Evaluation of SARS-CoV-2 Seroprevalence and Variant Distribution During the Delta–Omicron Transmission Waves in Greater Accra, Ghana, 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
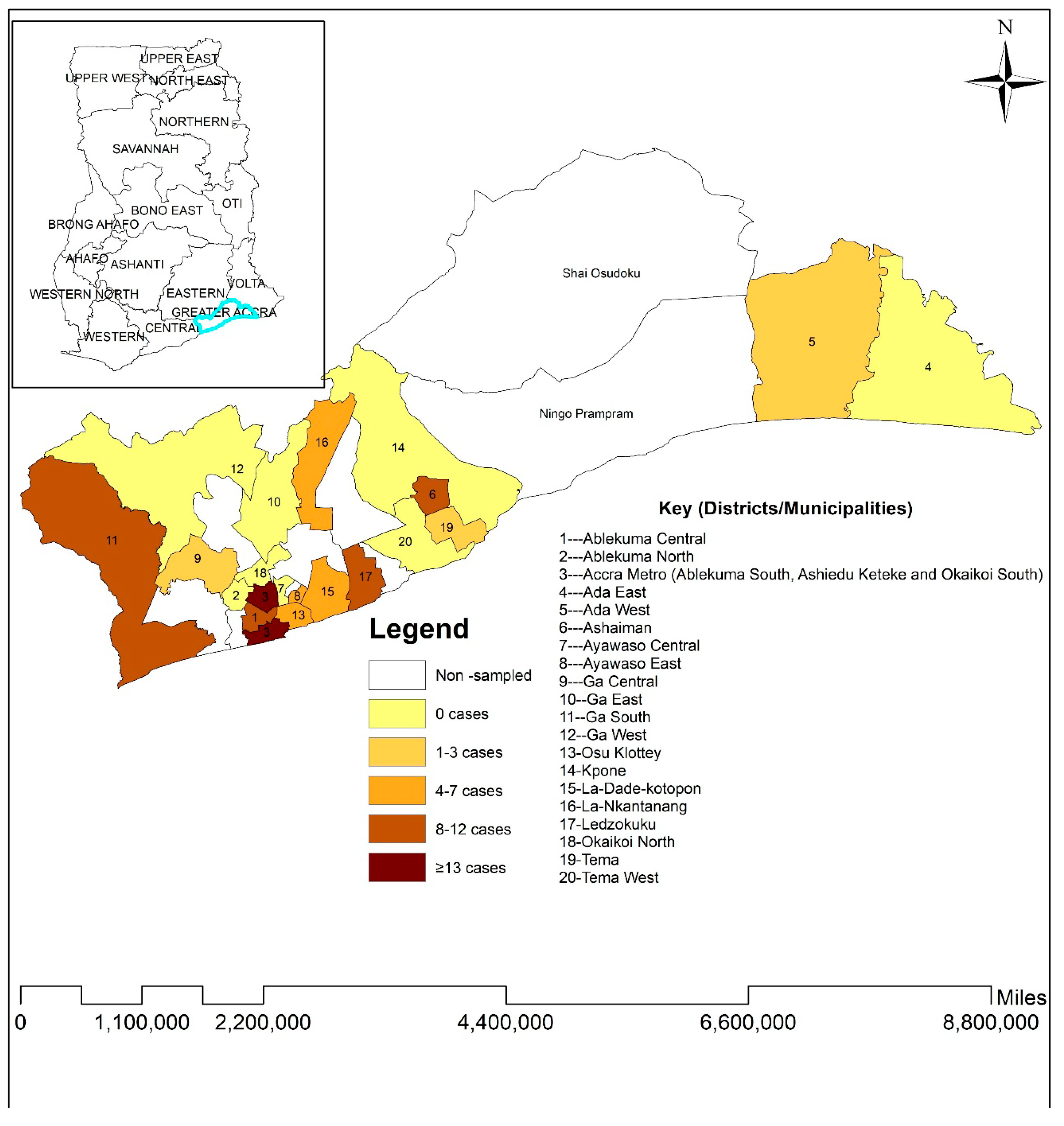
2.2. Study Setting, Eligibility, and Sampling
2.3. Sample Collection
2.4. Detection of (IgG/IgM) Antibodies Against SARS-CoV-2
2.5. Molecular Detection of SARS-CoV-2
2.6. Molecular Detection of Delta and Omicron Variants
2.7. Data Analysis
3. Results
3.1. Background Characteristics of the Study Participants
3.2. SARS-CoV-2 Seroprevalence, Infection, and Circulating Variants
3.3. Factors Associated with SARS-CoV-2 Seropositive Status in the Study Population
3.4. Factors Associated with SARS-CoV-2 Seropositive Status Among Non-Vaccinated Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 2 February 2025).
