Worldwide Trend Observation and Analysis of Sheep Pox and Goat Pox Disease: A Descriptive 18-Year Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Variables
2.2. Data Processing
2.2.1. Country Classification Based on Their SGP Reporting
2.2.2. Region Classification
2.2.3. Data Validation
2.3. Statistical Analysis
2.4. Trends Analysis
3. Results
3.1. Description of Countries with the Presence of SGP
3.2. Level of Income, Sheep and Goat Population, and Countries’ SGP Status
3.3. Outbreak Trend
4. Discussion
- Characteristics of the agent
- Vaccine and vaccination strategies
- Livestock production systems
- Political instability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDP | Gross Domestic Product |
| GTP | Goat pox |
| SPP | Sheep pox |
| SPPV | Sheeppox virus |
| SGP | Sheep and goat pox |
| GTPV | Goatpox virus |
| LSDV | Lumpy Skin Disease Virus |
| WAHIS | World Animal Health Information System |
| WOAH | World Organisation for Animal Health |
Appendix A
| Region | Country | Number of Reports | n of Times Reported | % of Number of Time | Outbreaks Total | Cases Total | Year Where Reports Is Missing | Income Group Classification ** | |
|---|---|---|---|---|---|---|---|---|---|
| Africa | |||||||||
| North Africa | Algeria | 18 | 17 | 94.4% | 1720 | 15,546 | LMI | ||
| Egypt | 18 | 3 | 16.7% | 7 | 20 | LMI | |||
| Libya | 15 | 7 | 46.7% | 67 | 38,242 | 2020, 2021, 2022 | UMI | ||
| Morocco | 18 | 18 | 100% | 4766 | 30,726 | LMI | |||
| Tunisia | 16 | 16 | 100% | 2555 | 13,487 | 2021, 2022 | LMI | ||
| Sub-Saharan | Burkina Faso | 15 | 6 | 40% | 31 | 1628 | 2020, 2021, 2022 | LI | |
| Burundi | 11 | 1 | 9.1% | 5 | 220 | 2011, 2013, 2014, 2019, 2020, 2021, 2022 | |||
| Cameroon | 13 | 6 | 46.2% | 49 | 1147 | 2018, 2019, 2020, 2021, 2022 | LMI | ||
| Chad | 17 | 2 | 11.8% | 2 | 37 | 2022 | LI | ||
| Congo Rep. of the | 17 | 1 | 5.9% | 2 | 229 | LMI | |||
| Cote D’Ivoire | 17 | 2 | 11.8% | 2 | 445 | 2021 | LMI | ||
| Eritrea | 17 | 17 | 100% | 85 | 36,196 | 2022 | LI | ||
| Ethiopia | 18 | 18 | 100% | 3371 | 269,802 | LI | |||
| Gambia | 5 | 1 | 20% | 7 | 16 | 2005–2007, 2010–2015, 2019–2022 | |||
| Ghana | 15 | 4 | 26.7% | 26 | 32 | 2020, 2021, 2022 | LMI | ||
| Kenya | 18 | 4 | 22.2% | 5 | 18 | LMI | |||
| Lesotho | 18 | 1 | 5.6% | 1 | 25 | LMI | |||
| Mali | 15 | 7 | 46.7% | 11 | 1531 | 2020, 2021, 2022 | LI | ||
| Mauritania | 15 | 1 | 6.7% | 8 | 113 | 2020, 2021, 2022 | LMI | ||
| Niger | 16 | 11 | 68.8% | 2445 | 31,075 | 2020, 2021, 2022 | LI | ||
| Nigeria | 18 | 6 | 33.3% | 117 | 1498 | 2022 | LMI | ||
| Rwanda * | 12 | 6 | |||||||
| Senegal | 15 | 14 | 93.3% | 112 | 2993 | 2020, 2021, 2022 | LMI | ||
| Sierra Leone | 10 | 0 | 0% | 0 | 458 | 2005–2008, 2019–2022 | |||
| Somalia | 18 | 11 | 61.1% | 568 | 21,348 | LI | |||
| Sudan | 18 | 18 | 100% | 296 | 19,694 | LI | |||
| Tanzania | 15 | 4 | 26.7% | 25 | 600 | 2020, 2021, 2022 | LMI | ||
| Togo * | 16 | 1 | |||||||
