Assessment of Relative Contributions of Lifestyle, Behavioral and Biological Risk Factors for Cervical Human Papillomavirus Infections in Female Sex Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population, and Study Site
2.2. Study Procedures
2.2.1. Sampling and Enrolment of Study Participants
2.2.2. Interview, Clinical Examination, and Sample Collection
2.2.3. HPV Genotyping
2.3. Data Management and Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CC | Cervical cancer |
| FSWs | Linear dichroism |
| HIV | Human Immunodeficiency Virus |
| HPV | Human papillomavirus |
| hrHPV | High-risk human papillomavirus |
| LGAs | Local Government Areas |
| QGC | Quantile-based G-Computation |
| SHINI | Sexual behavior and HPV infection in Nigerians in Ibadan |
| STIs | Sexually Transmitted Infections |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.who.int/today (accessed on 6 August 2024).
- Lawson, O.; Ameyan, L.; Tukur, Z.; Dunu, S.; Kerry, M.; Okuyemi, O.O.; Yusuf, Z.; Fasawe, O.; Wiwa, O.; Hebert, K.S.; et al. Cervical cancer screening outcomes in public health facilities in three states in Nigeria. BMC Public Health 2023, 23, 1688. [Google Scholar] [CrossRef]
- Adeyinka, A.; Popoola, B.; Aderibigbe, A.; Olofin, O.; Adeleke, K.; Oni, S.; Aihonsu, J. Predictors of cervical precancerous lesions among women attending an urban Primary Health Centre in Lagos State, Nigeria. J. Community Med. Prim. Health Care 2024, 36, 22–34. [Google Scholar]
- zur Hausen, H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989, 49, 4677–4681. [Google Scholar] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar]
- Tjalma, W.A.; Fiander, A.; Reich, O.; Powell, N.; Nowakowski, A.M.; Kirschner, B.; Koiss, R.; O’Leary, J.; Joura, E.A.; Rosenlund, M.; et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int. J. Cancer 2013, 132, 854–867. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Day, A.T.; Sturgis, E.M. (Eds.) Prevention and screening of HPV malignancies. In Seminars in Radiation Oncology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Bowden, S.J.; Doulgeraki, T.; Bouras, E.; Markozannes, G.; Athanasiou, A.; Grout-Smith, H.; Kechagias, K.S.; Ellis, L.B.; Zuber, V.; Chadeau-Hyam, M. Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: An umbrella review and follow-up Mendelian randomisation studies. BMC Med. 2023, 21, 274. [Google Scholar]
- Delam, H.; Izanloo, S.; Bazrafshan, M.-R.; Eidi, A. Risk factors for cervical cancer: An epidemiological review. J. Health Sci. Surveill. Syst. 2020, 8, 105–109. [Google Scholar]
- Tchouaket, M.C.T.; Ka’e, A.C.; Semengue, E.N.J.; Sosso, S.M.; Simo, R.K.; Yagai, B.; Nka, A.D.; Chenwi, C.A.; Abba, A.; Fainguem, N. Variability of High-Risk Human Papillomavirus and Associated Factors among Women in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Pathogens 2023, 12, 1032. [Google Scholar] [CrossRef]
- Olusanya, O.; Wigfall, L.; Rossheim, M.; Tomar, A.; Barry, A. Binge drinking, HIV/HPV co-infection risk, and HIV testing: Factors associated with HPV vaccination among young adults in the United States. Prev. Med. 2020, 134, 106023. [Google Scholar]
- Morhason-Bello, I.O.; Baisley, K.; Pavon, M.A.; Adewole, I.F.; Bakare, R.A.; de Sanjosé, S.; Francis, S.C.; Watson-Jones, D. Oral, genital and anal human papillomavirus infections among female sex workers in Ibadan, Nigeria. PLoS ONE 2022, 17, e0265269. [Google Scholar]
- Farahmand, M.; Moghoofei, M.; Dorost, A.; Abbasi, S.; Monavari, S.H.; Kiani, S.J.; Tavakoli, A. Prevalence and genotype distribution of genital human papillomavirus infection in female sex workers in the world: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1455. [Google Scholar]
- Tounkara, F.K.; Téguété, I.; Guédou, F.A.; Talbot, D.; Traoré, C.B.; Béhanzin, L.; Traoré, S.; Goma-Matsétsé, E.; Aza-Gnandji, M.; Keita, B. Type-specific incidence, persistence and factors associated with human papillomavirus infection among female sex workers in Benin and Mali, West Africa. Int. J. Infect. Dis. 2021, 106, 348–357. [Google Scholar]
- Morhason-Bello, I.O.; Baisley, K.; Pavon, M.A.; Adewole, I.F.; Bakare, R.; de Sanjosé, S.; Francis, S.C.; Watson-Jones, D. Prevalence and genotype specific concordance of oro-genital and anal human papillomavirus infections among sexually active Nigerian women. Infect. Agents Cancer Res. 2021, 16, 59. [Google Scholar]
- Bakare, R.; Olayinka, A.; Gadzama, G.; Adinma, A.; Ofondu, E.; Abdullahi, A.; Obunge, O.; Adeniran, A.; Oduyebo, O. National Guidelines on the Syndromic Management of Sexually Transmitted Infections (STIs) and Other Reproductive Tract Infections; Federal Ministry of Health: Abuja, Nigeria, 2016. Available online: https://nascp.gov.ng/resources/get_resource_doc/4 (accessed on 6 August 2024).