- COVID-19 Pandemic—Our World in Data. Available online: https://ourworldindata.org/coronavirus (accessed on 24 February 2025).
- Ioannidis, J.P.A. Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur. J. Clin. Investig. 2020, 50, e13423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Cabore, J.W.; Karamagi, H.C.; Kipruto, H.K.; Mungatu, J.K.; Asamani, J.A.; Droti, B.; Titi-Ofei, R.; Seydi, A.B.W.; Kidane, S.N.; Balde, T.; et al. COVID-19 in the 47 countries of the WHO African region: A modelling analysis of past trends and future patterns. Lancet Glob. Health 2022, 10, e1099–e1114. [Google Scholar] [CrossRef]
- McKay, T.; Robinson, R.S.; Musungu, S.; Padi-Adjirackor, N.A.; Angotti, N. The Missing Millions: Uncovering the Burden of COVID-19 Cases and Deaths in the African Region. Popul. Dev. Rev. 2024, 50, 7–58. [Google Scholar] [CrossRef]
- Odikro, M.A.; Kenu, E.; Malm, K.L.; Asiedu-Bekoe, F.; Noora, C.L.; Frimpong, J.; Calys-Tagoe, B.; Koram, K.A. Epidemiology of COVID-19 outbreak in Ghana, 2020. Ghana Med. J. 2020, 54 (Suppl. S5), 5–15. [Google Scholar] [CrossRef]
- Paleker, M.; A Tembo, Y.; Davies, M.-A.; Mahomed, H.; Pienaar, D.; Madhi, S.A.; McCarthy, K. Asymptomatic COVID-19 in South Africa—Implications for the control of transmission. Public Health Action 2021, 11, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Tapela, K.; Oyawoye, F.O.; Olwal, C.O.; Opurum, P.C.; Amponsah, J.A.; Segbedzi, K.A.L.; Tetteh, B.; Kumi-Ansah, F.; Mutungi, J.K.; Obodai, E.; et al. Probing SARS-CoV-2-positive plasma to identify potential factors correlating with mild COVID-19 in Ghana, West Africa. BMC Med. 2022, 20, 370. [Google Scholar] [CrossRef]
- Lewis, H.C.; Ware, H.; Whelan, M.; Subissi, L.; Li, Z.; Ma, X.; Nardone, A.; Valenciano, M.; Cheng, B.; Noel, K.; et al. SARS-CoV-2 infection in Africa: A systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021. BMJ Glob. Health 2022, 7, 8793. [Google Scholar] [CrossRef]
- Kosuge, M.; Furusawa-Nishii, E.; Ito, K.; Saito, Y.; Ogasawara, K. Point mutation bias in SARS-CoV-2 variants results in increased ability to stimulate inflammatory responses. Sci. Rep. 2020, 10, 17766. [Google Scholar] [CrossRef]
- Martínez-González, B.; Soria, M.E.; Vázquez-Sirvent, L.; Ferrer-Orta, C.; Lobo-Vega, R.; Mínguez, P.; de la Fuente, L.; Llorens, C.; Soriano, B.; Ramos, R.; et al. SARS-CoV-2 Point Mutation and Deletion Spectra and Their Association with Different Disease Outcomes. Microbiol. Spectr. 2022, 10, e00221–e00222. [Google Scholar] [CrossRef]
