Molecular Epidemiology of Human Adenovirus from Acute Gastroenteritis Cases in Brazil After the COVID-19 Pandemic Period, 2021–2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Stool Samples and Ethical Aspects
2.2. Viral Nucleic Acid Extraction
2.3. HAdV Detection and Quantification
2.4. Rotavirus and Norovirus Detection
2.5. HAdV Molecular Characterization and Genotyping
2.6. Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
3.1. Epidemiologic Features of HAdV
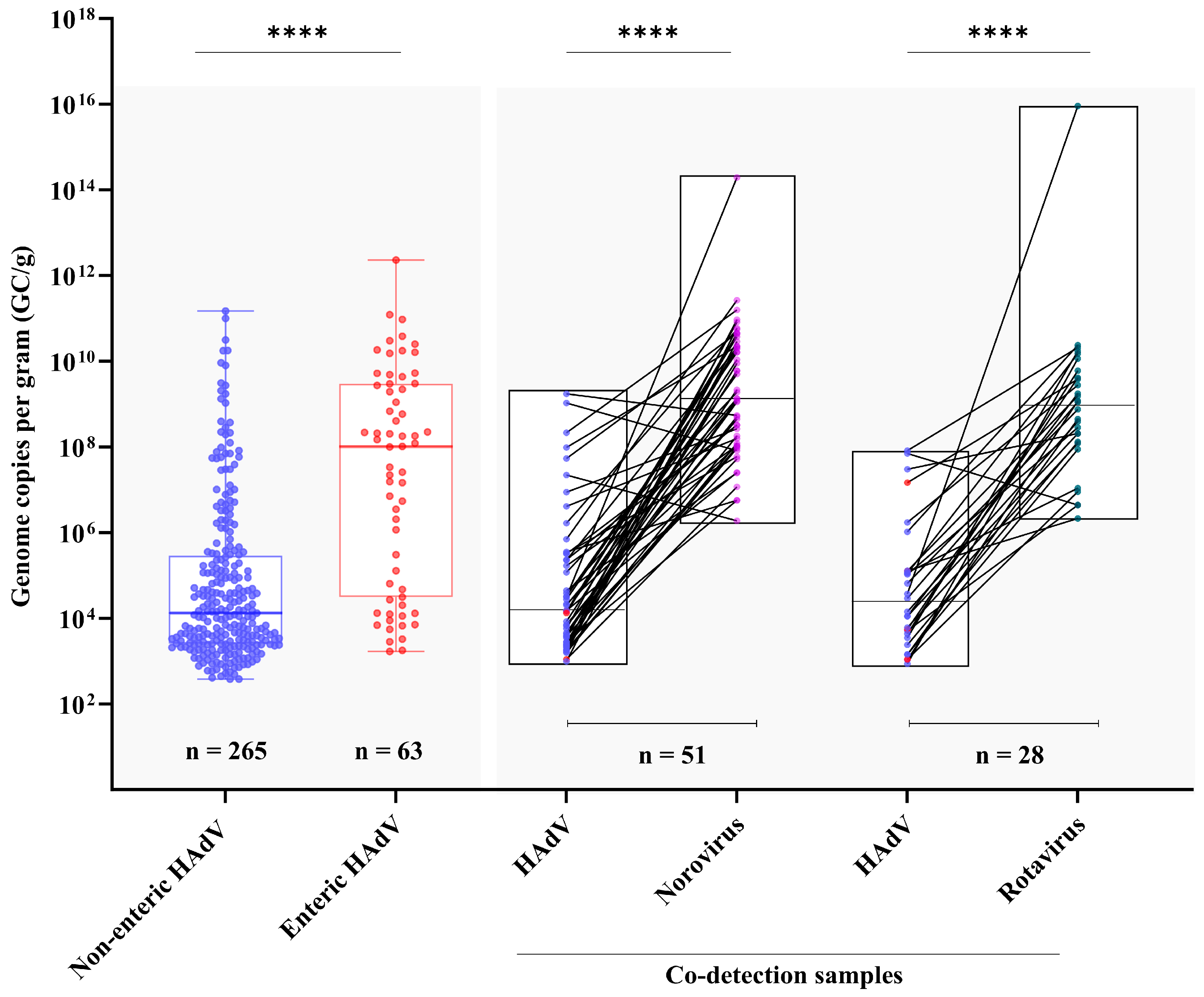
3.2. Viral Load and Co-Detection Rates
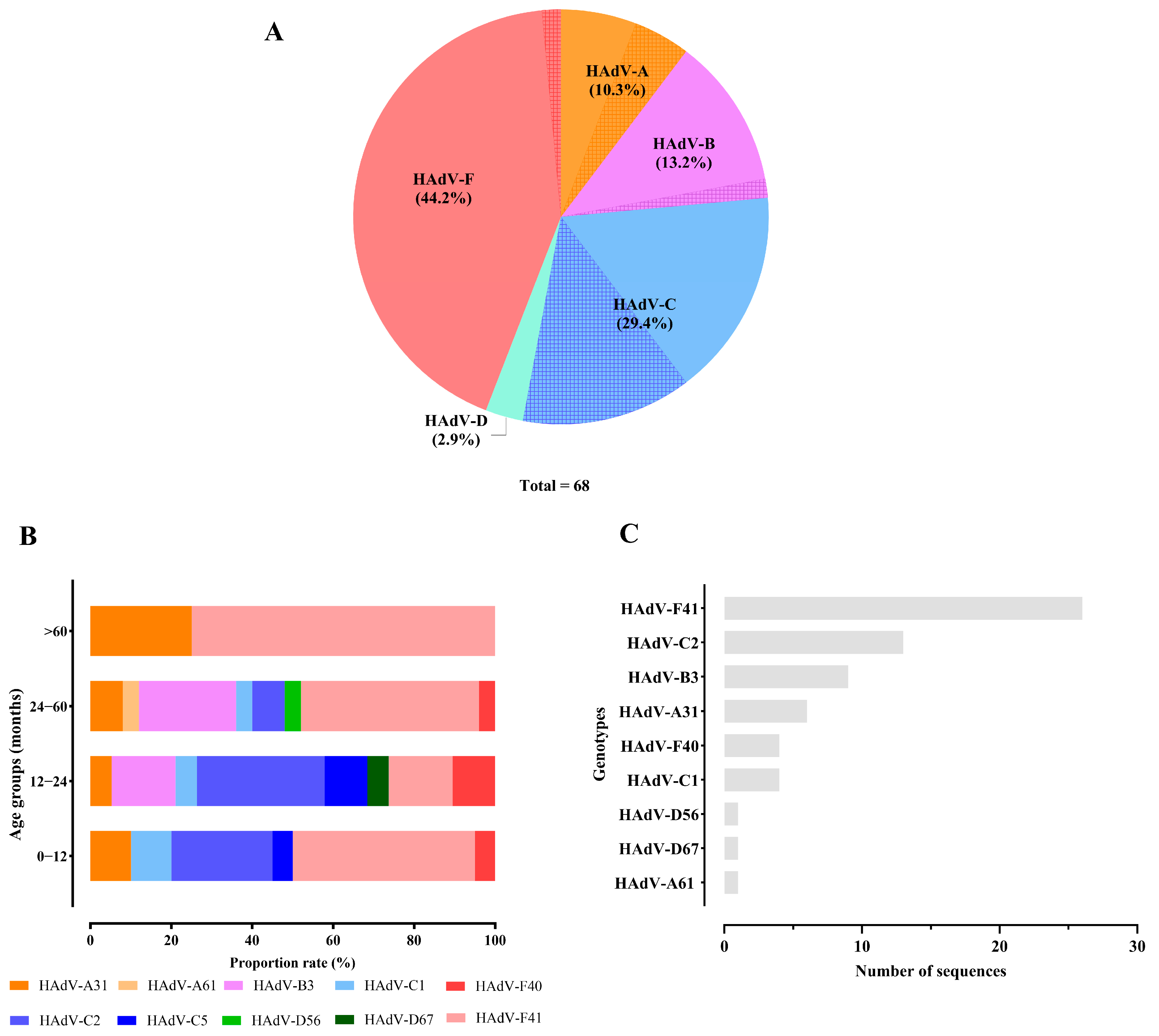
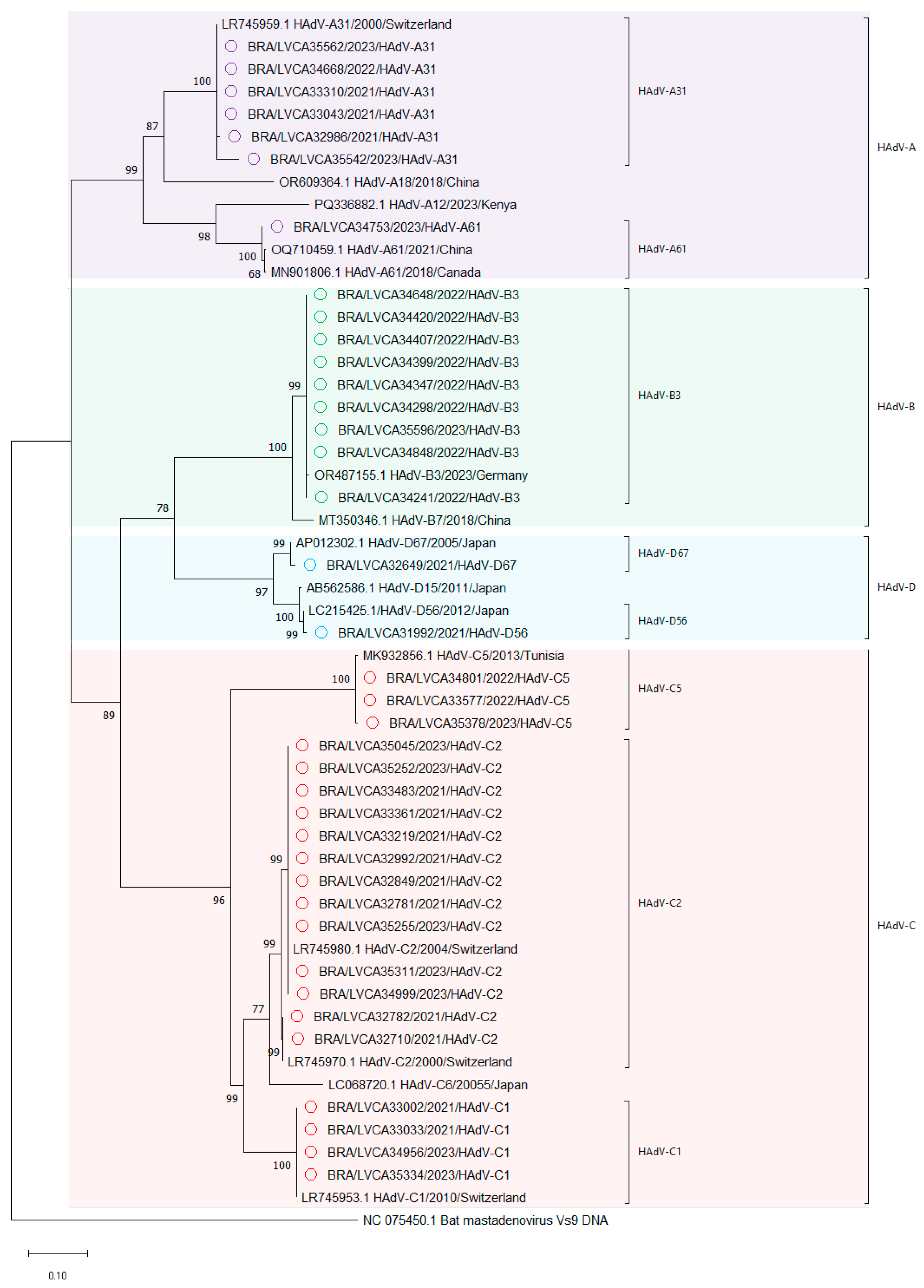
3.3. Genetic Characterization HAdV
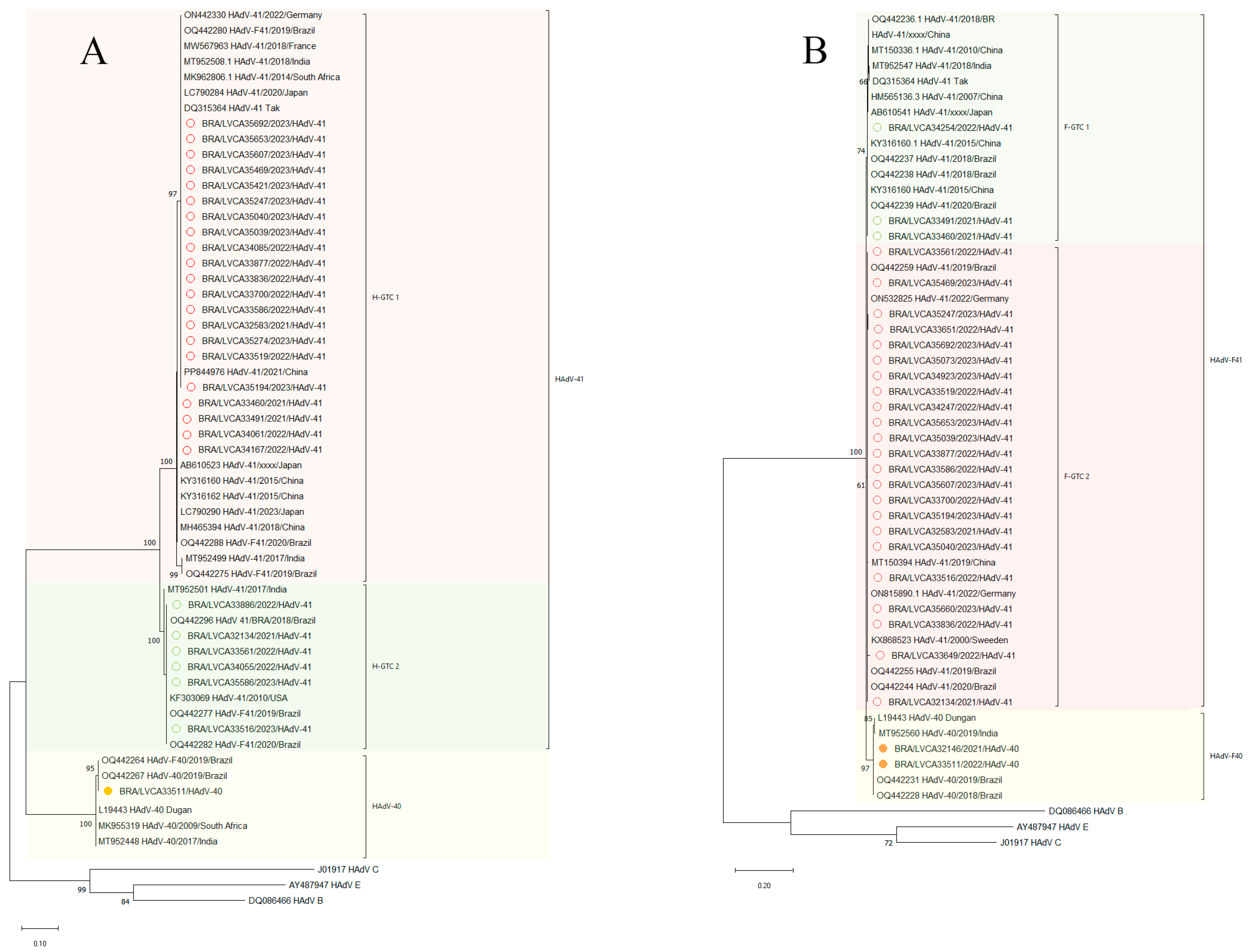
3.4. Diversity of Enteric HAdV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 30 December 2023).
- Number of Under-Five Deaths—By Cause. Available online: https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/indicator-explorer-new/MCA/number-of-under-five-deaths---by-cause (accessed on 25 March 2024).
- World Health Organization Diarrhoeal Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 22 March 2024).
- Akdag, A.I.; Gupta, S.; Khan, N.; Upadhayay, A.; Ray, P. Epidemiology and Clinical Features of Rotavirus, Adenovirus, and Astrovirus Infections and Coinfections in Children with Acute Gastroenteritis Prior to Rotavirus Vaccine Introduction in Meerut, North India. J. Med. Virol. 2020, 92, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.G.; Olortegui, M.P.; Kosek, M.N. Viral Gastroenteritis. Lancet 2024, 403, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Tousimis, A.J.; Hilleman, M.R. Electron Microscopy of Type 4 Adenovirus Strain RI-67. Virology 1957, 4, 499–508. [Google Scholar] [CrossRef]
- Valentine, R.C.; Hopper, P.K. Polyhedral Shape of Adenovirus Particles as Shown by Electron Microscopy. Nature 1957, 180, 928. [Google Scholar] [CrossRef]
- Davison, A.J.; Benkő, M.; Harrach, B. Genetic Content and Evolution of Adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef]
- San Martín, C. Latest Insights on Adenovirus Structure and Assembly. Viruses 2012, 4, 847–877. [Google Scholar] [CrossRef]
- Robinson, C.M.; Singh, G.; Lee, J.Y.; Dehghan, S.; Rajaiya, J.; Liu, E.B.; Yousuf, M.A.; Betensky, R.A.; Jones, M.S.; Dyer, D.W.; et al. Molecular Evolution of Human Adenoviruses. Sci. Rep. 2013, 3, 1812. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gonzalez, G.; Harada, S.; Oosako, H.; Hanaoka, N.; Hinokuma, R.; Fujimoto, T. Recombinant Type Human Mastadenovirus D85 Associated with Epidemic Keratoconjunctivitis since 2015 in Japan. J. Med. Virol. 2018, 90, 881–889. [Google Scholar] [CrossRef]
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.M.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The Coxsackievirus-Adenovirus Receptor Protein Can Function as a Cellular Attachment Protein for Adenovirus Serotypes from Subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [CrossRef] [PubMed]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Frängsmyr, L.; Frank, M.; Blaum, B.S.; Chai, W.; Podgorski, I.I.; Harrach, B.; Benkő, M.; et al. Polysialic Acid Is a Cellular Receptor for Human Adenovirus 52. Proc. Natl. Acad. Sci. USA 2018, 115, E4264–E4273. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, I.I.; Pantó, L.; Papp, T.; Harrach, B.; Benkö, M. Genome Analysis of Four Old World Monkey Adenoviruses Supports the Proposed Species Classification of Primate Adenoviruses and Reveals Signs of Possible Homologous Recombination. J. Gen. Virol. 2016, 97, 1604–1614. [Google Scholar] [CrossRef]