- Pal, M.; Ali, M.M.; Woo, J. Exponentiated weibull distribution. Statistica 2006, 66, 139–147. [Google Scholar]
- Tewari, P.; Banka, P.; Kernan, N.; Reynolds, S.; White, C.; Pilkington, L.; O’Toole, S.; Sharp, L.; D’Arcy, T.; Murphy, C. Prevalence and concordance of oral HPV infections with cervical HPV infections in women referred to colposcopy with abnormal cytology. J. Oral Pathol. Med. 2021, 50, 692–699. [Google Scholar]
- Kedarisetty, S.; Orosco, R.K.; Hecht, A.S.; Chang, D.C.; Weissbrod, P.A.; Coffey, C.S. Concordant oral and vaginal human papillomavirus infection in the United States. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 457–465. [Google Scholar]
- Guler, T.; Uygur, D.; Uncu, M.; Yayci, E.; Atacag, T.; Bas, K.; Gunay, M.; Yakicier, C. Coexisting anal human papilloma virus infection in heterosexual women with cervical HPV infection. Arch. Gynecol. Obstet. 2013, 288, 667–672. [Google Scholar]
- Marra, E.; Kroone, N.; Freriks, E.; van Dam, C.; Alberts, C.J.; Hogewoning, A.; Bruisten, S.; van Dijk, A.; Kroone, M.M.; Waterboer, T. Vaginal and anal human papillomavirus infection and seropositivity among female sex workers in Amsterdam, the Netherlands: Prevalence, concordance and risk factors. J. Infect. 2018, 76, 393–405. [Google Scholar]
- Hernandez, B.Y.; McDuffie, K.; Zhu, X.; Wilkens, L.R.; Killeen, J.; Kessel, B.; Wakabayashi, M.T.; Bertram, C.C.; Easa, D.; Ning, L. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2550–2556. [Google Scholar]
- Steinau, M.; Hariri, S.; Gillison, M.L.; Broutian, T.R.; Dunne, E.F.; Tong, Z.-Y.; Markowitz, L.E.; Unger, E.R. Prevalence of cervical and oral human papillomavirus infections among US women. J. Infect. Dis. 2014, 209, 1739–1743. [Google Scholar] [PubMed]
- Sehnal, B.; Zikan, M.; Nipcova, M.; Dusek, L.; Cibula, D.; Slama, J. The association among cervical, anal, and oral HPV infections in high-risk and low-risk women. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100061. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.T.; Boemi, S.; Caruso, G.; Sgalambro, F.; Ferlito, S.; Cavallaro, A.; Sudano, M.C.; Palumbo, M. Oral HPV infection in women with HPV-positive cervix is Closely related to oral sex. Diagnostics 2023, 13, 2096. [Google Scholar] [CrossRef] [PubMed]
