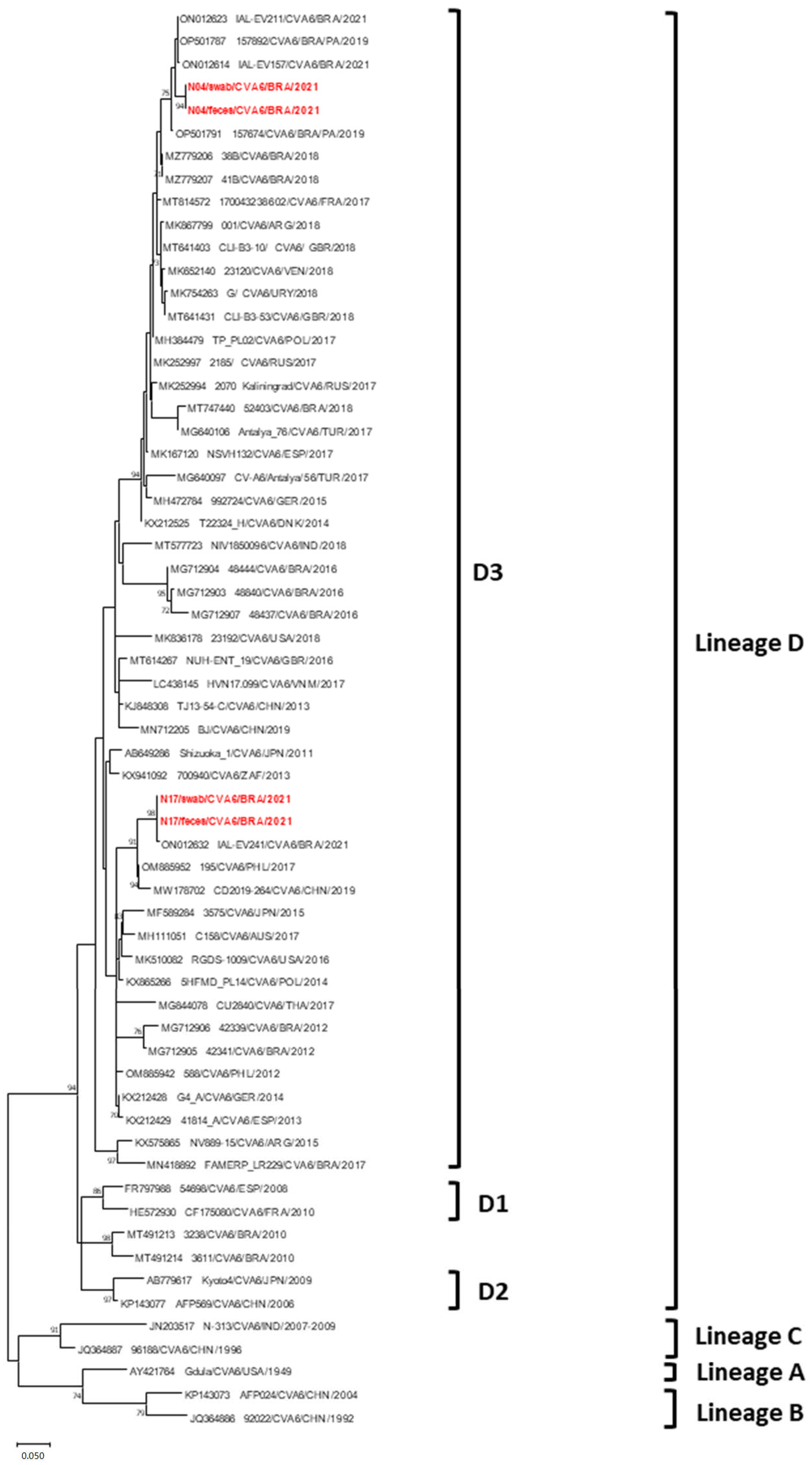
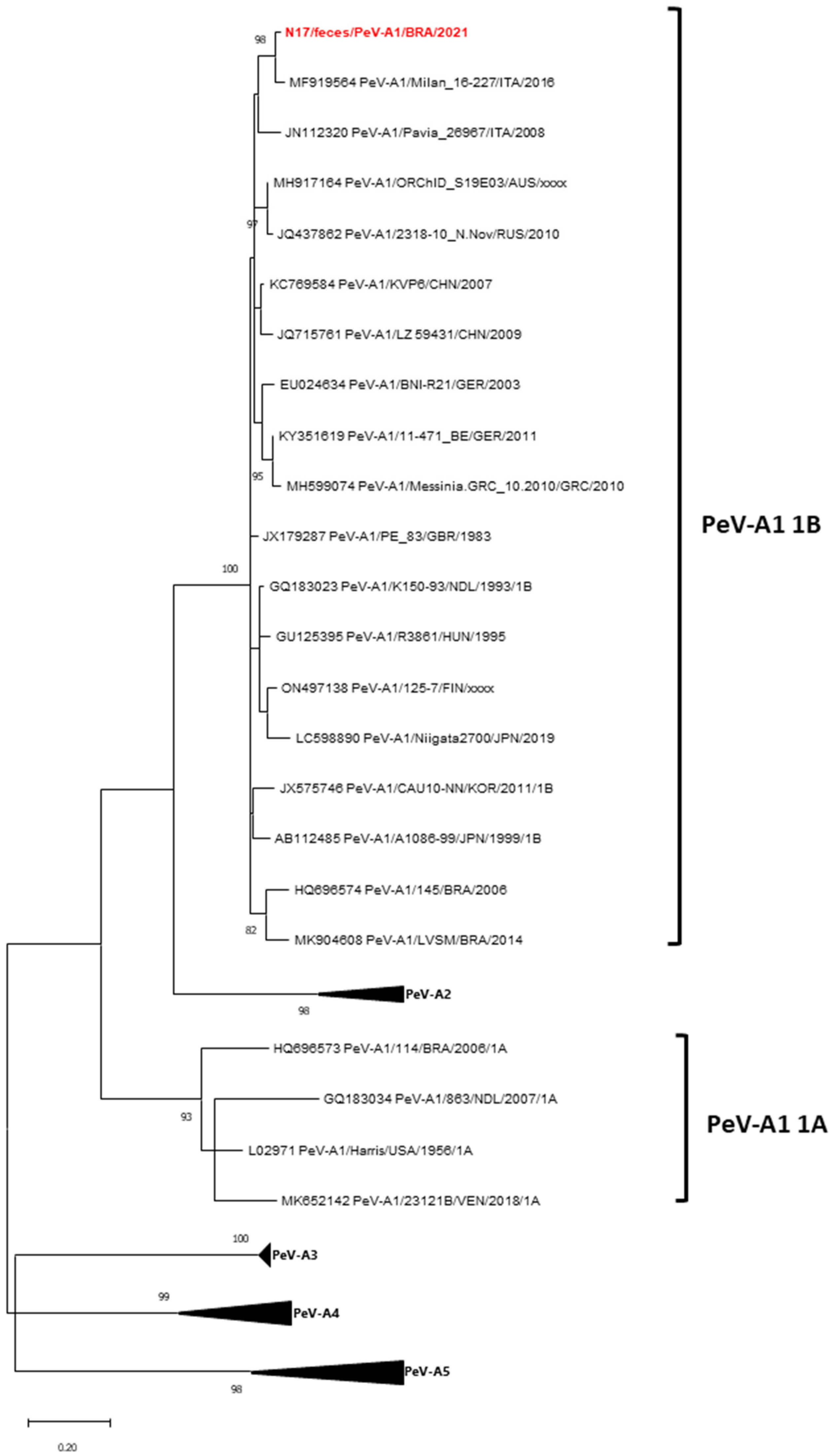
2.4. Phylogenetic Analysis
3. Results
3.1. Clinical Findings
3.2. Viral Diagnosis
3.3. Genotypic Data
3.4. Phylogenetic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Shen, L.; Yan, H.; Li, W.; Tian, Y.; Lin, C.; Liu, B.; Wang, Y.; Jia, L.; Zhang, D.; Yang, P.; et al. Occurrence of respiratory viruses among outpatients with diarrhea in Beijing, China, 2019–2020. Front. Microbiol. 2023, 13, 1073980. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Wei, H.; Lian, Z.X.; Sun, R.; Tian, Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014, 211, 2397–2410. [Google Scholar] [CrossRef]
- Rao, C.D. Enteroviruses in gastrointestinal diseases. Rev. Med. Virol. 2021, 31, 1–12. [Google Scholar] [CrossRef]
- Pérez-Martínez, Z.; Álvarez-Argüelles, M.E.; Rojo-Alba, S.; Castello-Abietar, C.; Boga, J.A.; Morilla-Morilla, A.; Vivanco-Allende, A.; Rodríguez-Suárez, J.; Alonso-Álvarez, M.A.; Melón, S. Incidence of enterovirus in patients with acute gastroenteritis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Guido, M.; Tumolo, M.R.; Verri, T.; Romano, A.; Serio, F.; De Giorgi, M.; De Donno, A.; Bagordo, F.; Zizza, A. Human bocavirus: Current knowledge and future challenges. World J. Gastroenterol. 2016, 22, 8684–8697. [Google Scholar] [CrossRef] [PubMed]
- de Crom, S.C.; Rossen, J.W.; van Furth, A.M.; Obihara, C.C. Enterovirus and parechovirus infection in children: A brief overview. Eur. J. Pediatr. 2016, 175, 1023–1029. [Google Scholar] [CrossRef]
- Sarmento, S.K.; de Andrade, J.D.S.R.; Miagostovich, M.P.; Fumian, T.M. Virological and Epidemiological Features of Norovirus Infections in Brazil, 2017–2018. Viruses 2021, 13, 1724. [Google Scholar] [CrossRef]
- Silva, A.D.D.; Veiga, A.B.G.D.; Cruz, O.G.; Bastos, L.S.; Gomes, M.F.D.C. Severe acute respiratory infection surveillance in Brazil: The role of public, private and philanthropic healthcare units. Health Policy Plan. 2022, 37, 1075–1085. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Halkosalo, A.; Salminen, M.; Szakal, E.D.; Puustinen, L.; Vesikari, T. One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. J. Virol. Methods 2008, 153, 238–240. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Yan, Q.; He, S.; Zhou, W.; Ge, S.; Xia, N. A one-step, triplex, real-time RT-PCR assay for the simultaneous detection of enterovirus 71, coxsackie A16 and pan-enterovirus in a single tube. PLoS ONE. 2014, 9, e102724. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A.; Maher, K.; Johansson, E.S.; Niklasson, B.; Lindberg, A.M.; Pallansch, M.A.; Oberste, M.S. Detection of all known parechoviruses by real-time PCR. J. Clin. Microbiol. 2008, 46, 2519–2524. [Google Scholar] [CrossRef]