| Uganda | 18 | 0 | 0% | 0 | 78 | LI | |||
| Zambia * | 17 | 1 | |||||||
| Asia | |||||||||
| Central Asia | Afghanistan | 18 | 10 | 55.6% | 284 | 12,064 | LI | ||
| Azerbaijan | 18 | 1 | 5.6% | 1 | 1 | UMI | |||
| Iran | 17 | 17 | 100% | 4777 | 71,344 | 2022 | LMI | ||
| Kazakhstan | 16 | 5 | 31.3% | 8 | 515 | 2020, 2021 | UMI | ||
| Kyrgyzstan | 17 | 8 | 47.1% | 24 | 226 | 2022 | LMI | ||
| Pakistan | 18 | 5 | 27.8% | 33 | 64,444 | LMI | |||
| Tajikistan | 15 | 9 | 60% | 33 | 270 | 2020, 2021, 2022 | LMI | ||
| Turkey | 18 | 18 | 100% | 2137 | 87,718 | UMI | |||
| Turkmenistan | 17 | 1 | 5.9% | 1 | 245 | 2005 | UMI | ||
| East Asia | Bhutan | 18 | 2 | 11.1% | 3 | 29 | LMI | ||
| Bangladesh * | 14 | 12 | |||||||
| China | 15 | 15 | 100% | 1949 | 68,520 | 2020, 2021, 2022 | UMI | ||
| Chinese Taipei | 18 | 4 | 22.2% | 583 | 7289 | ||||
| India | 18 | 18 | 100% | 3938 | 91,618 | LMI | |||
| Indonesia * | 18 | 5 | |||||||
| Korea Rep. of | 16 | 1 | 6.3% | 1 | 1 | 2021, 2022 | HI | ||
| Laos * | 18 | 3 | |||||||
| Mongolia | 16 | 8 | 50% | 161 | 36,146 | 2020, 2021 | LMI | ||
| Nepal | 18 | 6 | 33.3% | 48 | 5441 | LMI | |||
| Sri Lanka | 18 | 1 | 5.6% | 1 | 1186 | LMI | |||
| Vietnam | 17 | 2 | 11.8% | 16 | 4300 | 2022 | LMI | ||
| Middle East | Bahrain | 18 | 10 | 55.6% | 601 | 3460 | HI | ||
| Iraq | 18 | 17 | 94.4% | 1561 | 24,341 | UMI | |||
| Israel | 18 | 13 | 72.2% | 77 | 2026 | HI | |||
| Jordan | 17 | 17 | 100% | 129 | 1907 | 2021 | LMI | ||
| Kuwait | 18 | 9 | 50% | 106 | 1967 | HI | |||
| Lebanon * | 17 | 7 | |||||||
| Qatar * | 18 | 3 | |||||||
| Oman | 17 | 14 | 82.4% | 3538 | 42,461 | 2022 | HI | ||
| Palestine | 18 | 18 | 100% | 989 | 8978 | ||||
| Saudi Arabia * | 18 | 7 | |||||||
| United Arab Emirates | 18 | 4 | 22.2% | 27 | 1915 | HI | |||
| Yemen | 18 | 9 | 50% | 442 | 13,290 | 2022 | LI | ||
| Europe | |||||||||
| Europe | Bulgaria | 16 | 1 | 6.3% | 4 | 64 | 2021, 2022 | UMI | |
| Greece | 15 | 8 | 53.3% | 150 | 2120 | 2020, 2021, 2022 | HI | ||
| Russia | 18 | 12 | 66.7% | 86 | 4176 | UMI | |||
| Spain | 18 | 1 | 5.6% | 23 | 464 | HI | |||
| Sheep | Region | ||||
|---|---|---|---|---|---|
| East Asia | Europe | Middle East | North Africa | Sub-Saharan Africa | |
| Central Asia | p < 0.0033 | p < 0.0033 | p < 0.0033 | ||
| East Asia | p < 0.0033 | p < 0.0033 | |||
| Europe | p < 0.0033 | p < 0.0033 | p < 0.0033 | ||
| Middle East | |||||
| North Africa | |||||
| Goats | Region | ||||
|---|---|---|---|---|---|
| East Asia | Europe | Middle East | North Africa | Sub-Saharan Africa | |
| Central Asia | p < 0.0033 | p < 0.0033 | p < 0.0033 | ||
| East Asia | p < 0.0033 | ||||
| Europe | p < 0.0033 | p < 0.0033 | p < 0.0033 | ||
| Middle East | p < 0.0033 | p < 0.0033 | |||
| North Africa | p < 0.0033 | ||||
References
- World Organisation for Animal Health. Terrestrial Animal Health Code, 23rd ed.; OIE: Paris, France, 2024. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 1 January 2024).
