Clinical Predictors and Determinants of Mpox Complications in Hospitalized Patients: A Prospective Cohort Study from Burundi
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Settings
2.2. Participant Selection and Case Definitions
2.3. Data Collection
2.4. Statistical Methods
2.5. Ethics Approval
3. Results
3.1. Participant Characteristics
3.2. Transmission Patterns
3.3. Clinical Manifestations
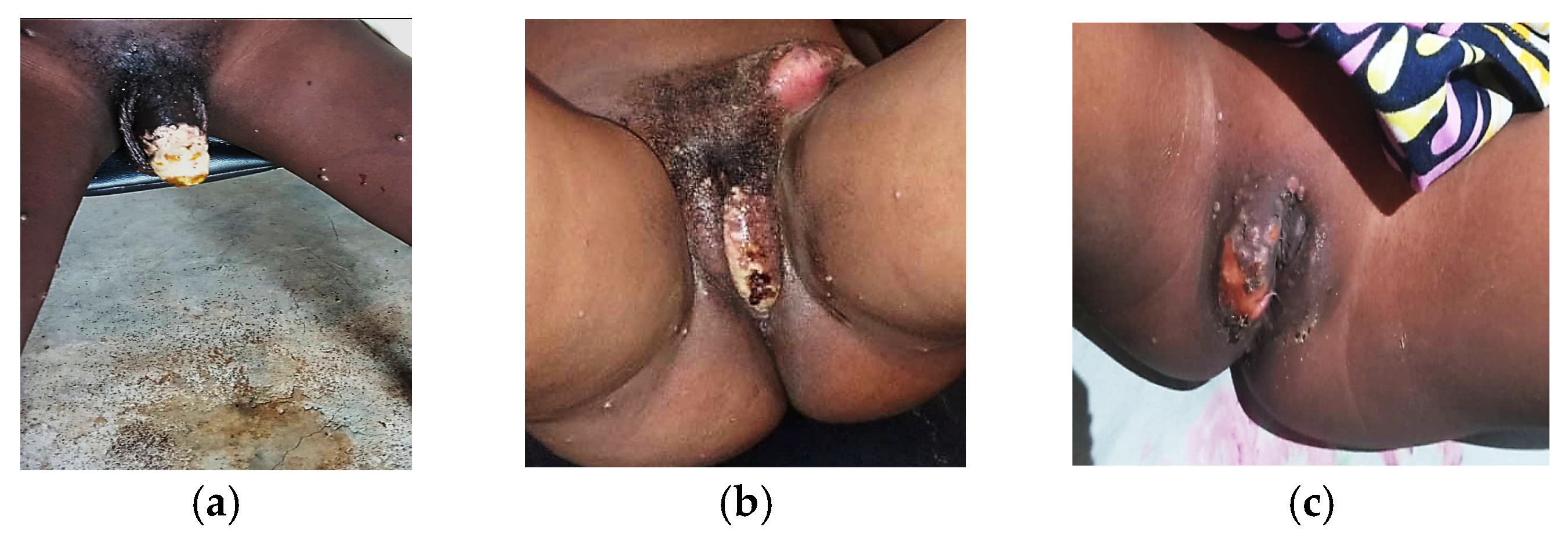
3.4. Clinical Complications and Comorbidities
3.5. Risk Factors for Mpox Complications
3.6. Disease Severity
4. Discussion
4.1. Study Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immunodeficiency Syndrome |
| CD4 | Cluster of Differentiation 4 |
| CI | Confidence Interval |
| DNA | Deoxyribonucleic Acid |
| DRC | Democratic Republic of the Congo |
| E E-value | (statistical measure) |
| HIV | Human Immunodeficiency Virus |
| IQR | Interquartile Range |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| MPXV | Orthopoxvirus monkeypox |
| OR | Odds Ratio |
| p | Probability Value |
| PCR | Polymerase Chain Reaction |
| RNA | Ribonucleic Acid |
| SD | Standard Deviation |
| STI | Sexually Transmitted Infection |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| U.S. | United States |
| UCL | University College London |
| USAID | United States Agency for International Development |
| WHO | World Health Organization |
References
- World Health Organization. Mpox. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 1 March 2025).
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Izibewule, J.H.; Ogunleye, A.; Ederiane, E.; Anebonam, U.; Neni, A.; Oyeyemi, A.; Etebu, E.N.; Ihekweazu, C. The 2017 human monkeypox outbreak in Nigeria: Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE 2019, 14, e0214229. [Google Scholar] [CrossRef]
- Africa Centers for Disease Control and Prevention. Africa CDC Declares Mpox a Public Health Emergency of Continental Security, Mobilizing Resources Across the Continent [Internet]. 2024. Available online: https://africacdc.org/news-item/africa-cdc-declares-mpox-a-public-health-emergency-of-continental-security-mobilizing-resources-across-the-continent/ (accessed on 26 November 2024).