- Stasiak, A.C.; Stehle, T. Human Adenovirus Binding to Host Cell Receptors: A Structural View. Med. Microbiol. Immunol. 2020, 209, 325–333. [Google Scholar] [CrossRef]
- Ballmann, M.Z.; Raus, S.; Engelhart, R.; Kaján, G.L.; Beqqali, A.; Hadoke, P.W.F.; Van Der Zalm, C.; Papp, T.; John, L.; Khan, S.; et al. Human AdV-20-42-42, a Promising Novel Adenoviral Vector for Gene Therapy and Vaccine Product Development. J. Virol. 2021, 95, e00387-21. [Google Scholar] [CrossRef]
- Shieh, W.-J. Human Adenovirus Infections in Pediatric Population—An Update on Clinico–Pathologic Correlation. Biomed. J. 2022, 45, 38–49. [Google Scholar] [CrossRef]
- Lenaerts, L.; De Clercq, E.; Naesens, L. Clinical Features and Treatment of Adenovirus Infections. Rev. Med. Virol. 2008, 18, 357–374. [Google Scholar] [CrossRef]
- Lopez, S.M.C.; Michaels, M.G.; Green, M. Adenovirus Infection in Pediatric Transplant Recipients: Are Effective Antiviral Agents Coming Our Way? Curr. Opin. Organ Transplant. 2018, 23, 395–399. [Google Scholar] [CrossRef]
- Kuwatsuka, Y.; Atsuta, Y.; Hirakawa, A.; Uchida, N.; Inamoto, Y.; Najima, Y.; Ikegame, K.; Eto, T.; Ozawa, Y.; Ichinohe, T.; et al. Use of Unapproved or Off-Label Drugs in Japan for the Treatment of Graft-versus-Host Disease and Post-Transplant Viral Infection. Int. J. Hematol. 2020, 112, 841–850. [Google Scholar] [CrossRef]
- Uhnoo, I.; Wadell, G.; Svensson, L.; Johansson, M.E. Importance of Enteric Adenoviruses 40 and 41 in Acute Gastroenteritis in Infants and Young Children. J. Clin. Microbiol. 1984, 20, 365–372. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Losonsky, G.A.; Morris, J.G.; Wasserman, S.S.; Singh-Naz, N.; Levine, M.M. Enteric Adenovirus Infection and Childhood Diarrhea: An Epidemiologic Study in Three Clinical Settings. Pediatrics 1989, 84, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Damon, C.F.; Platts-Mills, J.A. Pediatric Acute Gastroenteritis Associated with Adenovirus 40/41 in Low-Income and Middle-Income Countries. Curr. Opin. Infect. Dis. 2020, 33, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhong, H.; Xu, M.; Su, L.; Cao, L.; Jia, R.; Xu, J. Molecular and Epidemiological Characterization of Human Adenovirus and Classic Human Astrovirus in Children with Acute Diarrhea in Shanghai, 2017–2018. BMC Infect. Dis. 2021, 21, 713. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hu, Y.; Zhong, X.; Xu, H. Molecular Epidemiology of Human Adenovirus, Astrovirus, and Sapovirus Among Outpatient Children With Acute Diarrhea in Chongqing, China, 2017–2019. Front. Pediatr. 2022, 10, 826600. [Google Scholar] [CrossRef]
- Cohen, A.L.; Platts-Mills, J.A.; Nakamura, T.; Operario, D.J.; Antoni, S.; Mwenda, J.M.; Weldegebriel, G.; Rey-Benito, G.; De Oliveira, L.H.; Ortiz, C.; et al. Aetiology and Incidence of Diarrhoea Requiring Hospitalisation in Children under 5 Years of Age in 28 Low-Income and Middle-Income Countries: Findings from the Global Pediatric Diarrhea Surveillance Network. BMJ Glob. Health 2022, 7, e009548. [Google Scholar] [CrossRef]
- Liu, L.; Qian, Y.; Jia, L.; Dong, H.; Deng, L.; Huang, H.; Zhao, L.; Zhu, R. Genetic Diversity and Molecular Evolution of Human Adenovirus Serotype 41 Strains Circulating in Beijing, China, during 2010–2019. Infect. Genet. Evol. 2021, 95, 105056. [Google Scholar] [CrossRef]
- Lambisia, A.W.; Makori, T.O.; Mutunga, M.; Cheruiyot, R.; Murunga, N.; Quick, J.; Githinji, G.; Nokes, D.J.; Houldcroft, C.J.; Agoti, C.N. Genomic Epidemiology of Human Adenovirus F40 and F41 in Coastal Kenya: A Retrospective Hospital-Based Surveillance Study (2013–2022). Virus Evol. 2023, 9, vead023. [Google Scholar] [CrossRef]