- Babiuk, S.; Bowden, T.R.; Boyle, D.B.; Wallace, D.B.; Kitching, R.P. Capripoxviruses: An emerging worldwide threat to sheep, goats and cattle. Transbound. Emerg. Dis. 2008, 55, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017, 64, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.G.; Sawarkar, S.D.; Brett, E.K.; Edwards, J.R.; Kulkarni, V.B.; Boyle, D.B.; Singh, S.N. The extent and impact of sheep pox and goat pox in the state of Maharashtra, India. Trop. Anim. Health Prod. 2000, 32, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, J.; Munyanduki, H.; Omari Tadlaoui, K.; El Harrak, M.; Fassi Fihri, O. Capripoxvirus Infections in Ruminants: A Review. Microorganisms 2021, 9, 902. [Google Scholar] [CrossRef]
- Bhanuprakash, V.; Indrani, B.K.; Hosamani, M.; Singh, R.K. The current status of sheep pox disease. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 27–60. [Google Scholar] [CrossRef]
- Zro, K.; Zakham, F.; Melloul, M.; El Fahime, E.; Ennaji, M.M. A sheeppox outbreak in Morocco: Isolation and identification of virus responsible for the new clinical form of disease. BMC Vet. Res. 2014, 10, 31. [Google Scholar] [CrossRef]
- Lafar, S.; Zro, K.; Haegeman, A.; Khayli, M.; Clercq, K.D.; Lancelot, R.; Ennaji, M.M. Clinical and Epidemiological Evolution of Sheep Pox in Morocco. J. Agric. Sci. Technol. A 2019, 9, 103–113. [Google Scholar] [CrossRef]
- Garner, M.G.; Lack, M.B. Modelling the potential impact of exotic diseases on regional Australia. Aust. Vet. J. 1995, 72, 81–87. [Google Scholar] [CrossRef]
- Mangana, O.; Kottaridi, C.; Nomikou, K. The epidemiology of sheep pox in Greece from 1987 to 2007. Rev. Sci. Tech. (Int. Off. Epizoot.) 2008, 27, 899–905. [Google Scholar] [CrossRef]
- WAHIS: World Animal Health Information System. 2024. Available online: https://wahis.woah.org/#/home (accessed on 2 February 2024).
- FAO. Food and Agriculture Organization of the United Nations. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 January 2023).
- The World Bank (WB). 2017. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.KD (accessed on 1 January 2022).
- Aminikhanghahi, S.; Cook, D.J. A Survey of Methods for Time Series Change Point Detection. Knowl. Inf. Syst. 2017, 51, 339–367. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Scientific Opinion on sheep and goat pox. EFSA J. 2014, 12, 3885. [Google Scholar] [CrossRef]
- Boshra, H.; Truong, T.; Babiuk, S.; Hemida, M.G. Seroprevalence of Sheep and Goat Pox, Peste Des Petits Ruminants and Rift Valley Fever in Saudi Arabia. PLoS ONE 2015, 10, e0140328. [Google Scholar] [CrossRef]
- Ben Chehida, F.; Ayari-Fakhfakh, E.; Caufour, P.; Amdouni, J.; Nasr, J.; Messaoudi, L.; Haj Ammar, H.; Sghaier, S.; Bernard, C.; Ghram, A.; et al. Sheep pox in Tunisia: Current status and perspectives. Transbound. Emerg. Dis. 2018, 65, 50–63. [Google Scholar] [CrossRef]
- Tadesse, B.; Aregahagn, S.; Muluneh, B.T.; Worku, Y. Spatio-temporal ditribution and transmission dynamics of sheep pox and goat pox diseases in South Wollo zone north East Ethiopia. Heliyon 2024, 10, e27470. [Google Scholar] [CrossRef]
- Achour, H.A.; Bouguedour, R. Epidemiology of sheep pox in Algeria. Rev. Sci. Tech. (Int. Off. Epizoot.) 1999, 18, 606–617. [Google Scholar]
- Maksyutov, R.A.; Gavrilova, E.V.; Agafonov, A.P.; Taranov, O.S.; Glotov, A.G.; Miheev, V.N.; Shchelkunov, S.N.; Sergeev, A.N. An Outbreak of Sheep Pox in Zabajkalskij kray of Russia. Transbound. Emerg. Dis. 2015, 62, 453–456. [Google Scholar] [CrossRef]
- Krotova, A.; Shalina, K.; Mazloum, A.; Kwon, D.; Van Schalkwyk, A.; Byadovskaya, O.; Sprygin, A. Genetic characterization of sheep pox virus strains from outbreaks in Central Russia in 2018–2019. Transbound. Emerg. Dis. 2022, 69, e3430–e3435. [Google Scholar] [CrossRef]