- Vakaniaki, E.H.; Kacita, C.; Kinganda-Lusamaki, E.; O’toole, Á.; Wawina-Bokalanga, T.; Mukadi-Bamuleka, D.; Amuri-Aziza, A.; Malyamungu-Bubala, N.; Mweshi-Kumbana, F.; Mutimbwa-Mambo, L.; et al. Sustained human outbreak of a new MPXV clade I lineage in eastern Democratic Republic of the Congo. Nat. Med. 2024, 30, 2791–2795. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-Odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical course and outcome of human monkeypox in Nigeria. Clin. Infect. Dis. 2020, 71, e210–e214. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Zucker, J.; McLean, J.; Huang, S.; DeLaurentis, C.; Gunaratne, S.; Stoeckle, K.; Glesby, M.J.; Wilkin, T.J.; Fischer, W.; Damon, I.; et al. Development and Pilot of an Mpox Severity Scoring System. J. Infect. Dis. 2024, 229, S229–S233. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods 2010, 169, 223–227. [Google Scholar] [CrossRef]
- Schuele, L.; Masirika, L.M.; Udahemuka, J.C.; Siangoli, F.B.; Mbiribindi, J.B.; Ndishimye, P.; Aarestrup, F.M.; Koopmans, M.; Munnink, B.B.O.; Molenkamp, R.; et al. Real-time PCR assay to detect the novel Clade Ib monkeypox virus, September 2023 to May 2024. Euro Surveill. 2024, 29, 2400486. [Google Scholar] [CrossRef]
- Seyed Alinaghi, S.; Afsahi, A.M.; Afzalian, A.; Shahidi, R.; Zadeh, S.S.T.; Varshochi, S.; Dashti, M.; Ghasemzadeh, A.; Pashaei, A.; Paranjkhoo, P.; et al. Monkeypox: A systematic review of epidemiology, pathogenesis, manifestations, and outcomes. Russ. J. Infect. Immun. 2023, 13, 851–867. [Google Scholar] [CrossRef]
- Okoli, G.N.; Van Caeseele, P.; Askin, N.; Abou-Setta, A.M. Comparative evaluation of the clinical presentation and epidemiology of the 2022 and previous mpox outbreaks: A rapid review and meta-analysis. Infect. Dis. 2023, 55, 490–508. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Ayada, I.; Avan, A.; Zheng, Q.; Peppelenbosch, M.P.; de Vries, A.C.; Pan, Q. Clinical features, antiviral treatment and patient outcomes: A systematic review and comparative analysis of the previous and the 2022 mpox outbreaks. J. Infect. Dis. 2023, 228, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Yon, H.; Shin, H.; Shin, J.I.; Shin, J.U.; Shin, Y.H.; Lee, J.; Rhee, S.Y.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Clinical manifestations of human mpox infection: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2446. [Google Scholar] [CrossRef] [PubMed]
- Domínguez García, L.; Gutierrez-Arroyo, A.; Miguel-Buckley, R.; Sanchez-Vicente, J.L.; Muñoz-Sanz, A.; Cordero Coma, M. Persistent and Severe Mpox Keratitis Despite Systemic and Topical Treatment. Cornea, 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Casas, C.G.; Strahan, A.G.; Fuller, L.C.; Peebles, K.; Carugno, A.; Leslie, K.S.; Harp, J.L.; Pumnea, T.; McMahon, D.E.; et al. A dermatologic assessment of 101 mpox cases from 13 countries during the 2022 outbreak: Skin lesion morphology, clinical course, and scarring. J. Am. Acad. Dermatol. 2023, 88, 1066–1073. [Google Scholar] [CrossRef]
- De la Herrán-Arita, A.K.; González-Galindo, C.; Inzunza-Leyva, G.K.; Valdez-Flores, M.A.; Norzagaray-Valenzuela, C.D.; Camacho-Zamora, A.; Batiz-Beltrán, J.C.; Urrea-Ramírez, F.J.; Romero-Utrilla, A.; Angulo-Rojo, C.; et al. Clinical Predictors of Monkeypox Diagnosis: A Case-Control Study in a Nonendemic Region during the 2022 Outbreak. Microorganisms 2023, 11, 2287. [Google Scholar] [CrossRef]
- Álvarez-Moreno, C.A.; Alzate-Ángel, J.C.; De La Hoz-Siegler, I.H.; Bareño, A.; Mantilla, M.; Sussman, O.; Valderrama-Beltrán, S.; Rodriguez, J.Y.; Arévalo, L.; Andrade-Sierra, J.; et al. Clinical and epidemiological characteristics of mpox: A descriptive cases series in Colombia. Travel. Med. Infect. Dis. 2023, 53, 102594. [Google Scholar] [CrossRef]