- Maes, M.; Khokhar, F.; Wilkinson, S.A.J.; Smith, A.D.; Kovalenko, G.; Dougan, G.; Quick, J.; Loman, N.J.; Baker, S.; Curran, M.D.; et al. Multiplex MinION Sequencing Suggests Enteric Adenovirus F41 Genetic Diversity Comparable to Pre-COVID-19 Era. Microb. Genom. 2023, 9, 000920. [Google Scholar] [CrossRef]
- Carvalho-Costa, F.A.; De Assis, R.M.S.; Fialho, A.M.; Araújo, I.T.; Silva, M.F.; Gómez, M.M.; Andrade, J.S.; Rose, T.L.; Fumian, T.M.; Volotão, E.M.; et al. The Evolving Epidemiology of Rotavirus A Infection in Brazil a Decade after the Introduction of Universal Vaccination with Rotarix®. BMC Pediatr. 2019, 19, 42. [Google Scholar] [CrossRef]
- Do Nascimento, L.G.; Fialho, A.M.; De Andrade, J.D.S.R.; De Assis, R.M.S.; Fumian, T.M. Human Enteric Adenovirus F40/41 as a Major Cause of Acute Gastroenteritis in Children in Brazil, 2018 to 2020. Sci. Rep. 2022, 12, 11220. [Google Scholar] [CrossRef]
- Sarmento, S.K.; De Andrade, J.D.S.R.; Miagostovich, M.P.; Fumian, T.M. Virological and Epidemiological Features of Norovirus Infections in Brazil, 2017–2018. Viruses 2021, 13, 1724. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, R.; Luchs, A.; Tardy, K.; Hefford, P.M.; Tinker, R.J.; Eilami, O.; De Padua Milagres, F.A.; Brustulin, R.; Teles, M.D.A.R.; Dos Santos Morais, V.; et al. Viral Gastroenteritis in Tocantins, Brazil: Characterizing the Diversity of Human Adenovirus F through next-Generation Sequencing and Bioinformatics. J. Gen. Virol. 2020, 101, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Souza, Y.F.V.P.D.; Souza, E.V.D.; Azevedo, L.S.D.; Medeiros, R.S.; Timenetsky, M.D.C.S.T.; Luchs, A. Enteric Adenovirus Epidemiology from Historical Fecal Samples in Brazil (1998–2005): Pre-Rotavirus Vaccine Era. Infect. Genet. Evol. 2021, 94, 105007. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.C.D.S.; Teixeira, D.M.; Siqueira, J.A.M.; Deus, D.R.D.; Oliveira, D.D.S.; Ferreira, J.L.; Lobo, P.D.S.; Soares, L.D.S.; Tavares, F.N.; Gabbay, Y.B. Epidemiology and Molecular Detection of Human Adenovirus and Non-Polio Enterovirus in Fecal Samples of Children with Acute Gastroenteritis: A Five-Year Surveillance in Northern Brazil. PLoS ONE 2024, 19, e0296568. [Google Scholar] [CrossRef]
- Gutierrez Sanchez, L.H.; Shiau, H.; Baker, J.M.; Saaybi, S.; Buchfellner, M.; Britt, W.; Sanchez, V.; Potter, J.L.; Ingram, L.A.; Kelly, D.; et al. A Case Series of Children with Acute Hepatitis and Human Adenovirus Infection. N. Engl. J. Med. 2022, 387, 620–630. [Google Scholar] [CrossRef]
- Baker, J.M.; Buchfellner, M.; Britt, W.; Sanchez, V.; Potter, J.L.; Ingram, L.A.; Shiau, H.; Sanchez, L.H.G.; Saaybi, S.; Kelly, D.; et al. Acute Hepatitis and Adenovirus Infection Among Children—Alabama, October 2021–February 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 638–640. [Google Scholar] [CrossRef]
- Hernroth, B.E.; Conden-Hansson, A.-C.; Rehnstam-Holm, A.-S.; Girones, R.; Allard, A.K. Environmental Factors Influencing Human Viral Pathogens and Their Potential Indicator Organisms in the Blue Mussel, Mytilus Edulis: The First Scandinavian Report. Appl. Environ. Microbiol. 2002, 68, 4523–4533. [Google Scholar] [CrossRef]
- Liu, J.; Gratz, J.; Amour, C.; Nshama, R.; Walongo, T.; Maro, A.; Mduma, E.; Platts-Mills, J.; Boisen, N.; Nataro, J.; et al. Optimization of Quantitative PCR Methods for Enteropathogen Detection. PLoS ONE 2016, 11, e0158199. [Google Scholar] [CrossRef]