- Baisley, K.J.; Andreasen, A.; Irani, J.; Nnko, S.; Changalucha, J.; Crucitti, T.; Francis, S.; Hansen, C.H.; Hayes, R.J.; Buvé, A. HPV prevalence around the time of sexual debut in adolescent girls in Tanzania. Sex. Transm. Infect. 2020, 96, 211–219. [Google Scholar]
- Chelimo, C.; Wouldes, T.A.; Cameron, L.D.; Elwood, J.M. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J. Infect. 2013, 66, 207–217. [Google Scholar]
- Meyer, M.F.; Huebbers, C.U.; Siefer, O.G.; Vent, J.; Engbert, I.; Eslick, G.D.; Valter, M.; Klussmann, J.P.; Preuss, S.F. Prevalence and risk factors for oral human papillomavirus infection in 129 women screened for cervical HPV infection. Oral Oncol. 2014, 50, 27–31. [Google Scholar]

| Variables | Mean (SD) |
|---|---|
| Age | 30.9 (6.4) |
| Ethnic groups, n (%) | |
| Igbo | 57 (31.3) |
| Yoruba | 14 (7.7) |
| Other Nigerian | 111 (61.0) |
| Body Mass Index | 27.5 (5.9) |
| Weekly income from sex work in Naira (USD 1—NGN 357) | 10,560.4 (7295.4) |
| Duration of involving commercial sex activity (yr) | 2.4 (2.9) |
| Years of completed formal education | 10.2 (3.0) |
| Number of vaginal sex partners reported throughout life | 119.3 (165.3) |
| Age at first vaginal sex | 17.5 (2.7) |
| Age of first vaginal sex partner | 23.5 (5.5) |
| Number of self-estimated weekly transactional sex | 26.6 (14.2) |
| Alcohol consumption (frequency of consumption of six or more units of alcohol), N (%) | |
| Less than 1–2 times per month | 35 (19.2) |
| 2–4 times per month | 44 (24.2) |
| 2–3 times per week | 66 (36.3) |
| 4 or more times per week | 37 (20.3) |
| Number of sites with HPV (other than cervix) | 1.8 (0.8) |
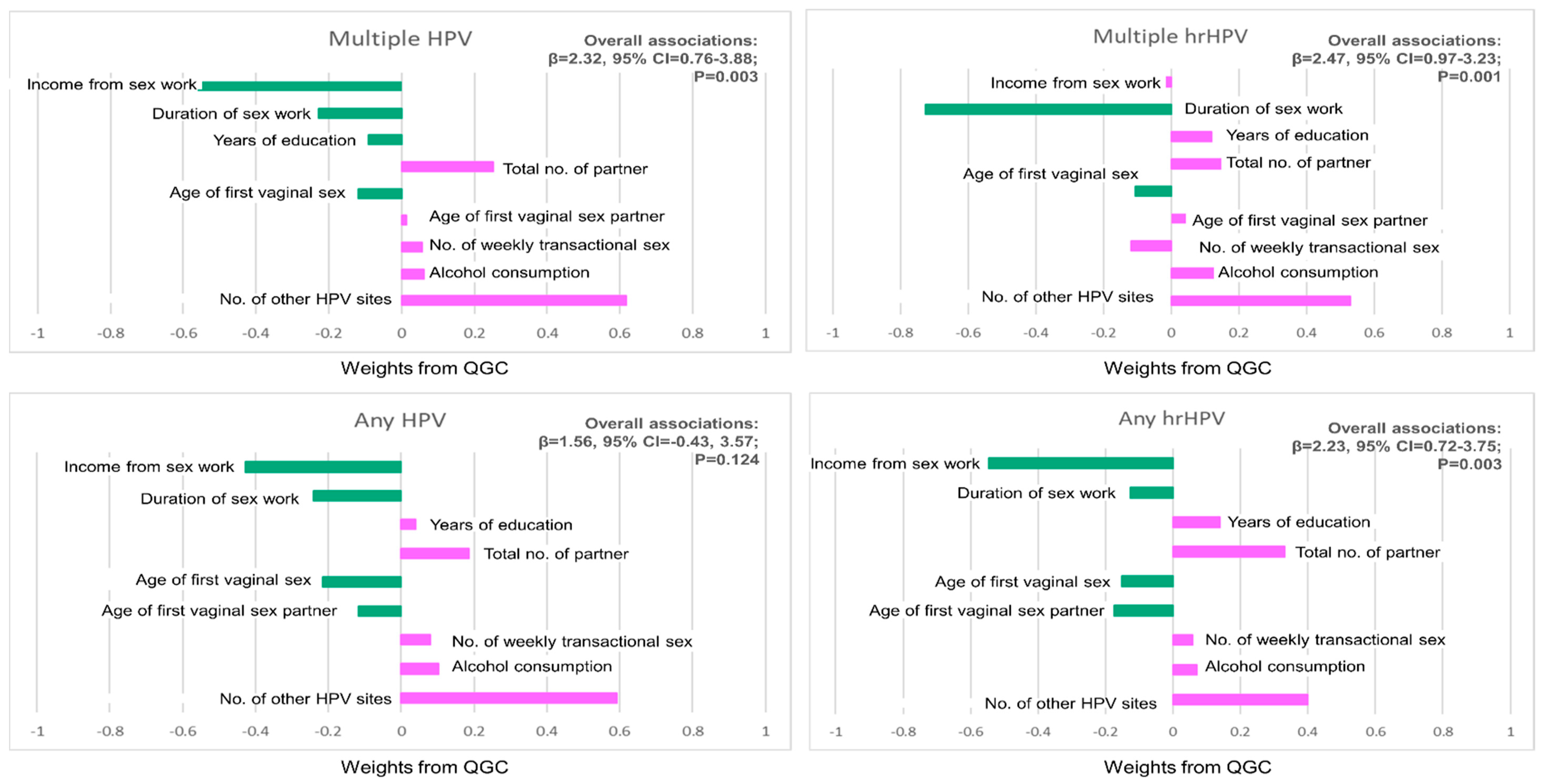
| Risk Factors | Any HPV | Any hrHPV |
|---|---|---|
| Income from sex work in naira | −0.427 | −0.547 |
| Duration of sex work | −0.240 | −0.126 |
| Total years of education | 0.039 | 0.138 |
| Total number of partners | 0.185 | 0.331 |
| Age at first vaginal sex | −0.215 | −0.151 |
| Age of first vaginal sex partner | −0.118 | −0.175 |
| No. of other HPV sites | 0.592 | 0.399 |
| No. of weekly men who pay for sex | 0.080 | 0.058 |
| Alcohol consumption (quantile) | 0.102 | 0.071 |
| β (95% CI) for combined factors | 1.56 (−0.43, 3.57), p = 0.124 | 2.23 (0.72, 3.75), p = 0.003 |