- Cho, W.; Park, S.; Kim, H.J.; Lee, M.; Choi, Y.S.; Yeo, S.G.; Lee, J.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Clinical characteristics and outcomes of patients with mpox during the 2022 mpox outbreak compared with those before the outbreak: A systematic review and meta-analysis. Rev. Med. Virol. 2024, 34, e2508. [Google Scholar] [CrossRef]
- Ogoina, D.; Dalhat, M.M.; Denue, B.A.; Okowa, M.; Chika-Igwenyi, N.M.; Yusuff, H.A.; Christian, U.C.; Adekanmbi, O.; Ojimba, A.O.; Aremu, J.T.; et al. Clinical characteristics and predictors of human mpox outcome during the 2022 outbreak in Nigeria: A cohort study. Lancet Infect. Dis. 2023, 23, 1418–1428. [Google Scholar] [CrossRef]
- Cices, A.; Prasad, S.; Akselrad, M.D.; Sells, N.; Woods, K.L.; Silverberg, N.B.; Camins, B. Mpox Update: Clinical Presentation, Vaccination Guidance, and Management. Cutis 2023, 111, 197–202. [Google Scholar] [CrossRef]
- Yu, P.A.; Elmor, R.; Muhammad, K.; Yu, Y.C.; Rao, A.K. Tecovirimat Use under Expanded Access to Treat Mpox in the United States, 2022–2023. NEJM Evid. 2024, 3, e2400189. [Google Scholar] [CrossRef]
- National Institutes of Health. The Antiviral Tecovirimat Is Safe but Did Not Improve Clade I Mpox Resolution in Democratic Republic of the Congo. Available online: https://www.nih.gov/news-events/news-releases/antiviral-tecovirimat-safe-did-not-improve-clade-i-mpox-resolution-democratic-republic-congo (accessed on 12 March 2025).
- Suspène, R.; Raymond, K.A.; Boutin, L.; Guillier, S.; Lemoine, F.; Ferraris, O.; Tournier, J.-N.; Iseni, F.; Simon-Lorière, E.; Vartanian, J.-P. APOBEC3F Is a Mutational Driver of the Human Monkeypox Virus Identified in the 2022 Outbreak. J. Infect. Dis. 2023, 228, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jhanwar, P.; Roohani, B.; Gulati, A.; Tatu, U. Genomic analyses of recently emerging clades of mpox virus reveal gene deletion and single nucleotide polymorphisms that correlate with altered virulence and transmission. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kinganda-Lusamaki, E.; Amuri-Aziza, A.; Fernandez-Nuñez, N.; Makangara-Cigolo, J.-C.; Pratt, C.; Vakaniaki, E.H.; Hoff, N.A.; Luakanda-Ndelemo, G.; Akil-Bandali, P.; Nundu, S.S.; et al. Clade I mpox virus genomic diversity in the Democratic Republic of the Congo, 2018–2024: Predominance of zoonotic transmission. Cell 2024, 189, 834–846. [Google Scholar] [CrossRef]
- McGrail, J.P.; Mondolfi, A.P.; Ramírez, J.D.; Vidal, S.; García-Sastre, A.; Palacios, G.; Sanchez-Seco, M.P.; Guerra, S. Comparative Analysis of 2022 Outbreak MPXV and Previous Clade II MPXV. J. Med. Virol. 2024, 96, 11. [Google Scholar] [CrossRef]
- Nizigiyimana, A.; Ndikumwenayo, F.; Houben, S.; Manirakiza, M.; van Lettow, M.; Liesenborghs, L.; Mbala-Kingebeni, P.; Rimoin, A.W.; Bogoch, I.I.; Kindrachuk, J. Epidemiological analysis of confirmed mpox cases, Burundi, 3 July to 9 September 2024. Euro Surveill. 2024, 29, 2400647. [Google Scholar] [CrossRef]
- Laurenson-Schafer, H.; Sklenovská, N.; Hoxha, A.; Kerr, S.M.; Ndumbi, P.; Fitzner, J.; Almiron, M.; de Sousa, L.A.; Briand, S.; Cenciarelli, O.; et al. Description of the First Global Outbreak of Mpox: An Analysis of Global Surveillance Data. Lancet Glob. Health 2023, 11, e1012–e1023. [Google Scholar] [CrossRef]
- World Health Organization. Multi-Country Outbreak of Mpox, External Situation Report #48; WHO: Geneva, Switzerland, 2025. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report--48---10-march-2025 (accessed on 19 March 2025).