- Salyer, S.J.; Maeda, J.; Sembuche, S.; Kebede, Y.; Tshangela, A.; Moussif, M.; Ihekweazu, C.; Mayet, N.; Abate, E.; Ouma, A.O.; et al. The first and second waves of the COVID-19 pandemic in Africa: A cross-sectional study. Lancet 2021, 397, 1265–1275. [Google Scholar] [CrossRef]
- Daria, S.; Islam, M.R. The SARS-CoV-2 omicron wave is indicating the end of the pandemic phase but the COVID-19 will continue. J. Med. Virol. 2022, 94, 2343–2345. [Google Scholar] [CrossRef]
- Butt, A.A.; Dargham, S.R.; Chemaitelly, H.; Al Khal, A.; Tang, P.; Hasan, M.R.; Coyle, P.V.; Thomas, A.G.; Borham, A.M.; Concepcion, E.G.; et al. Severity of Illness in Persons Infected With the SARS-CoV-2 Delta Variant vs Beta Variant in Qatar. JAMA Intern. Med. 2022, 182, 197–205. [Google Scholar] [CrossRef]
- Kenu, E.; Frimpong, J.A.; Koram, K.A. Responding to the COVID-19 pandemic in Ghana. Ghana Med. J. 2020, 54, 72–73. [Google Scholar] [CrossRef]
- Quashie, P.K.; Mutungi, J.K.; Dzabeng, F.; Oduro-Mensah, D.; Opurum, P.C.; Tapela, K.; Udoakang, A.J.; COVID, W.; Asante, I.; Paemka, L.; et al. Trends of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody prevalence in selected regions across Ghana. Wellcome Open Res. 2021, 6, 173. [Google Scholar] [CrossRef]
- Morang’a, C.M.; Ngoi, J.M.; Gyamfi, J.; Amuzu, D.S.; Nuertey, B.D.; Soglo, P.M.; Appiah, V.; Asante, I.A.; Owusu-Oduro, P.; Armoo, S.; et al. Genetic diversity of SARS-CoV-2 infections in Ghana from 2020–2021. Nat. Commun. 2022, 13, 2494. [Google Scholar] [CrossRef]
- Ofori, S.K.; Schwind, J.S.; Sullivan, K.L.; Cowling, B.J.; Chowell, G.; Fung, I.C.H. Transmission Dynamics of COVID-19 in Ghana and the Impact of Public Health Interventions. Am. J. Trop. Med. Hyg. 2022, 107, 175–179. [Google Scholar] [CrossRef]
- Ghana Health Service (GHS). COVID-19 Updates Ghana. Available online: https://ghs.gov.gh/covid19/ (accessed on 25 February 2025).
- Population-Based Age-Stratified Seroepidemiological Investigation Protocol for Coronavirus 2019 (COVID-19) Infection. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Seroepidemiology-2020.2 (accessed on 25 February 2025).
- Németh, R. Respondent selection within the household-A modification of the Kish grid. In Proceedings of the Sixth Austrian, Hungarian, Italian, and Slovenian Meeting of Yung Statisticians, Ossiach, Austria, 5–7 October 2002. [Google Scholar]
- Coden, E.; Russo, F.; Arosio, A.D.; Castelnuovo, P.; Karligkiotis, A.; Volpi, L. Optimum Naso-oropharyngeal Swab Procedure for COVID-19: Step-by-Step Preparation and Technical Hints. Laryngoscope 2020, 130, 2564–2567. [Google Scholar] [CrossRef]
- Wantai SARS-CoV-2 Diagnostics WANTAI SARS-CoV-2 Ab ELISA for Total Antibody to SARS-CoV-2; Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.: Beijing, China, 2020.