- Heim, A.; Ebnet, C.; Harste, G.; Pring-Akerblom, P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 2003, 70, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chittaganpitch, M.; Olsen, S.J.; Mackay, I.M.; Sloots, T.P.; Fry, A.M.; Erdman, D.D. Real-time PCR assays for detection of bocavirus in human specimens. J. Clin. Microbiol. 2006, 44, 3231–3235. [Google Scholar] [CrossRef]
- Chu, D.K.; Poon, L.L.; Guan, Y.; Peiris, J.S. Novel astroviruses in insectivorous bats. J. Virol. 2008, 82, 9107–9114. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; McDonough, M.C.; Erdman, D.D. Species-specific identification of human adenoviruses by a multiplex PCR assay. J. Clin. Microbiol. 2000, 38, 4114–4120. [Google Scholar] [CrossRef]
- Durigon, G.S.; Oliveira, D.B.; Vollet, S.B.; Storni, J.G.; Felício, M.C.; Finelli, C.; Piera, J.; Magalhães, M.; Caldeira, R.N.; Barbosa, M.L.; et al. Hospital-acquired human bocavirus in infants. J. Hosp. Infect. 2010, 76, 171–173. [Google Scholar] [CrossRef]
- Chhabra, P.; Browne, H.; Huynh, T.; Diez-Valcarce, M.; Barclay, L.; Kosek, M.N.; Ahmed, T.; Lopez, M.R.; Pan, C.Y.; Vinjé, J. Single-step RT-PCR assay for dual genotyping of GI and GII norovirus strains. J. Clin. Virol. 2021, 134, 104689. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Megson, B.; Gray, J. Molecular detection and characterization of human enteroviruses directly from clinical samples using RT-PCR and DNA sequencing. J. Med. Virol. 2006, 78, 243–253. [Google Scholar] [CrossRef]
- Ghanem-Zoubi, N.; Shiner, M.; Shulman, L.M.; Sofer, D.; Wolf, D.; Marva, E.; Kra-Oz, Z.; Shachor-Meyouhas, Y.; Averbuch, D.; Bechor-Fellner, A.; et al. Human parechovirus type 3 central nervous system infections in Israeli infants. J. Clin. Virol. 2013, 58, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hellferscee, O.; Tempia, S.; Walaza, S.; Variava, E.; Dawood, H.; Wolter, N.; Madhi, S.A.; du Plessis, M.; Cohen, C.; Treurnicht, F.K. Enterovirus genotypes among patients with severe acute respiratory illness, influenza-like illness, and asymptomatic individuals in South Africa, 2012–2014. J. Med. Virol. 