- Do Nascimento, L.G.; Sarmento, S.K.; Röpke Junior, R.; Fumian, T.M. Evaluation of qPCR for the Selective Detection of Enteric Adenovirus Followed by Sequence-Based Genetic Characterization of F Strains Circulating in Brazil. Appl. Microbiol. 2024, 4, 1016–1029. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly Reactive and Highly Sensitive Assay for Norwalk-Like Viruses Based on Real-Time Quantitative Reverse Transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef]
- Zeng, S.-Q.; Halkosalo, A.; Salminen, M.; Szakal, E.D.; Puustinen, L.; Vesikari, T. One-Step Quantitative RT-PCR for the Detection of Rotavirus in Acute Gastroenteritis. J. Virol. Methods 2008, 153, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, H.; Johnson, G.; Brown, M.; Petric, M.; Tellier, R. Comprehensive Detection and Serotyping of Human Adenoviruses by PCR and Sequencing. J. Clin. Microbiol. 2004, 42, 3963–3969. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; McDonough, M.C.; Erdman, D.D. Species-Specific Identification of Human Adenoviruses by a Multiplex PCR Assay. J. Clin. Microbiol. 2000, 38, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shimizu, H.; Doan, L.T.P.; Tung, P.G.; Okitsu, S.; Nishio, O.; Suzuki, E.; Seo, J.K.; Kim, K.S.; Müller, W.E.G.; et al. Characterizations of Adenovirus Type 41 Isolates from Children with Acute Gastroenteritis in Japan, Vietnam, and Korea. J. Clin. Microbiol. 2004, 42, 4032–4039. [Google Scholar] [CrossRef]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K. aLeaves Facilitates On-Demand Exploration of Metazoan Gene Family Trees on MAFFT Sequence Alignment Server with Enhanced Interactivity. Nucleic Acids Res. 2013, 41, W22–W28. [Google Scholar] [CrossRef]
- Springer. Multiple Sequence Alignment: Methods and Protocols. In Methods in Molecular Biology; Katoh, K., Ed.; Springer: New York, NY, USA, 2021; Volume 2231, ISBN 978-1-07-161035-0. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating of the Human-Ape Splitting by a Molecular Clock of Mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Afrad, M.H.; Avzun, T.; Haque, J.; Haque, W.; Hossain, M.E.; Rahman, A.R.; Ahmed, S.; Faruque, A.S.G.; Rahman, M.Z.; Rahman, M. Detection of Enteric- and Non-enteric Adenoviruses in Gastroenteritis Patients, Bangladesh, 2012–2015. J. Med. Virol. 2018, 90, 677–684. [Google Scholar] [CrossRef]
- Kumthip, K.; Khamrin, P.; Ushijima, H.; Maneekarn, N. Enteric and Non-Enteric Adenoviruses Associated with Acute Gastroenteritis in Pediatric Patients in Thailand, 2011 to 2017. PLoS ONE 2019, 14, e0220263. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, J.; Li, N.; Zhang, R.; Jiang, L.; Zhou, X.; Xiang, Y.; Cun, J.; Qiao, E. Detection and Complete Genome Sequence Analysis of Human Adenovirus in Children with Acute Diarrhea in Yunnan, China, 2015–2021. Arch. Virol. 2024, 169, 34. [Google Scholar] [CrossRef] [PubMed]
- Khales, P.; Razizadeh, M.H.; Ghorbani, S.; Moattari, A.; Sarvari, J.; Saadati, H.; Sayyahfar, S.; Salavatiha, Z.; Hasanabad, M.H.; Poortahmasebi, V.; et al. Human Adenoviruses in Children with Gastroenteritis: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2024, 24, 478. [Google Scholar] [CrossRef]
- Primo, D.; Pacheco, G.T.; Timenetsky, M.D.C.S.T.; Luchs, A. Surveillance and Molecular Characterization of Human Adenovirus in Patients with Acute Gastroenteritis in the Era of Rotavirus Vaccine, Brazil, 2012–2017. J. Clin. Virol. 2018, 109, 35–40. [Google Scholar] [CrossRef]
- Black, R.E.; Perin, J.; Yeung, D.; Rajeev, T.; Miller, J.; Elwood, S.E.; Platts-Mills, J.A. Estimated Global and Regional Causes of Deaths from Diarrhoea in Children Younger than 5 Years during 2000–21: A Systematic Review and Bayesian Multinomial Analysis. Lancet Glob. Health 2024, 12, e919–e928. [Google Scholar] [CrossRef]