- GeurtsvanKessel, C.H.; Okba, N.M.A.; Igloi, Z.; Bogers, S.; Embregts, C.W.E.; Laksono, B.M.; Leijten, L.; Rokx, C.; Rijnders, B.; Rahamat-Langendoen, J.; et al. An Evaluation of COVID-19 Serological Assays Informs Future Diagnostics and Exposure Assessment. Nat. Commun. 2020, 11, 3436. [Google Scholar] [CrossRef]
- Owusu Donkor, I.; Mensah, S.K.; Dwomoh, D.; Akorli, J.; Abuaku, B.; Ashong, Y.; Opoku, M.; Andoh, N.E.; Sumboh, J.G.; Ohene, S.A.; et al. Modeling SARS-CoV-2 antibody seroprevalence and its determinants in Ghana: A nationally representative cross-sectional survey. PLoS Glob. Public Health 2023, 3, e0001851. [Google Scholar] [CrossRef]
- Struck, N.S.; Lorenz, E.; Deschermeier, C.; Eibach, D.; Kettenbeil, J.; Loag, W.; Brieger, S.A.; Ginsbach, A.M.; Obirikorang, C.; Maiga-Ascofare, O.; et al. High Seroprevalence of SARS-CoV-2 in Burkina-Faso, Ghana and Madagascar in 2021: A Population-Based Study. BMC Public Health 2022, 22, 1676. [Google Scholar] [CrossRef]
- Busch, M.P.; Stramer, S.L.; Stone, M.; Yu, E.A.; Grebe, E.; Notari, E.; Saa, P.; Ferg, R.; Manrique, I.M.; Weil, N.; et al. Population-Weighted Seroprevalence from SARS-CoV-2 Infection, Vaccination, and Hybrid Immunity among U.S. Blood Donations from January-December 2021. Clin. Infect. Dis. 2022, 75, ciac470. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta Variant of SARS-CoV-2: A Comparative Computational Study of Spike Protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef]
- Rana, R.; Kant, R.; Huirem, R.S.; Bohra, D.; Ganguly, N.K. Omicron Variant: Current Insights and Future Directions. Microbiol. Res. 2022, 265, 127204. [Google Scholar] [CrossRef]
- Paton, R.S.; Overton, C.E.; Ward, T. The Rapid Replacement of the SARS-CoV-2 Delta Variant by Omicron (B.1.1.529) in England. Sci. Transl. Med. 2022, 14, 5395. [Google Scholar] [CrossRef]
- van Doremalen, N.; Schulz, J.E.; Adney, D.R.; Saturday, T.A.; Fischer, R.J.; Yinda, C.K.; Thakur, N.; Newman, J.; Ulaszewska, M.; Belij-Rammerstorfer, S.; et al. ChAdOx1 NCoV-19 (AZD1222) or NCoV-19-Beta (AZD2816) Protect Syrian Hamsters against Beta Delta and Omicron Variants. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Fischer, C.; Maponga, T.G.; Yadouleton, A.; Abílio, N.; Aboce, E.; Adewumi, P.; Afonso, P.; Akorli, J.; Andriamandimby, S.F.; Anga, L.; et al. Emergence and Spread of the SARS-CoV-2 Omicron (BA.1) Variant across Africa: An Observational Study. Lancet Glob. Health 2025, 13, e256–e267. [Google Scholar] [CrossRef]