2017, 89, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.C.; Varella, R.B.; Santos, N. Acute respiratory viral infections in children in Rio de Janeiro and Teresópolis, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Swets, M.C.; Russell, C.D.; Harrison, E.M.; Docherty, A.B.; Lone, N.; Girvan, M.; Hardwick, H.E.; ISARIC4C Investigators; Visser, L.G.; Openshaw, P.J.M.; et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022, 399, 1463–1464. [Google Scholar] [CrossRef]
- Velasco, J.M.; Yoon, I.K.; Mason, C.J.; Jarman, R.G.; Bodhidatta, L.; Klungthong, C.; Silapong, S.; Valderama, M.T.; Wongstitwilairoong, T.; Torres, A.G.; et al. Applications of PCR (real-time and MassTag) and enzyme-linked immunosorbent assay in diagnosis of respiratory infections and diarrheal illness among deployed U.S. military personnel during exercise Balikatan 2009, Philippines. Mil. Med. 2011, 176, 1096–1100. [Google Scholar] [CrossRef]
- Silva, P.E.; Figueiredo, C.A.; Luchs, A.; de Paiva, T.M.; Pinho, M.A.B.; Paulino, R.S.; da Silva, D.B.B.; de Oliveira Santos, K.C.; Afonso, A.M.S.; de Oliveira, M.I. Human bocavirus in hospitalized children under 5 years with acute respiratory infection, São Paulo, Brazil, 2010. Arch. Virol. 2018, 163, 1325–1330. [Google Scholar] [CrossRef]
- Viana, E.; França, Y.; de Azevedo, L.S.; Medeiros, R.S.; Guiducci, R.; Guadagnucci, S.; Luchs, A. Genotypic diversity and long-term impact of human bocavirus on diarrheal disease: Insights from historical fecal samples in Brazil. J. Med. Virol. 2024, 96, e29429. [Google Scholar] [CrossRef]
- Kapoor, A.; Simmonds, P.; Slikas, E.; Li, L.; Bodhidatta, L.; Sethabutr, O.; Triki, H.; Bahri, O.; Oderinde, B.S.; Baba, M.M.; et al. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J. Infect. Dis. 2010, 201, 1633–1643. [Google Scholar] [CrossRef]
- Zheng, G.L.; Zhu, Z.X.; Cui, J.L.; Yu, J.M. Evolutionary analyses of emerging GII.2[P16] and GII.4 Sydney [P16] noroviruses. Virus Evol. 2022, 8, veac030. [Google Scholar] [CrossRef]
- Sarmento, S.K.; de Andrade, J.D.S.R.; Malta, F.C.; Fialho, A.M.; Mello, M.S.; Burlandy, F.M.; Fumian, T.M. Norovirus Epidemiology and Genotype Circulation during the COVID-19 Pandemic in Brazil, 2019–2022. Pathogens 2023, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Iritani, N.; Kaida, A.; Abe, N.; Sekiguchi, J.; Kubo, H.; Takakura, K.; Goto, K.; Ogura, H.; Seto, Y. Increase of GII.2 norovirus infections during the 2009-2010 season in Osaka City, Japan. J. Med. Virol. 2012, 84, 517–525. [Google Scholar] [CrossRef]