- Dixon, J.; Gulliver, A.; Gibbon, D. Farming Systems and Poverty: Improving Farmers’ Livelihoods in a Changing World; FAO and World Bank: Rome, Italy, 2001; 412p. [Google Scholar]
- Mahmah, A.; Amar, A. Food Security in the MENA Region: Does Agriculture Performance Matter? In Emerging Challenges to Food Production and Security in Asia, Middle East, and Africa, Climate Risks and Resource Scarcity, 1st ed.; Springer: Cham, Switzerland, 2021; Chapter 14; pp. 101–125. [Google Scholar] [CrossRef]
- Dubie, T.; Dagnew, B.; Hamid, M.; Bizuayehu, F.; Fentahun, G. Seroprevalence and associated risk factors of pox infection among sheep and goats in selected districts of Afar region, Ethiopia. Heliyon 2022, 8, e12394. [Google Scholar] [CrossRef]
- Kitching, R.P.; McGrane, J.J.; Taylor, W.P. Capripox in the Yemen Arab Republic and the Sultanate of Oman. Trop. Anim. Health Prod. 1986, 18, 115–122. [Google Scholar] [CrossRef]
- Bhanuprakash, V.; Moorthy, A.R.; Krishnappa, G.; Srinivasa Gowda, R.N.; Indrani, B.K. An epidemiological study of sheep pox infection in Karnataka State, India. Rev. Sci. Tech. (Int. Off. Epizoot.) 2005, 24, 909–920. [Google Scholar]
- Bhanuprakash, V.; Venkatesan, G.; Balamurugan, V.; Hosamani, M.; Yogisharadhya, R.; Chauhan, R.S.; Pande, A.; Mondal, B.; Singh, R.K. Pox outbreaks in sheep and goats at Makhdoom (Uttar Pradesh), India: Evidence of sheeppox virus infection in goats. Transbound. Emerg. Dis. 2010, 57, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.M.; Chu, Y.F.; Wu, G.H.; Zhao, Z.X.; Li, J.; Zhu, H.X.; Zhang, Q. An outbreak of sheep pox associated with goat poxvirus in Gansu province of China. Vet. Microbiol. 2012, 156, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, E.; Belay, A.; Ayelet, G.; Jenberie, S.; Yami, M.; Loitsch, A.; Tuppurainen, E.; Grabherr, R.; Diallo, A.; Lamien, C.E. Capripox disease in Ethiopia: Genetic differences between field isolates and vaccine strain, and implications for vaccination failure. Antivir. Res. 2015, 119, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Alwan, H.; Torabi, M.; Nourani, H.; Al-Shuhaib, M.B.S. The emergence of novel Iranian variants in sheeppox and goatpox viral envelope proteins with remarkably altered putative binding affinities with the host receptor. Virus Genes 2023, 59, 437–448. [Google Scholar] [CrossRef]
- Babiuk, S.; Bowden, T.R.; Parkyn, G.; Dalman, B.; Hoa, D.M.; Long, N.T.; Vu, P.P.; Bieu do, X.; Copps, J.; Boyle, D.B. Yemen and Vietnam capripoxviruses demonstrate a distinct host preference for goats compared with sheep. J. Gen. Virol. 2009, 90 Pt 1, 105–114. [Google Scholar] [CrossRef]
- Balinsky, C.A.; Delhon, G.; Smoliga, G.; Prarat, M.; French, R.A.; Geary, S.J.; Rock, D.L.; Rodriguez, L.L. Rapid preclinical detection of sheeppox virus by a real-time PCR assay. J. Clin. Microbiol. 2008, 46, 438–442. [Google Scholar] [CrossRef]
- Kardjadj, M. Prevalence, distribution, and risk factor for sheep pox and goat pox (SPGP) in Algeria. Trop. Anim. Health Prod. 2017, 49, 649–652. [Google Scholar] [CrossRef]
- Nizeyimana, G.; Vudriko, P.; Erume, J.; Mubiru, F.; Eneku, W.; Biryomumaisho, S.; Mwebe, R.; Arinaitwe, E.; Ademun, R.; Atim, S.; et al. Spatio-temporal analysis of sheep and goat pox outbreaks in Uganda during 2011–2022. BMC Vet. Res. 2023, 19, 224. [Google Scholar] [CrossRef]
- FAO. RADISCON Phase Two Project Presented to Donors. Food and Agriculture Organization of the United Nations. 2024. Available online: https://www.fao.org/4/y3428e/y3428e08.htm (accessed on 1 December 2024).