- Singh, P.; Sridhar, S.B.; Shareef, J.; Talath, S.; Mohapatra, P.; Khatib, M.N.; Ballal, S.; Kaur, M.; Nathiya, D.; Sharma, S.; et al. The Resurgence of Monkeypox: Epidemiology, Clinical Features, and Public Health Implications in the Post-Smallpox Eradication Era. New Microbes New Infect. 2024, 62, 101487. [Google Scholar] [CrossRef]
- Allan-Blitz, L.; Gandhi, M.; Adamson, P.C.; Park, I.U.; Bolan, G.; Klausner, J.D. A Position Statement on Mpox as a Sexually Transmitted Disease. Clin. Infect. Dis. 2022, 75, e1243–e1247. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. How it Spreads|Mpox|Poxvirus. Available online: https://www.cdc.gov/mpox/causes/index.html (accessed on 19 March 2025).
- Titanji, B.K.; Hazra, A.; Zucker, J. Mpox Clinical Presentation, Diagnostic Approaches, and Treatment Strategies: A Review. JAMA 2024, 332, 1652–1662. [Google Scholar] [CrossRef]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef] [PubMed]


| Occupation | Count (n = 465 *) | Percent (%) |
|---|---|---|
| Primary and secondary students | 162 | 34.8 |
| Merchants and sellers | 65 | 14.0 |
| Commercial sex workers | 22 | 4.7 |
| Drivers | 19 | 4.1 |
| Military and police | 16 | 3.4 |
| Health care professionals | 4 | 0.9 |
| University students | 13 | 2.8 |
| Mechanics | 11 | 2.4 |
| Other | 153 | 32.9 |
| Age Group (Years) | Total Cases n (%) 1 | Genital Lesions 2 n (%) 3 | Anorectal Lesions n(%) 3 | Oral Lesions n (%) 3 |
|---|---|---|---|---|
| 0–4 | 115 (13.8) | 10 (8.7) | 13 (11.3) | 24 (20.9) |
| 5–15 | 213 (25.5) | 49 (23.0) | 19 (8.9) | 41 (19.2) |
| 16–19 | 71 (8.5) | 39 (54.9) | 14 (19.7) | 12 (16.9) |
| 20–29 | 209 (25.0) | 151 (72.2) | 45 (21.5) | 48 (23.0) |
| 30–39 | 153 (18.3) | 100 (65.4) | 51 (33.3) | 57 (37.3) |
| 40–49 | 62 (7.4) | 38 (61.3) | 26 (41.9) | 38 (61.3) |
| 50–59 | 9 (1.1) | 5 (55.6) | 9 (100.0) | 9 (100.0) |
| ≥60 | 3 (0.4) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Total | 835 (100.0) | 392 (46.9) | 178 (21.3) | 230 (27.5) |
| Primary Complication | Count | Percent (%) |
|---|---|---|
| No complications | 820 | 96.5 |
| Any complication | 26 | 3.1 |
| Vaginitis | 9 | 1.1 |
| Ulceration in the genital area | 4 | 0.5 |
| Conjunctivitis | 2 | 0.2 |
| Necrotic lesions in the scrotum/penis | 2 | 0.2 |
| Other specific complications (e.g., cellulitis, human immunodeficiency virus-related ocular lesions, genital necrosis, complicated pyelonephritis with vaginitis, Fournier gangrene) | ≤1 each | <0.2 each |
| Secondary complication | ||
| No secondary complications | 807 | 94.9 |
| Any secondary complication | 43 | 5.1 |
| Specific cases (e.g., complicated pyelonephritis, Fournier gangrene) | ≤1 each | <0.2 each |
| Category | Subcategory | Count | Percent (%) |
|---|---|---|---|
| Comorbidity | STI history (No) | 710 | 58.1 |
| STI history (Yes) | 67 | 5.49 | |
| Hypertension (No) | 871 | 68.6 | |
| Hypertension (Yes) | 1 | 0.08 | |
| Diabetes (No) | 814 | 66.7 | |
| Diabetes (Yes) | 3 | 0.25 | |
| Cancer (No) | 844 | 69.1 | |
| Cancer (Yes) | 1 | 0.08 | |
| Kidney failure (No) | 843 | 69.0 | |