| Characteristic | Frequency (N = 1027) | Percentage (%) |
|---|---|---|
| Area | ||
| Rural | 61 | 5.94% |
| Urban | 966 | 94.06% |
| Age group | ||
| <20 | 390 | 37.97% |
| 20–39 | 353 | 34.37% |
| 40–59 | 180 | 17.53% |
| 60+ | 104 | 10.13% |
| Sex | ||
| Female | 575 | 55.99% |
| Male | 452 | 44.01% |
| Educational level | ||
| Never attended school | 94 | 9.15% |
| Primary | 353 | 34.37% |
| Secondary+ | 580 | 56.48% |
| Employment status | ||
| Employed | 491 | 47.81% |
| Unemployed | 536 | 52.19% |
| Vaccination status | ||
| No | 731 | 71.18% |
| Yes | 296 | 28.82% |
| Pre-existing medical conditions | ||
| No | 1000 | 97.37% |
| Yes | 27 | 2.63% |
| Have you had contact with anyone with flu-like symptoms in the last 7 days? | ||
| No | 955 | 92.99% |
| Unknown | 24 | 2.34% |
| Yes | 48 | 4.67% |
| Adherence to COVID-19 protocols ɸ | ||
| High | 183 | 17.82% |
| Low | 393 | 38.27% |
| Moderate | 158 | 15.38% |
| No adherence | 293 | 28.53% |
| Characteristic | Frequency (N) | Percentage (%) | Vaccination Status | p-Value | |
|---|---|---|---|---|---|
| No, N = 731 | Yes, N = 296 | ||||
| SARS-CoV-2 infection | 0.079 | ||||
| Negative | 922 | 89.78% | 664 (72.0%) | 258 (28.0%) | |
| Positive | 105 | 10.22% | 67 (63.8%) | 38 (36.2%) | |
| SARS-CoV-2 serostatus | <0.001 | ||||
| Negative | 136 | 13.24% | 130 (95.6%) | 6 (4.4%) | |
| Positive | 891 | 86.76% | 601 (67.5%) | 290 (32.5%) | |
| Circulating variants | 0.042 | ||||
| Omicron | 45 | 42.86% | 29 (64.4%) | 16 (35.6%) | |
| Delta | 9 | 8.57% | 9 (100.0%) | 0 (0.0%) | |
| Not genotyped | 51 | 48.47% | 29 (56.9%) | 22 (43.1%) | |
| Characteristic | Serostatus | Univariate Regression | Multivariate Regression | |||
|---|---|---|---|---|---|---|
| Negative, N = 136 | Positive, N = 891 | cOR (95% CI) 1 | p-Value | aOR (95% CI) 2 | p-Value | |
| Area | ||||||
| Rural | 14 (23.0%) | 47 (77.0%) | — | — | ||
| Urban | 122 (12.6%) | 844 (87.4%) | 2.06 (1.07–3.76) | 0.024 | 1.88 (0.93–3.60) | 0.065 |
| Age group | ||||||
| <20 | 76 (19.5%) | 314 (80.5%) | — | — | ||
| 20–39 | 39 (11.0%) | 314 (89.0%) | 1.95 (1.29–2.98) | 0.002 | 0.75 (0.40–1.44) | 0.4 |
| 40–59 | 17 (9.4%) | 163 (90.6%) | 2.32 (1.36–4.18) | 0.003 | 0.68 (0.30–1.59) | 0.4 |
| 60+ | 4 (3.8%) | 100 (96.2%) | 6.05 (2.44–20.2) | <0.001 | 2.26 (0.81–8.14) | 0.2 |
| Sex | ||||||
| Female | 75 (13.0%) | 500 (87.0%) | — | — | ||
| Male | 61 (13.5%) | 391 (86.5%) | 0.96 (0.67–1.39) | 0.8 | 0.98 (0.67–1.43) | 0.9 |
| Educational level | ||||||