- Cáceres, G.G.; Romero, G.L.; Bonilla, G.S.; Guerrero, C.F.; Fernandez, M.M.; Capilla, G.J.; Tejero, C.J. Description of sheep pox outbreak in Spain in 2022–2023: Challenges found and lessons learnt in relation with control and eradication of this disease. Viruses 2024, 16, 1164. [Google Scholar] [CrossRef]
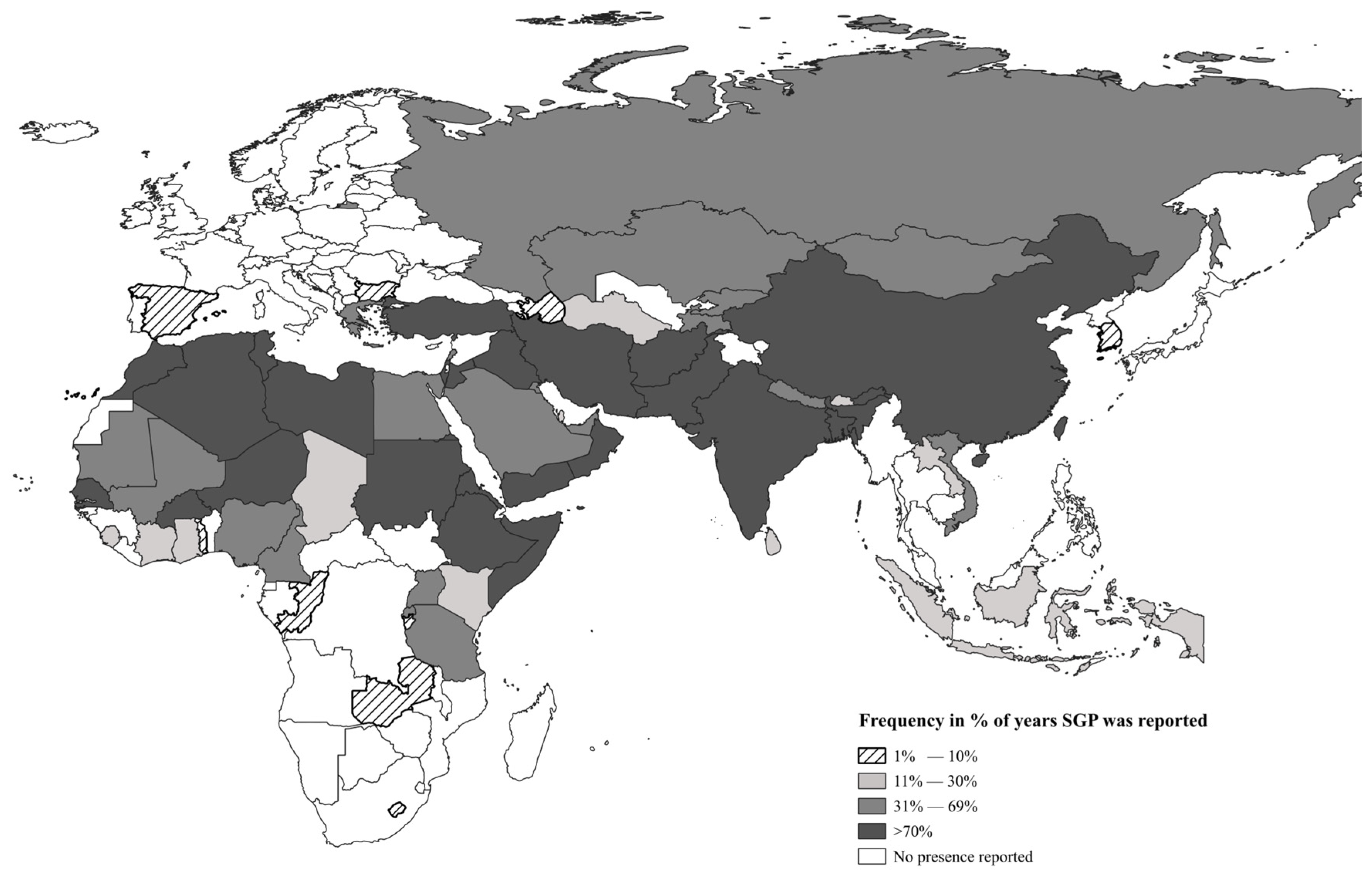
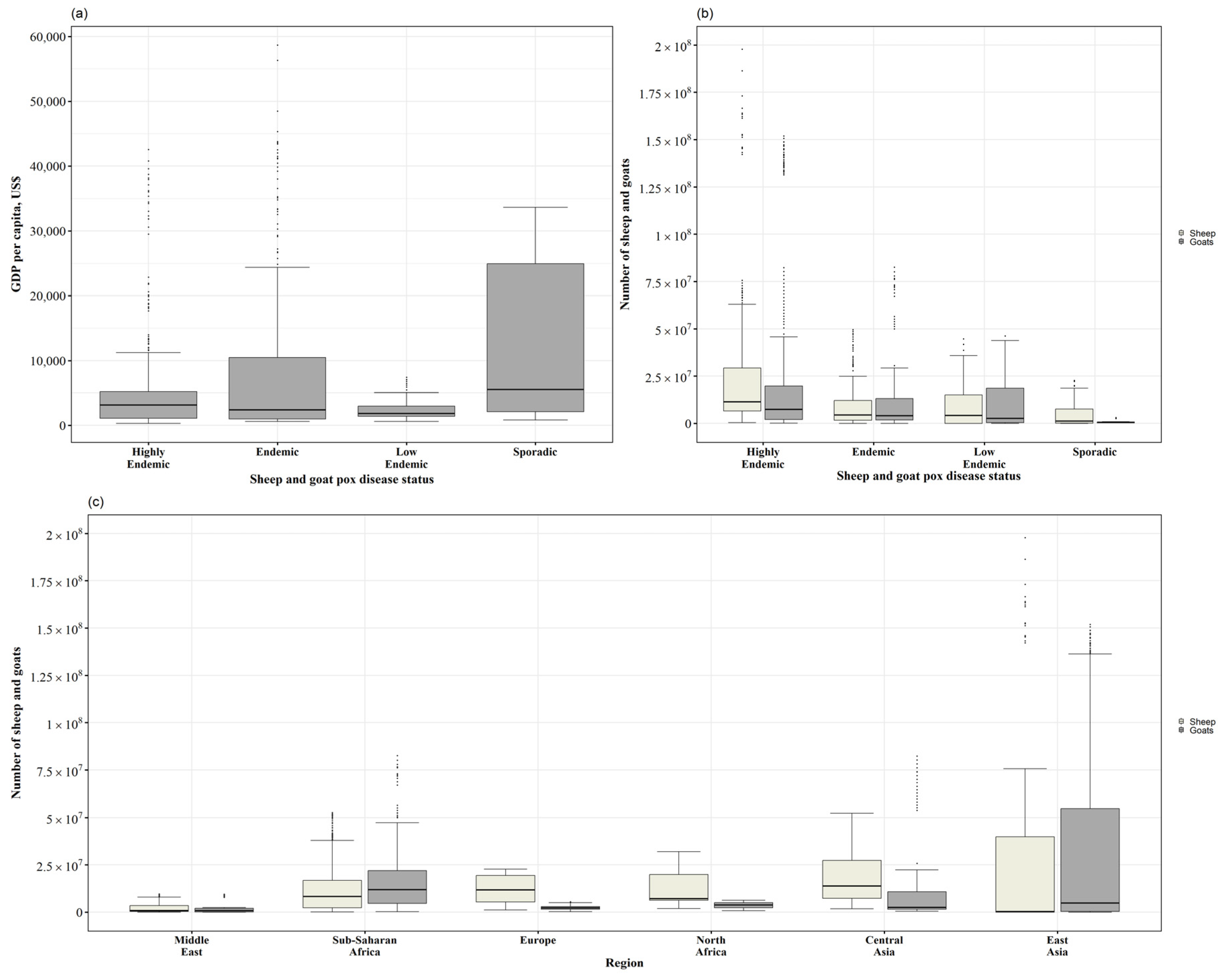

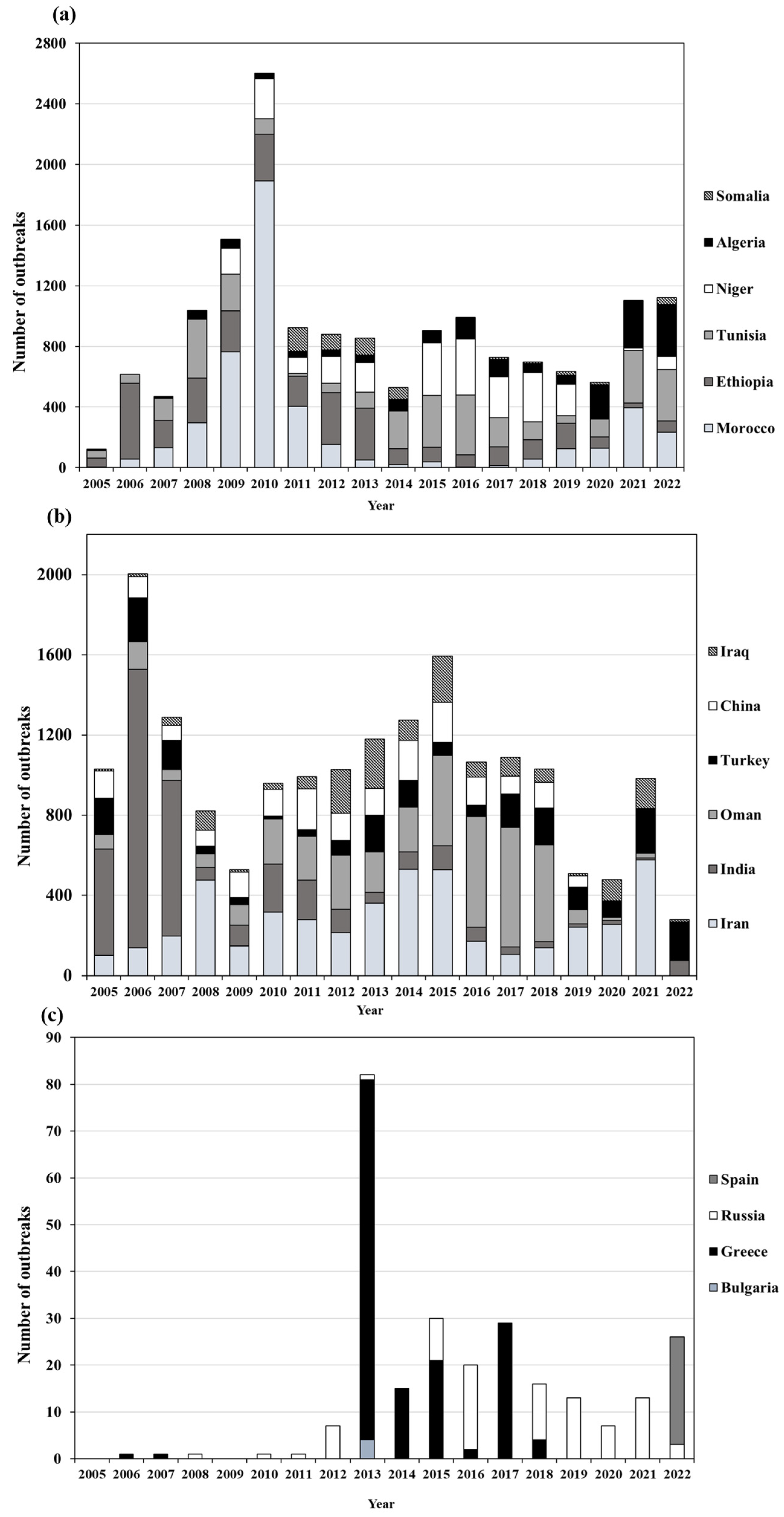

| Number of Reports | Africa | Asia | Europe | Total n of Countries | |||
|---|---|---|---|---|---|---|---|
| North Africa | Sub-Saharan Africa | Middle East | Central Asia | East Asia | |||
| 18 | Algeria, Egypt, Morocco, Tunisia | Rep. of the Congo, Ethiopia, Kenya, Lesotho, Nigeria, Somalia, Sudan, Uganda | Bahrain, Iraq, Israel, Kuwait, Palestine, United Arab Emirates, Qatar, Saudi Arabia | Afghanistan, Azerbaijan, Kyrgyzstan, Pakistan, Republic of Türkiye | Bhutan, Taiwan, India, Laos, Mongolia, Nepal, Sri Lanka, Indonesia | Russia, Spain | 35 |
| 17 | Tanzania, Chad, Cote d’ Ivoire, Eritrea, Níger, Zambia | Jordan, Oman, Lebanon | Iran, Turkmenistan | Rep. of Korea, Vietnam | 13 | ||