- Khan, A.I.; Amin, M.A. Understanding Deaths from Diarrhoea in Children Younger than 5 Years. Lancet Glob. Health 2024, 12, e891–e892. [Google Scholar] [CrossRef]
- Souza, E.V.D.; De Souza, Y.F.V.P.; Medeiros, R.S.; De Azevedo, L.S.; De Queiroz, T.G.A.; Sanz-Duro, R.L.; Marinho, R.D.S.S.; Komninakis, S.V.; Timenetsky, M.D.C.S.T.; Luchs, A. Diversity of Enteric and Non-Enteric Human Adenovirus Strains in Brazil, 2006–2011. Arch. Virol. 2021, 166, 897–903. [Google Scholar] [CrossRef]
- Pratte-Santos, R.; Miagostovich, M.P.; Fumian, T.M.; Maciel, E.L.; Martins, S.A.; Cassini, S.T.; Keller, R. High Prevalence of Enteric Viruses Associated with Acute Gastroenteritis in Pediatric Patients in a Low-income Area in Vitória, Southeastern Brazil. J. Med. Virol. 2019, 91, 744–750. [Google Scholar] [CrossRef]
- Olivares, A.I.O.; Leitão, G.A.A.; Pimenta, Y.C.; Cantelli, C.P.; Fumian, T.M.; Fialho, A.M.; Da Silva E Mouta, S.; Delgado, I.F.; Nordgren, J.; Svensson, L.; et al. Epidemiology of Enteric Virus Infections in Children Living in the Amazon Region. Int. J. Infect. Dis. 2021, 108, 494–502. [Google Scholar] [CrossRef]
- Olsen, S.J.; Winn, A.K.; Budd, A.P.; Prill, M.M.; Steel, J.; Midgley, C.M.; Kniss, K.; Burns, E.; Rowe, T.; Foust, A.; et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic—United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1013–1019. [Google Scholar] [CrossRef]
- Angoulvant, F.; Ouldali, N.; Yang, D.D.; Filser, M.; Gajdos, V.; Rybak, A.; Guedj, R.; Soussan-Banini, V.; Basmaci, R.; Lefevre-Utile, A.; et al. Coronavirus Disease 2019 Pandemic: Impact Caused by School Closure and National Lockdown on Pediatric Visits and Admissions for Viral and Nonviral Infections—A Time Series Analysis. Clin. Infect. Dis. 2021, 72, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, D.K.; Foley, D.A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Blyth, C.C.; Moore, H.C. Impact of Coronavirus Disease 2019 Public Health Measures on Detections of Influenza and Respiratory Syncytial Virus in Children During the 2020 Australian Winter. Clin. Infect. Dis. 2021, 72, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Kraay, A.N.M.; Han, P.; Kambhampati, A.K.; Wikswo, M.E.; Mirza, S.A.; Lopman, B.A. Impact of Nonpharmaceutical Interventions for Severe Acute Respiratory Syndrome Coronavirus 2 on Norovirus Outbreaks: An Analysis of Outbreaks Reported By 9 US States. J. Infect. Dis. 2021, 224, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.J.; Uri, H.; Bojkova, D.; Cinatl, J.; Wass, M.N.; Michaelis, M. Impact of the COVID-19 Pandemic on the Circulation of Other Pathogens in England. J. Med. Virol. 2023, 95, e28401. [Google Scholar] [CrossRef]
- Knudsen, P.K.; Lind, A.; Klundby, I.; Dudman, S. The Incidence of Infectious Diseases and Viruses Other than SARS-CoV-2 amongst Hospitalised Children in Oslo, Norway during the Covid-19 Pandemic 2020–2021. J. Clin. Virol. Plus 2022, 2, 100060. [Google Scholar] [CrossRef]
- Yorsaeng, R.; Suntronwong, N.; Thongpan, I.; Chuchaona, W.; Lestari, F.B.; Pasittungkul, S.; Puenpa, J.; Atsawawaranunt, K.; Sharma, C.; Sudhinaraset, N.; et al. The Impact of COVID-19 and Control Measures on Public Health in Thailand, 2020. PeerJ 2022, 10, e12960. [Google Scholar] [CrossRef]
- Maison, N.; Peck, A.; Illi, S.; Meyer-Buehn, M.; Von Mutius, E.; Hübner, J.; Von Both, U. The Rising of Old Foes: Impact of Lockdown Periods on “Non-SARS-CoV-2” Viral Respiratory and Gastrointestinal Infections. Infection 2022, 50, 519–524. [Google Scholar] [CrossRef]