| Never attended school | 19 (20.2%) | 75 (79.8%) | — | — | ||
| Primary | 58 (16.4%) | 295 (83.6%) | 1.29 (0.71–2.26) | 0.4 | 1.68 (0.89–3.11) | 0.10 |
| Secondary+ | 59 (10.2%) | 521 (89.8%) | 2.24 (1.24–3.90) | 0.006 | 2.10 (1.12–3.83) | 0.018 |
| Employment status | ||||||
| Employed | 44 (9.0%) | 447 (91.0%) | — | — | ||
| Unemployed | 92 (17.2%) | 444 (82.8%) | 0.48 (0.32–0.69) | <0.001 | 0.52 (0.28–0.98) | 0.040 |
| Vaccination status | ||||||
| No | 130 (17.8%) | 601 (82.2%) | — | — | ||
| Yes | 6 (2.0%) | 290 (98.0%) | 10.5 (4.97–26.9) | <0.001 | 8.53 (3.87–22.6) | <0.001 |
| Pre-existing medical conditions | ||||||
| No | 135 (13.5%) | 865 (86.5%) | — | — | ||
| Yes | 1 (3.7%) | 26 (96.3%) | 4.06 (0.85–72.7) | 0.2 | 1.90 (0.34–35.7) | 0.5 |
| Have you had contact with anyone with flu-like symptoms in the last 7 days? | ||||||
| No | 130 (13.6%) | 825 (86.4%) | — | — | ||
| Unknown | 2 (8.3%) | 22 (91.7%) | 1.73 (0.50–10.9) | 0.5 | 1.97 (0.53–12.8) | 0.4 |
| Yes | 4 (8.3%) | 44 (91.7%) | 1.73 (0.69–5.83) | 0.3 | 1.63 (0.61–5.70) | 0.4 |
| Adherence to COVID-19 protocols | ||||||
| High | 24 (13.1%) | 159 (86.9%) | — | — | ||
| Low | 56 (14.2%) | 337 (85.8%) | 0.91 (0.54–1.50) | 0.7 | 1.04 (0.58–1.81) | 0.9 |
| Moderate | 19 (12.0%) | 139 (88.0%) | 1.10 (0.58–2.12) | 0.8 | 1.11 (0.55–2.24) | 0.8 |
| No adherence | 37 (12.6%) | 256 (87.4%) | 1.04 (0.60–1.80) | 0.9 | 1.64 (0.89–2.98) | 0.11 |
| Characteristic | Serostatus | Univariate Regression | Multivariate Regression | |||
|---|---|---|---|---|---|---|
| Negative, N = 130 | Positive, N = 601 | cOR 1 (95% CI) | p-Value | aOR 2 (95% CI) | p-Value | |
| Area | ||||||
| Rural | 14 (23.3%) | 46 (76.7%) | — | — | ||
| Urban | 116 (17.3%) | 555 (82.7%) | 1.46 (0.75–2.67) | 0.2 | 1.90 (0.94–3.66) | 0.063 |
| Age group | ||||||
| <20 | 76 (20.4%) | 296 (79.6%) | — | — | ||
| 20–39 | 34 (15.0%) | 193 (85.0%) | 1.46 (0.94–2.29) | 0.10 | 0.69 (0.36–1.35) | 0.3 |
| 40–59 | 16 (17.8%) | 74 (82.2%) | 1.19 (0.67–2.22) | 0.6 | 0.49 (0.21–1.22) | 0.12 |
| 60+ | 4 (9.5%) | 38 (90.5%) | 2.44 (0.94–8.32) | 0.10 | 1.75 (0.61–6.40) | 0.3 |
| Sex | ||||||
| Female | 69 (17.2%) | 333 (82.8%) | — | — | ||
| Male | 61 (18.5%) | 268 (81.5%) | 0.91 (0.62–1.33) | 0.6 | 0.90 (0.61–1.34) | 0.6 |
| Educational level | ||||||
| Never attended school | 19 (27.1%) | 51 (72.9%) | — | — | ||
| Primary | 57 (18.8%) | 247 (81.3%) | 1.61 (0.87–2.91) | 0.12 | 1.78 (0.93–3.32) | 0.075 |
| Secondary+ | 54 (15.1%) | 303 (84.9%) | 2.09 (1.13–3.77) | 0.016 | 2.23 (1.17–4.17) | 0.013 |