| 16 | Togo | Kazakhstan | Bulgaria | 3 | |||
| 15 | Libya | Ghana, Mali, Mauritania, Senegal, Burkina Faso | People’s Rep. of China | 7 | |||
| 14 | Yemen | Tajikistan | Bangladesh | Greece | 4 | ||
| 13 | Cameroon | 1 | |||||
| 12 | Rwanda | ||||||
| 11 | Burundi | 1 | |||||
| 10 | Sierra Leone | 1 | |||||
| 5 | Gambia | 1 | |||||
| Total | 5 | 25 | 12 | 9 | 12 | 4 | 67 |
| Variable | Category | OR (95% CI) | p-Value |
|---|---|---|---|
| Number of sheep | Low | Ref | – |
| Middle low | 6.76 (4.27–10.70) | <0.001 | |
| Middle high | 2.96 (2.01–4.36) | <0.001 | |
| High | 7.66 (4.77–12.30) | <0.001 | |
| Number of goats | Low | Ref | – |
| Middle low | 4.03 (2.71–5.99) | <0.001 | |
| Middle high | 13.97 (8.11–24.06) | <0.001 | |
| High | 6.79 (4.37–10.53) | <0.001 | |
| Income category | Low | Ref | – |
| Lower middle | 0.26 (0.15–0.44) | <0.001 | |
| Upper middle | 0.2 (0.11–0.36) | <0.001 | |
| High | 0.3 (0.16–0.55) | <0.001 |
| Country Income Category | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Income | Lower Middle Income | Upper Middle Income | High Income | |||||||||