- Jin, M.; Wu, S.; Kong, X.; Xie, H.; Fu, J.; He, Y.; Feng, W.; Liu, N.; Li, J.; Rainey, J.J.; et al. Norovirus Outbreak Surveillance, China, 2016–2018. Emerg. Infect. Dis. 2020, 26, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Bidalot, M.; Théry, L.; Kaplon, J.; De Rougemont, A.; Ambert-Balay, K. Emergence of new recombinant noroviruses GII.p16-GII.4 and GII.p16-GII.2, France, winter 2016 to 2017. Euro Surveill. 2017, 22, 30508. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, L.D.; Triantafilou, M.J.; Marshall, J.A. The molecular epidemiology of norovirus outbreaks in Victoria, 2016. Commun Dis Intell. 2018, 42, s2209–s6051. [Google Scholar]
- Van Beek, J.; de Graaf, M.; Al-Hello, H.; Allen, D.J.; Ambert-Balay, K.; Botteldoorn, N.; Brytting, M.; Buesa, J.; Cabrerizo, M.; Chan, M.; et al. Molecular surveillance of norovirus, 2005–16: An epidemiological analysis of data collected from the NoroNet network. Lancet Infect. Dis. 2018, 18, 545–553. [Google Scholar] [CrossRef]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in Norovirus Genotype Distribution among Children with Acute Gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef]
- Shen, C.F.; Wang, S.M.; Wang, J.R.; Hu, Y.S.; Ho, T.S.; Liu, C.C. Comparative study of clinical and epidemiological characteristics of major pediatric adenovirus epidemics in southern Taiwan. BMC Infect. Dis. 2019, 19, 681. [Google Scholar] [CrossRef]
- Souza, E.V.; de Souza, Y.F.V.P.; Medeiros, R.S.; de Azevedo, L.S.; de Queiroz, T.G.A.; Sanz-Duro, R.L.; Marinho, R.D.S.S.; Komninakis, S.V.; Timenetsky, M.D.C.S.T.; Luchs, A. Diversity of enteric and non-enteric human adenovirus strains in Brazil, 2006–2011. Arch. Virol. 2021, 166, 897–903. [Google Scholar] [CrossRef]
- Kimmis, B.D.; Downing, C.; Tyring, S. Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Cutis 2018, 102, 353–356. [Google Scholar]
- Luchs, A.; Azevedo, L.S.; Souza, E.V.; Medeiros, R.S.; Souza, Y.F.V.P.; Teixeira, D.L.F.; Carneiro, T.F.O.; Alencar, G.M.F.; Morais, F.L.S.L.; Pinto, D.F.A.; et al. Coxsackievirus A6 strains causing an outbreak of hand-foot-and-mouth disease in Northeastern Brazil in 2018. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e16. [Google Scholar] [CrossRef]