- Grochowska, M.; Ambrożej, D.; Wachnik, A.; Demkow, U.; Podsiadły, E.; Feleszko, W. The Impact of the COVID-19 Pandemic Lockdown on Pediatric Infections—A Single-Center Retrospective Study. Microorganisms 2022, 10, 178. [Google Scholar] [CrossRef]
- La Rosa, G.; Della Libera, S.; Petricca, S.; Iaconelli, M.; Donia, D.; Saccucci, P.; Cenko, F.; Xhelilaj, G.; Divizia, M. Genetic Diversity of Human Adenovirus in Children with Acute Gastroenteritis, Albania, 2013–2015. BioMed Res. Int. 2015, 2015, 142912. [Google Scholar] [CrossRef]
- Qiu, F.; Shen, X.; Li, G.; Zhao, L.; Chen, C.; Duan, S.; Guo, J.; Zhao, M.; Yan, T.; Qi, J.-J.; et al. Adenovirus Associated with Acute Diarrhea: A Case-Control Study. BMC Infect. Dis. 2018, 18, 450. [Google Scholar] [CrossRef]
- Harb, A.; Abraham, S.; Rusdi, B.; Laird, T.; O’Dea, M.; Habib, I. Molecular Detection and Epidemiological Features of Selected Bacterial, Viral, and Parasitic Enteropathogens in Stool Specimens from Children with Acute Diarrhea in Thi-Qar Governorate, Iraq. Int. J. Environ. Res. Public Health 2019, 16, 1573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, Y. Effect of COVID-19 Protective Measures on the Epidemiology Characteristics of Rotavirus, Adenovirus, and Coinfections among Pediatric Patients with Acute Gastroenteritis in Hangzhou, China. Microbiol. Spectr. 2024, 12, e04007-23. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Islam, K.; Sayeed, M.A.; Platts-Mills, J.A.; Islam, M.T.; Khabir, M.I.U.; Rahman, M.; Khan, Z.H.; Begum, Y.A.; Khanam, F.; et al. Etiology of Diarrhea Requiring Hospitalization in Bangladesh by Quantitative Polymerase Chain Reaction, 2014–2018. Clin. Infect. Dis. 2021, 73, e2493–e2499. [Google Scholar] [CrossRef]
- Varghese, T.; Mills, J.A.P.; Revathi, R.; Antoni, S.; Soeters, H.M.; Emmanuel Njambe, T.O.; Houpt, E.R.; Tate, J.E.; Parashar, U.D.; Kang, G. Etiology of Diarrheal Hospitalizations Following Rotavirus Vaccine Implementation and Association of Enteric Pathogens with Malnutrition among Under-Five Children in India. Gut Pathog. 2024, 16, 22. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of Quantitative Molecular Diagnostic Methods to Identify Causes of Diarrhoea in Children: A Reanalysis of the GEMS Case-Control Study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Liu, J.; Rogawski, E.T.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of Quantitative Molecular Diagnostic Methods to Assess the Aetiology, Burden, and Clinical Characteristics of Diarrhoea in Children in Low-Resource Settings: A Reanalysis of the MAL-ED Cohort Study. Lancet Glob. Health 2018, 6, e1309–e1318. [Google Scholar] [CrossRef]
- Meier, J.L. Viral Acute Gastroenteritis in Special Populations. Gastroenterol. Clin. North Am. 2021, 50, 305–322. [Google Scholar] [CrossRef]
- Goldar, S.; Rajbongshi, G.; Chamuah, K.; Alam, S.T.; Sharma, A. Occurrence of Viral Gastroenteritis in Children below 5 Years: A Hospital-Based Study from Assam, India. Indian J. Med. Microbiol. 2019, 37, 415–417. [Google Scholar] [CrossRef]
- Borkakoty, B.; Bali, N.K.; Jakaria, A.; Hazarika, R.; Temsu, T.; Gohain, M.; Kaur, H. Norovirus Gastroenteritis in Children Under-Five Years Hospitalized for Diarrhea in Two Cities of Northeast India: A Retrospective Study. Indian J. Med. Microbiol. 2023, 45, 100397. [Google Scholar] [CrossRef]