| Employment status | ||||||
| Employed | 38 (13.1%) | 251 (86.9%) | — | — | ||
| Unemployed | 92 (20.8%) | 350 (79.2%) | 0.58 (0.38–0.86) | 0.009 | 0.41 (0.21–0.80) | 0.009 |
| Pre-existing medical conditions | ||||||
| No | 129 (18.0%) | 587 (82.0%) | — | — | ||
| Yes | 1 (6.7%) | 14 (93.3%) | 3.08 (0.61–56.0) | 0.3 | 1.81 (0.31–34.4) | 0.6 |
| Have you had contact with anyone with flu-like symptoms in the last 7 days? | ||||||
| No | 124 (18.1%) | 561 (81.9%) | — | — | ||
| Unknown | 2 (10.0%) | 18 (90.0%) | 1.99 (0.56–12.6) | 0.4 | 1.92 (0.51–12.6) | 0.4 |
| Yes | 4 (15.4%) | 22 (84.6%) | 1.22 (0.46–4.21) | 0.7 | 1.52 (0.55–5.41) | 0.5 |
| Adherence to COVID-19 protocols | ||||||
| High | 23 (19.3%) | 96 (80.7%) | — | — | ||
| Low | 54 (20.7%) | 207 (79.3%) | 0.92 (0.53–1.57) | 0.8 | 1.02 (0.56–1.81) | >0.9 |
| Moderate | 17 (16.2%) | 88 (83.8%) | 1.24 (0.62–2.50) | 0.5 | 1.20 (0.58–2.52) | 0.6 |
| No adherence | 36 (14.6%) | 210 (85.4%) | 1.40 (0.78–2.47) | 0.3 | 1.69 (0.90–3.12) | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomotey, E.S.; Akorli, J.; Opoku, M.; Odumang, D.A.; Nketia, K.; Gyekye, E.F.; Sedzro, K.M.; Andoh, N.E.; Ashong, Y.; Abuaku, B.; et al. Evaluation of SARS-CoV-2 Seroprevalence and Variant Distribution During the Delta–Omicron Transmission Waves in Greater Accra, Ghana, 2021. Viruses 2025, 17, 487. https://doi.org/10.3390/v17040487
Lomotey ES, Akorli J, Opoku M, Odumang DA, Nketia K, Gyekye EF, Sedzro KM, Andoh NE, Ashong Y, Abuaku B, et al. Evaluation of SARS-CoV-2 Seroprevalence and Variant Distribution During the Delta–Omicron Transmission Waves in Greater Accra, Ghana, 2021. Viruses. 2025; 17(4):487. https://doi.org/10.3390/v17040487
Chicago/Turabian StyleLomotey, Elvis Suatey, Jewelna Akorli, Millicent Opoku, Daniel Adjei Odumang, Kojo Nketia, Emmanuel Frimpong Gyekye, Kojo Mensah Sedzro, Nana Efua Andoh, Yvonne Ashong, Benjamin Abuaku, and et al. 2025. "Evaluation of SARS-CoV-2 Seroprevalence and Variant Distribution During the Delta–Omicron Transmission Waves in Greater Accra, Ghana, 2021" Viruses 17, no. 4: 487. https://doi.org/10.3390/v17040487
APA StyleLomotey, E. S., Akorli, J., Opoku, M., Odumang, D. A., Nketia, K., Gyekye, E. F., Sedzro, K. M., Andoh, N. E., Ashong, Y., Abuaku, B., Koram, K. A., & Owusu Donkor, I. (2025). Evaluation of SARS-CoV-2 Seroprevalence and Variant Distribution During the Delta–Omicron Transmission Waves in Greater Accra, Ghana, 2021. Viruses, 17(4), 487. https://doi.org/10.3390/v17040487