- Justino, M.C.A.; Mesquita, D.S.; Souza, M.F.; Farias, F.P.; Alves, J.C.D.S.; Ferreira, J.L.; Lopes, D.P.; Tavares, F.N. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J. Clin. Virol. 2020, 126, 104307. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.C.D.; Estofolete, C.F.; Milhim, B.H.G.A.; Augusto, M.T.; Zini, N.; Silva, G.C.D.D.; Ferraz-Junior, H.C.; Brienze, V.M.S.; Liso, E.; Cunha, M.S.; et al. Enteric viruses circulating in undiagnosed central nervous system infections at tertiary hospital in São José do Rio Preto, São Paulo, Brazil. J. Med. Virol. 2021, 93, 3539–3548. [Google Scholar] [CrossRef]
- Foronda, J.L.M.; Jiao, M.M.A.D.; Climacosa, F.M.M.; Oshitani, H.; Apostol, L.N.G. Epidemiological and molecular characterization of Coxsackievirus A6 causing hand, foot, and mouth disease in the Philippines, 2012–2017. Infect. Genet. Evol. 2023, 114, 105498. [Google Scholar] [CrossRef]
- Malasao, R.; Khamrin, P.; Kumthip, K.; Ushijima, H.; Maneekarn, N. Molecular epidemiology and genetic diversity of human parechoviruses in children hospitalized with acute diarrhea in Thailand during 2011–2016. Arch. Virol. 2019, 164, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Skanke, L.H.; Lysvand, H.; Heimdal, I.; Moe, N.; Krokstad, S.; Christensen, A.; Risnes, K.; Nordbø, S.A.; Døllner, H. Parechovirus A in Hospitalized Children with Respiratory Tract Infections: A 10-Year-Long Study from Norway. J. Pediatric Infect. Dis. Soc. 2021, 10, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Grywna, K.; Stöcker, A.; Almeida, P.S.; Medrado-Ribeiro, T.C.; Eschbach-Bludau, M.; Petersen, N.; da Costa-Ribeiro, H.J.; Drosten, C. Novel human parechovirus from Brazil. Emerg. Infect. Dis. 2009, 15, 310–313. [Google Scholar] [CrossRef]
- Leal, É.; Luchs, A.; Milagres, F.A.P.; Komninakis, S.V.; Gill, D.E.; Lobato, M.C.A.B.S.; Brustulin, R.; Chagas, R.T.D.; Abrão, M.F.N.D.S.; Soares, C.V.D.A.; et al. Recombinant Strains of Human Parechovirus in Rural Areas in the North of Brazil. Viruses 2019, 11, 488. [Google Scholar] [CrossRef]
- Soares, G.D.S.; Morais, L.V.; Silva, K.C.N.; Ferreira, E.M.; Shio, M.T.; Romano, C.M.; Conde, C.R.; Sabino, E.C.; França, C.N.; Nali, L.H. Low frequency of SARS-CoV2 infection in daycare centers during the reopening of school activities in the Southeast’s poor area of Brazil. Rev. Inst. Med. Trop. São Paulo 2022, 64, e46. [Google Scholar] [CrossRef]
- Yum, S.; Hong, K.; Sohn, S.; Kim, J.; Chun, B.C. Trends in Viral Respiratory Infections During COVID-19 Pandemic, South Korea. Emerg. Infect. Dis. 2021, 27, 1685–1688. [Google Scholar] [CrossRef]