| Risk Factors | Multiple HPV | Multiple hrHPV |
|---|---|---|
| Income from sex work in naira | −0.561 | −0.049 |
| Duration of sex work | −0.227 | −0.725 |
| Total years of education | −0.091 | 0.119 |
| Total number of partners | 0.252 | 0.174 |
| Age at first vaginal sex | −0.120 | −0.107 |
| Age of first vaginal sex partner | 0.012 | 0.044 |
| No. of other HPV sites | 0.616 | 0.529 |
| No. of weekly men who pay for sex | 0.057 | −0.118 |
| Alcohol consumption (quantile) | 0.061 | 0.133 |
| β (95% CI) for combined factors | 2.32 (0.76, 3.88), p = 0.003 | 2.47 (0.97, 3.23), p = 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morhason-Bello, I.; Kim, K.; Bello, Y.; Zheng, Y.; Oyerinde, S.; Idowu, O.C.; Pavón, M.Á.; Baisley, K.; Wang, J.; Fowotade, A.; et al. Assessment of Relative Contributions of Lifestyle, Behavioral and Biological Risk Factors for Cervical Human Papillomavirus Infections in Female Sex Workers. Viruses 2025, 17, 485. https://doi.org/10.3390/v17040485
Morhason-Bello I, Kim K, Bello Y, Zheng Y, Oyerinde S, Idowu OC, Pavón MÁ, Baisley K, Wang J, Fowotade A, et al. Assessment of Relative Contributions of Lifestyle, Behavioral and Biological Risk Factors for Cervical Human Papillomavirus Infections in Female Sex Workers. Viruses. 2025; 17(4):485. https://doi.org/10.3390/v17040485
Chicago/Turabian StyleMorhason-Bello, Imran, Kyeezu Kim, Yusuf Bello, Yinan Zheng, Sunday Oyerinde, Oluwasegun Caleb Idowu, Miquel Ángel Pavón, Kathy Baisley, Jun Wang, Adeola Fowotade, and et al. 2025. "Assessment of Relative Contributions of Lifestyle, Behavioral and Biological Risk Factors for Cervical Human Papillomavirus Infections in Female Sex Workers" Viruses 17, no. 4: 485. https://doi.org/10.3390/v17040485
APA StyleMorhason-Bello, I., Kim, K., Bello, Y., Zheng, Y., Oyerinde, S., Idowu, O. C., Pavón, M. Á., Baisley, K., Wang, J., Fowotade, A., Maiga, M., Jonah, M., Christian, E. N., Ogunbiyi, O., Adewole, I., Hou, L., Francis, S. C., & Watson-Jones, D. (2025). Assessment of Relative Contributions of Lifestyle, Behavioral and Biological Risk Factors for Cervical Human Papillomavirus Infections in Female Sex Workers. Viruses, 17(4), 485. https://doi.org/10.3390/v17040485