- Fumian, T.M.; Malta, F.C.; Sarmento, S.K.; Fernandes, S.B.; Negri, C.M.; Belettini, S.A.D.A.; Machado, M.H.; Guimarães, M.A.A.M.; De Assis, R.M.S.; Baduy, G.A.; et al. Acute Gastroenteritis Outbreak Associated with Multiple and Rare Norovirus Genotypes after Storm Events in Santa Catarina, Brazil. J. Med. Virol. 2023, 95, e29205. [Google Scholar] [CrossRef]
- Hassan, F.; Kanwar, N.; Harrison, C.J.; Halasa, N.B.; Chappell, J.D.; Englund, J.A.; Klein, E.J.; Weinberg, G.A.; Szilagyi, P.G.; Moffatt, M.E.; et al. Viral Etiology of Acute Gastroenteritis in <2-Year-Old US Children in the Post–Rotavirus Vaccine Era. J. Pediatr. Infect. Dis. Soc. 2019, 8, 414–421. [Google Scholar] [CrossRef]
- Pabbaraju, K.; Tellier, R.; Pang, X.-L.; Xie, J.; Lee, B.E.; Chui, L.; Zhuo, R.; Vanderkooi, O.G.; Ali, S.; Tarr, P.I.; et al. A Clinical Epidemiology and Molecular Attribution Evaluation of Adenoviruses in Pediatric Acute Gastroenteritis: A Case-Control Study. J. Clin. Microbiol. 2020, 59, e02287-20. [Google Scholar] [CrossRef] [PubMed]
- Pavia, A.T. Viral Infections of the Lower Respiratory Tract: Old Viruses, New Viruses, and the Role of Diagnosis. Clin. Infect. Dis. 2011, 52, S284–S289. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Suzutani, T.; Aoki, K.; Kitaichi, N.; Ishida, S.; Ishiko, H.; Ohashi, T.; Okamoto, S.; Nakagawa, H.; Hinokuma, R.; et al. Epidemiological and Virological Features of Epidemic Keratoconjunctivitis Due to New Human Adenovirus Type 54 in Japan. Br. J. Ophthalmol. 2011, 95, 32–36. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, Z.; Zhang, J.; Pan, Y.; Chen, M.; Guo, Y.; Yu, N.; Chodosh, J.; Fu, N.; Che, X.; et al. Fatal Community-Acquired Pneumonia in Children Caused by Re-Emergent Human Adenovirus 7d Associated with Higher Severity of Illness and Fatality Rate. Sci. Rep. 2016, 6, 37216. [Google Scholar] [CrossRef]
- Gopalkrishna, V.; Ganorkar, N.N.; Patil, P.R. Identification and Molecular Characterization of Adenovirus Types (HAdV-8, HAdV-37, HAdV-4, HAdV-3) in an Epidemic of Keratoconjunctivitis Occurred in Pune, Maharashtra, Western India. J. Med. Virol. 2016, 88, 2100–2105. [Google Scholar] [CrossRef]
- Portal, T.M.; Reymão, T.K.A.; Quinderé Neto, G.A.; Fiuza, M.K.D.C.; Teixeira, D.M.; Lima, I.C.G.; Sousa Júnior, E.C.; Bandeira, R.D.S.; De Deus, D.R.; Justino, M.C.A.; et al. Detection and Genotyping of Enteric Viruses in Hospitalized Children with Acute Gastroenteritis in Belém, Brazil: Occurrence of Adenovirus Viremia by Species F, Types 40/41. J. Med. Virol. 2019, 91, 378–384. [Google Scholar] [CrossRef]
- Lun, J.H.; Crosbie, N.D.; White, P.A. Genetic Diversity and Quantification of Human Mastadenoviruses in Wastewater from Sydney and Melbourne, Australia. Sci. Total Environ. 2019, 675, 305–312. [Google Scholar] [CrossRef]
- Blanco, R.; Alcalá, A.C.; Fernández, R.; Ramírez, V.; Rosales, R.E.; Páez, M.G.; Alemán, H.; González, R.; Zerpa, J.; Maldonado, A.J.; et al. Molecular Characterization of Human Adenovirus Causing Infantile Acute Gastroenteritis in Venezuela before and after Rotavirus Vaccine Implementation. Diagn. Microbiol. Infect. Dis. 2023, 107, 116056. [Google Scholar] [CrossRef]
- Chandra, P.; Lo, M.; Mitra, S.; Banerjee, A.; Saha, P.; Okamoto, K.; Deb, A.K.; Ghosh, S.K.; Manna, A.; Dutta, S.; et al. Genetic Characterization and Phylogenetic Variations of Human adenovirus-F Strains Circulating in Eastern India during 2017–2020. J. Med. Virol. 2021, 93, 6180–6190. [Google Scholar] [CrossRef]