- Ippolito, G.; La Vecchia, A.; Umbrello, G.; Di Pietro, G.; Bono, P.; Scalia Catenacci, S.; Pinzani, R.; Tagliabue, C.; Bosis, S.; Agostoni, C.; et al. Disappearance of Seasonal Respiratory Viruses in Children Under Two Years Old During COVID-19 Pandemic: A Monocentric Retrospective Study in Milan, Italy. Front. Pediatr. 2021, 9, 721005. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Rhee, J.E.; Kang, D.; Choi, E.H.; Lee, N.J.; Woo, S.; Lee, J.; Lee, S.-W.; Kim, E.-J.; Yun, K.W. Epidemiology of Respiratory Viruses in Korean Children Before and After the COVID-19 Pandemic: A Prospective Study from National Surveillance System. J. Korean Med. Sci. 2024, 39, e171. [Google Scholar] [CrossRef] [PubMed]
- Fontes, V.; Ferreira, H.; Ribeiro, M.; Pinheiro, A.; Maramaldo, C.; Pereira, E.; Batista, L.; Júnior, A.; Lobato, L.; Silva, F.; et al. High Incidence of Respiratory Syncytial Virus in Children with Community-Acquired Pneumonia from a City in the Brazilian Pre-Amazon Region. Viruses 2023, 15, 1306. [Google Scholar] [CrossRef] [PubMed]


| ID | Gender | Age | Onset Date | Collection Date | City | Signs and Symptoms | HBoV, Type | RVA | HAdV, Type | EV, Type | SARS-CoV-2 | Flu A/B | RSV | HAstV | NoV, Group | NoV Strain | PeV-A, Type | Sample |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N01 | Male | 1 year | August 8 | August 30 | Barueri | Diarrhea | +, NT | - | - | - | - | - | + | - | - | - | Swab | |
| - | - | - | - | - | - | + | - | +, GII | GII.4 Sydney[P16] | - | Feces | |||||||
| N02 | Male | 1 year | August 28 | September 3 | Barueri | Diarrhea, vomiting, coryza, cough, fever | +, NT | - | - | - | - | - | - | - | - | - | Swab | |
| - | - | - | - | - | - | - | - | +, GII | GII.4 Sydney[P16] | - | Feces | |||||||
| N03 | Female | 1 year | August 31 | September 3 | Carapicuiba | Diarrhea | +, NT | - | - | - | - | - | + | - | - | - | Swab | |
| +, NT | - | - | - | - | - | - | - | +, GII | NT | - | Feces | |||||||
| N04 | Male | 2 years | September 5 | September 5 | Barueri | Diarrhea, vomiting | +, HBoV-1 | - | - | +, CVA6 | - | - | - | - | - | - | Swab | |
| +, HBoV-1 | - | - | +, CVA6 | - | - | - | - | +, GII | GII.4 Sydney[P31] | - | Feces | |||||||
| N05 | Female | 1 year | September 5 | September 6 | Osasco | Diarrhea, cough, fever | +, HBoV-1 | - | - | - | - | - | - | - | - | - | Swab | |
| +, HBoV-1 | - | - | - | - | - | - | - | +, GII | GII.2[P16] | - | Feces | |||||||
| N06 | Female | 6 months | September 6 | September 8 | Osasco | Diarrhea, cough, vomiting | - | - | - | - | - | - | - | - | +, GII | GII.4 Sydney[P16] | +, NT | Feces |