| Kidney failure (Yes) | 2 | 0.16 | |
| Others (No) | 204 | 16.67 | |
| Others (Yes) | 2 | 0.16 | |
| Erythematous-squamous dermatosis | 1 | 0.08 | |
| Pyelonephritis complicated by vaginitis | 1 | 0.08 | |
| HIV status | Negative | 785 | 92.4 |
| Unknown | 33 | 3.9 | |
| Positive | 28 | 3.3 | |
| Age (HIV-positive cases) | 16–19 years | 4 | |
| 20–29 years | 9 | ||
| 30–39 years | 9 | ||
| 40–49 years | 5 | ||
| 50+ years | 1 |
| Variables | Balanced Dataset | Unbalanced Dataset | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sore throat | 12.63 (5.78–30.21) | <0.001 * | 4.32 (1.15–19.61) | 0.030 |
| Conjunctivitis | 27.30 (7.67–122.23) | <0.001 * | 45.44 (5.98–464.05) | <0.001 * |
| Asthenia/fatigue | 0.33 (0.10–0.93) | 0.036 | 1.81 (0.35–9.58) | 0.477 |
| Muscle pain | 2.86 (1.07–8.00) | 0.037 | 1.06 (0.23–4.73) | 0.940 |
| Back pain | 0.05 (0.01–0.43) | 0.006 * | 0.21 (0.01–2.01) | 0.185 |
| Chills/sweats | 0.03 (0.00–0.30) | <0.001 * | 0.27 (0.00–3.23) | 0.344 |
| Local lymphadenopathy | 0.24 (0.08–0.62) | 0.003 * | 0.13 (0.01–1.06) | 0.058 |
| Genital edema | 5.66 (1.55–23.28) | 0.008 * | 3.67 (0.58–22.69) | 0.162 |
| Generalized lesions | 0.10 (0.04–0.24) | <0.001 * | 0.36 (0.05–1.69) | 0.203 |
| Oral lesions | 0.20 (0.07–0.55) | 0.001 * | 0.44 (0.07–2.01) | 0.304 |
| Vaginal lesions | 3.44 (1.68–7.14) | <0.001 * | 8.88 (2.45–39.30) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkengurutse, L.; Otshudiema, J.O.; Kamwenubusa, G.; Diallo, I.; Nsavyimana, O.; Mbonicura, J.C.; Nkurunziza, J.C.; Cishahayo, F.; Niyongere, D.; Havyarimana, B.; et al. Clinical Predictors and Determinants of Mpox Complications in Hospitalized Patients: A Prospective Cohort Study from Burundi. Viruses 2025, 17, 480. https://doi.org/10.3390/v17040480
Nkengurutse L, Otshudiema JO, Kamwenubusa G, Diallo I, Nsavyimana O, Mbonicura JC, Nkurunziza JC, Cishahayo F, Niyongere D, Havyarimana B, et al. Clinical Predictors and Determinants of Mpox Complications in Hospitalized Patients: A Prospective Cohort Study from Burundi. Viruses. 2025; 17(4):480. https://doi.org/10.3390/v17040480
Chicago/Turabian StyleNkengurutse, Liliane, John O. Otshudiema, Godefroid Kamwenubusa, Issa Diallo, Odette Nsavyimana, Jean Claude Mbonicura, Jean Claude Nkurunziza, Fidèle Cishahayo, Dieudonné Niyongere, Bonite Havyarimana, and et al. 2025. "Clinical Predictors and Determinants of Mpox Complications in Hospitalized Patients: A Prospective Cohort Study from Burundi" Viruses 17, no. 4: 480. https://doi.org/10.3390/v17040480
APA StyleNkengurutse, L., Otshudiema, J. O., Kamwenubusa, G., Diallo, I., Nsavyimana, O., Mbonicura, J. C., Nkurunziza, J. C., Cishahayo, F., Niyongere, D., Havyarimana, B., Simbarariye, D., Nimburanira, M., Ntiranyibagira, B., Nzeyimana, S. D., Ndelema, B., Nkezimana, D., Shingiro, P., Sibomana, A., Nduwimana, S., ... Harakandi, S. (2025). Clinical Predictors and Determinants of Mpox Complications in Hospitalized Patients: A Prospective Cohort Study from Burundi. Viruses, 17(4), 480. https://doi.org/10.3390/v17040480