| Numbers | Outbreaks | Countries/Regions | Outbreaks | Countries/ Regions | Outbreaks | Countries/ Regions | Outbreaks | Countries/ Regions | ||||
| SGP status classification | ||||||||||||
| Highly endemic | 7621 | 8 | CA = 1 SS = 6 ME = 1 | 18,805 | 8 | CA = 2 NA = 3 EA = 1 SS = 1 ME = 1 | 5725 | 4 | CA = 1 EA = 1 ME = 1 NA = 1 | 3840 | 2 | ME = 2 |
| Endemic | 16 | 2 | SS = 2 | 488 | 10 | CA = 2 NA = 1 EA = 3 SS = 4 | 92 | 2 | CA = 1 EU = 1 | 884 | 4 | EU = 1 ME = 3 |
| Low endemic | 2 | 1 | SS = 1 | 98 | 5 | EA = 2 SS = 3 | 1 | 1 | CA = 1 | |||
| Sporadic | 3 | 2 | SS = 2 | 5 | 2 | CA = 1 EU = 1 | 24 | 2 | EA = 1 EU = 1 | |||
| Total | 7639 | 11 | 19,394 | 25 | 5825 | 9 | 4748 | 8 | ||||
| Explanatory Variable | Category | IRR (95% CI) | p-Value |
|---|---|---|---|
| Number of sheep | Low | Ref | – |
| Middle low | 1.82 (0.95–3.48) | 0.071 | |
| Middle high | 4.55 (1.96–10.58) | <0.001 | |
| High | 9.87 (3.89–25.03) | <0.001 | |
| Number of goats | Low | Ref | – |
| Middle low | 2.05 (1.22–3.44) | 0.007 | |
| Middle high | 6.03 (2.80–12.98) | <0.001 | |
| High | 13.85 (5.43–35.29) | <0.001 | |
| Region | Europe | Ref | – |
| Central Asia | 2.26 (0.95–5.38) | 0.065 | |