| N07 | Female | 1 year | September 9 | September 10 | Barueri | Diarrhea | +, HBoV-1 | - | - | - | - | - | - | - | +, GII | GII.4 Sydney[P16] | - | Feces |
| N08 | Male | 2 years | September 12 | September 13 | Barueri | Diarrhea, vomiting, coryza, fever, productive cough, abdominal pain | +, HBoV-1 | - | - | - | - | - | - | - | +, GII | NT | - | Feces |
| N09 | Male | 3 years | September 20 | September 21 | Barueri | Diarrhea, vomiting | - | - | - | - | - | - | - | - | - | - | Swab | |
| +, NT | - | - | - | - | - | - | - | +, GII | GII.2[P16] | - | Feces | |||||||
| N10 | Male | 20 days | September 18 | September 21 | Barueri | Vomiting, fever | +, HBoV-1 | - | - | - | - | - | - | - | - | - | Swab | |
| +, NT | - | - | - | - | - | - | - | - | - | Feces | ||||||||
| N11 | Female | 1 year | September 12 | September 13 | Carapicuiba | Diarrhea, vomiting, coryza, cough, fever | - | - | - | - | - | - | - | - | - | - | Swab | |
| +, HBoV-1 | - | +, C6 | - | - | - | - | - | +, GII | GII.4 Sydney[P16] | - | Feces | |||||||
| N12 | Male | 2 years | September 20 | September 22 | Barueri | Diarrhea, vomiting, coryza, sore throat, abdominal pain | +, HBoV-1 | - | - | - | - | - | - | - | - | - | Swab | |
| +, NT | - | - | - | - | - | - | - | +, GII | GII.4 Sydney[P16] | - | Feces | |||||||
| N13 | Male | 11 days | September 20 | September 21 | Barueri | Fever | +, NT | - | - | - | - | - | - | - | - | - | Swab | |
| +, NT | - | - | - | - | - | - | - | - | - | Feces | ||||||||
| N14 | Female | 6 months | September 26 | September 28 | Carapicuiba | Diarrhea, cough | - | - | - | - | - | - | - | - | - | - | Swab | |
| - | - | - | - | - | - | - | - | - | - | Feces | ||||||||
| N15 | Male | 1 year | October 2 | October 8 | Sao Paulo | Diarrhea, vomiting, coryza, fever, sneeze, productive cough, abdominal pain | +, HBoV-1 | - | +, C6 | - | - | - | - | - | - | - | Swab | |
| +, NT | - | - | - | - | - | - | - | +, GII | GII.2[P16] | - | Feces | |||||||
| N16 | Female | 1 year | October 7 | October 9 | Barueri | Diarrhea, Fever | +, NT | - | - | - | - | - | - | - | - | - | Swab | |
| +, NT | - | - | - | - | - | - | - | +, GII | GII.4 Sydney[P16] | - | Feces | |||||||
| N17 | Female | 5 months | October 11 | October 11 | Osasco | Diarrhea, vomiting | - | - | - | +, CVA6 | - | - | - | - | - | - | Swab | |
| - | - | - | +, CVA6 | - | - | - | - | +, GII | GII.4 Sydney[P16] | +, PeV-A1 | Feces | |||||||