- Götting, J.; Cordes, A.K.; Steinbrück, L.; Heim, A. Molecular Phylogeny of Human Adenovirus Type 41 Lineages. Virus Evol. 2022, 8, veac098. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, J.C. Adenoviruses in the Immunocompromised Host. Clin. Microbiol. Rev. 1992, 5, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Lion, T. Adenovirus Infections in Immunocompetent and Immunocompromised Patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Kosulin, K.; Geiger, E.; Vécsei, A.; Huber, W.-D.; Rauch, M.; Brenner, E.; Wrba, F.; Hammer, K.; Innerhofer, A.; Pötschger, U.; et al. Persistence and Reactivation of Human Adenoviruses in the Gastrointestinal Tract. Clin. Microbiol. Infect. 2016, 22, 381.e1–381.e8. [Google Scholar] [CrossRef]

| States | No. Stool Samples—Positive/Total (%) | |||
|---|---|---|---|---|
| 2021 | 2022 | 2023 | Total | |
| Bahia | 8/55 (14.5) | 0/2 | 0/0 | 8/57 (14) |
| Paraíba | 0/0 | 1/2 | 10/28 (35.7) | 11/30 (36.7) |
| Pernambuco | 5/9 | 14/100 (14) | 18/94 (19.1) | 37/203 (18.2) |
| Sergipe | 0/1 | 2/2 | 3/6 | 5/9 |
| Espírito Santo | 3/22 (13.6) | 5/87 (5.7) | 0/5 | 8/114 (7) |
| Minas Gerais | 4/41(9.8) | 14/81 (17.3) | 66/230 (28.7) | 84/352 (23.9) |
| Rio de Janeiro | 0/18 | 6/39 (15.4) | 1/4 | 7/61 (11.5) |
| Rio Grande do Sul | 33/332 (10) | 44/241 (18.3) | 33/125 (26.4) | 110/698 (15.8) |
| Santa Catarina | 23/105 (21.9) | 21/120 (17.5) | 14/231 (6) | 58/456 (12.7) |
| Total | 76/583 (13) | 107/674 (15.9) | 145/723 (20.1) | 328/1980 (16.6) |
| Region | No. of Stool Samples—Positive/Total (%) | p-Value a (Fisher Test) | |||||
|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | Total | 2021 vs. 2022 | 2021 vs. 2023 | 2022 vs. 2023 | |
| Northeastern | 13/65 (20) | 17/106 (16) | 31/128 (24.2) | 61/299 (20.4) | 0.5387 | 0.5880 | 0.1443 |
| Southeastern | 7/81 (8.6) | 25/207 (12.1) | 67/239 (28) | 99/527 (18.8) | 0.5323 | 0.0002 | <0.0001 |
| Southern | 56/437 (12.8) | 65/361 (18) | 47/356 (13.2) | 168/1154 (14.6) | 0.0473 | 0.9156 | 0.0810 |
| Total | 76/583 (13) | 107/674 (15.9) | 145/723 (20.1) | 328/1980 (16.6) | 0.1728 | 0.0008 | 0.0437 |
| Age Groups a | No. of Fecal Samples—Positive/Tested (%) | Total | p-Value b | |||
|---|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | All HAdV | HAdV-F | ||
| 0–12 | 16/91 (17.6) | 22/101 (21.8) | 39/156 (25) | 77/348 (22.1) | 14/348 (4) | <0.0001 |
| 12–24 | 20/110 (18.2) | 29/132 (22) | 44/135 (32.6) | 93/377 (24.7) | 17/377 (4.5) | <0.0001 |
| 24–60 | 25/133 (18.8) | 41/157 (26.1) | 35/142 (24.7) | 101/432 (23.4) | 23/432 (5.3) | <0.0001 |
| >60 | 15/249 (6) | 15/284 (5.3) | 27/290 (9.3) | 57/823 (6.9) | 9/823 (1.1) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mello, M.d.S.; Malta, F.C.; Fialho, A.M.; Burlandy, F.M.; Fumian, T.M. Molecular Epidemiology of Human Adenovirus from Acute Gastroenteritis Cases in Brazil After the COVID-19 Pandemic Period, 2021–2023. Viruses 2025, 17, 577. https://doi.org/10.3390/v17040577
Mello MdS, Malta FC, Fialho AM, Burlandy FM, Fumian TM. Molecular Epidemiology of Human Adenovirus from Acute Gastroenteritis Cases in Brazil After the COVID-19 Pandemic Period, 2021–2023. Viruses. 2025; 17(4):577. https://doi.org/10.3390/v17040577
Chicago/Turabian StyleMello, Mateus de Souza, Fábio Correia Malta, Alexandre Madi Fialho, Fernanda Marcicano Burlandy, and Tulio Machado Fumian. 2025. "Molecular Epidemiology of Human Adenovirus from Acute Gastroenteritis Cases in Brazil After the COVID-19 Pandemic Period, 2021–2023" Viruses 17, no. 4: 577. https://doi.org/10.3390/v17040577
APA StyleMello, M. d. S., Malta, F. C., Fialho, A. M., Burlandy, F. M., & Fumian, T. M. (2025). Molecular Epidemiology of Human Adenovirus from Acute Gastroenteritis Cases in Brazil After the COVID-19 Pandemic Period, 2021–2023. Viruses, 17(4), 577. https://doi.org/10.3390/v17040577