| East Asia | 2.88 (1.12–7.45) | 0.029 | |
| Middle East | 76.63 (30.74–191.01) | <0.001 | |
| North Africa | 56.25 (21.60–146.54) | <0.001 | |
| Sub-Saharan Africa | 1.54 (0.65–3.68) | 0.328 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchini, J.; Filippitzi, M.-E.; Saegerman, C. Worldwide Trend Observation and Analysis of Sheep Pox and Goat Pox Disease: A Descriptive 18-Year Study. Viruses 2025, 17, 479. https://doi.org/10.3390/v17040479
Bianchini J, Filippitzi M-E, Saegerman C. Worldwide Trend Observation and Analysis of Sheep Pox and Goat Pox Disease: A Descriptive 18-Year Study. Viruses. 2025; 17(4):479. https://doi.org/10.3390/v17040479
Chicago/Turabian StyleBianchini, Juana, Maria-Eleni Filippitzi, and Claude Saegerman. 2025. "Worldwide Trend Observation and Analysis of Sheep Pox and Goat Pox Disease: A Descriptive 18-Year Study" Viruses 17, no. 4: 479. https://doi.org/10.3390/v17040479
APA StyleBianchini, J., Filippitzi, M.-E., & Saegerman, C. (2025). Worldwide Trend Observation and Analysis of Sheep Pox and Goat Pox Disease: A Descriptive 18-Year Study. Viruses, 17(4), 479. https://doi.org/10.3390/v17040479