| N18 | Male | 1 year | October 14 | October 17 | Carapicuiba | Diarrhea, vomiting, coryza, sneeze, productive cough | +, NT | - | - | - | - | - | - | - | - | - | Swab | |
| - | - | - | - | - | - | - | - | +, GII | GII.4 Sydney[P16] | - | Feces | |||||||
| N19 | Male | 1 year | November 30 | December 4 | Itapevi | Diarrhea, coryza, productive cough, fever | - | - | - | - | - | - | - | - | - | - | Swab | |
| - | - | - | - | - | - | - | - | - | - | Feces | ||||||||
| N20 | Male | 2 years | December 2 | December 6 | Carapicuiba | Diarrhea, vomiting, coryza, cough | - | - | - | - | - | Flu A | - | - | - | - | Swab | |
| - | - | - | - | - | - | - | - | - | - | Feces |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchs, A.; Adiwardana, N.S.; Rocha, L.C.d.; Viana, E.; Guadagnucci, S.; Parise, A.; Silva, V.C.M.; Azevedo, L.S.d.; Guiducci, R.; França, Y.; et al. Concurrent Circulation of Viral Agents in Pediatric Patients Presenting with Respiratory Illness and Diarrheal Symptoms in Metropolitan Region of São Paulo, Brazil, 2021. Viruses 2025, 17, 497. https://doi.org/10.3390/v17040497
Luchs A, Adiwardana NS, Rocha LCd, Viana E, Guadagnucci S, Parise A, Silva VCM, Azevedo LSd, Guiducci R, França Y, et al. Concurrent Circulation of Viral Agents in Pediatric Patients Presenting with Respiratory Illness and Diarrheal Symptoms in Metropolitan Region of São Paulo, Brazil, 2021. Viruses. 2025; 17(4):497. https://doi.org/10.3390/v17040497
Chicago/Turabian StyleLuchs, Adriana, Natanael Sutikno Adiwardana, Leonardo Cecilio da Rocha, Ellen Viana, Simone Guadagnucci, Adriana Parise, Vanessa Cristina Martins Silva, Lais Sampaio de Azevedo, Raquel Guiducci, Yasmin França, and et al. 2025. "Concurrent Circulation of Viral Agents in Pediatric Patients Presenting with Respiratory Illness and Diarrheal Symptoms in Metropolitan Region of São Paulo, Brazil, 2021" Viruses 17, no. 4: 497. https://doi.org/10.3390/v17040497
APA StyleLuchs, A., Adiwardana, N. S., Rocha, L. C. d., Viana, E., Guadagnucci, S., Parise, A., Silva, V. C. M., Azevedo, L. S. d., Guiducci, R., França, Y., Frank, N. L. P., Silva, A. L. N. d., Oliveira, A. L. V. d., Azevedo, A. H. S., Carreteiro, B. S., & Nogueira, M. L. (2025). Concurrent Circulation of Viral Agents in Pediatric Patients Presenting with Respiratory Illness and Diarrheal Symptoms in Metropolitan Region of São Paulo, Brazil, 2021. Viruses, 17(4), 497. https://doi.org/10.3390/v17040497








