Abstract
Domestic animals can share viral pathogens with humans, acting mainly as a bridge host. The Orthopoxvirus genus hosts important zoonotic species that have emerged in urban areas worldwide. Nevertheless, the role of companion animals, such as dogs and cats, in the circulation of orthopoxviruses in urban areas remains poorly understood. Therefore, the objective of this study was to evaluate the presence of neutralizing anti-orthopoxvirus antibodies in serum samples from owned dogs from three municipalities in Minas Gerais, as well as the presence of the C11R and A56R orthopoxviruses genes. The presence of neutralizing antibodies was detected in 14.3% of the animals investigated. However, no sample was positive for the presence of the genes investigated. Further study of the population of dogs in urban areas may prove a valuable tool for understanding the spread of orthopoxviruses in urbanized areas of Brazil.
1. Introduction
Zoonotic diseases are among the greatest threats to global public health [1]. More than 70% of emerging diseases originate from animals, with wildlife being the main source of these diseases. Domestic animals are the source of 25% of zoonoses [2,3,4].
Since the first human settlements, zoonoses have become more frequent after the beginning of the animal domestication process and have, in a way, shaped human evolution [5,6,7]. Several factors contribute to the importance of domestic animals in transmitting zoonotic pathogens, even livestock and companion animals. Domestic animals have close contact with humans, a wide geographical distribution, and high abundance. These characteristics of domestic animals increase the sharing of pathogens [8,9]. According to Morand, McIntyre and Baylis [10], the longer the domestication time, the greater the number of pathogens shared with humans and other domestic animals. The study by Johnson et al. [11] showed that domestic animals harbor 50% of the zoonotic viral species of mammals and harbor a median of 19.3 zoonotic viruses, while the mean for wild animals is 0.23. In addition, network analyses showed that domestic animals occupy a central position in the sharing of zoonotic pathogens from wild animals to humans [11,12,13], therefore acting as a bridge host [14,15].
Companion animals or pets are animals that humans have an emotional relationship with. These animals can help people with disabilities, perform tasks (as service animals), and treat psychological conditions (as emotional support animals) [16,17]. Dogs, belonging to the order Carnivora, are the most popular companion animals and are found in a high density in urban areas. These animals can be free-roaming or under the care of guardians/owners [18]. Most owned dogs access the outdoors without supervision [19,20]. The canine population had been growing in recent decades around the world [21,22]. According to Abinpet [23], there are 167.6 million pets in Brazil and dogs comprise 40.4% of this total.
Concerning viral zoonoses, dogs are implicated in the transmission of canine parvovirus, canine distemper virus, canine coronavirus, and canine herpesvirus to wild animals [24,25] and, notably, rabies virus to wildlife and humans, especially in urban areas [4,15]. Dias et al. [26] found dogs, horses, and cattle are being exposed to the Mayaro and Oropouche viruses in urban and peri-urban areas of the Brazilian Centro-Oeste region. Similarly, Davila et al. [27], found a high prevalence of neutralizing antibodies to West Nile virus in dogs from urban areas of Mexico. Despite this, the dogs showed no signs of the disease and there were no reports of infections in humans during the period in which they were sampled. These data show how domestic dogs can be important sentinels for human diseases in urban areas [4,15].
A group of zoonotic viruses impacting public health globally is the Orthopoxvirus genus. Wild and domestic animals participate in orthopoxvirus maintenance and transmission to humans in urban areas. The best-known member of this genus is the variola virus, which caused smallpox and had claimed thousands of human lives over the centuries. Zoonotic orthopoxviruses important to human and animal health are cowpox virus, monkeypox virus and vaccinia virus [28,29]. Other orthopoxviruses that have been reported in humans are the camelpox virus, Akhmeta virus, and Alaskapox virus [30,31].
The recent emergence of the monkeypox virus (species Orthopoxvirus monkeypox), which causes mpox, across the globe from 2022 highlights the importance of orthopoxviruses. Since the first descriptions of monkeypox virus infections in humans, the occurrence of cases has been concentrated in rural areas, close to wilderness areas. Most of these cases are associated with the zoonotic transmission of the monkeypox virus, mainly through contact with rodents, possible reservoirs, and wild primates [28,32]. As of 2017, the mpox outbreak in Nigeria, a country where there had been no recorded cases of the disease for almost 40 years, has shown important epidemiological changes that are also seen in the global outbreak. Among these changes are the fact that most human cases occur in urban areas and person-to-person transmission has become more common [32,33,34,35]. The involvement of companion animals in the transmission of the monkeypox virus lacks evidence. To date, there have been only two reports of domestic dogs that likely became infected after contact with mpox-positive owners [36,37]. Morgan et al. [38] collected skin swab or fur samples from the pets of infected owners, and four dogs and one cat tested positive for monkeypox virus and RNase-P DNA. No animals with positive monkeypox samples had a viable virus or orthopoxvirus antibodies, indicating that the animals were not infected but likely contaminated by infected humans within the household, according to the authors.
Cowpox virus (species Orthopoxvirus cowpox) circulates mainly in Europe, infecting cats, humans [39,40,41] and confined wild animals in urban areas [42]. The main form of transmission to humans reported is contact with domestic cats. Cats become infected by hunting and preying on rodents, which are reservoirs of the virus [39,40,41]. Cases of infection in dogs and other canids are less common, but these animals can show signs of the disease [43,44,45,46].
Vaccinia virus (species Orthopoxvirus vaccinia) circulates mainly in South America, with Brazil presenting the most reported cases. This virus causes bovine vaccinia in cattle and workers in rural areas [47,48,49,50]. Some recent studies have shown the silent circulation of the virus in wild animals, such as coatis [51] and capybaras [52,53], domestic dogs [51], domestic cats [54], Rattus rattus [55] and the human population present in urban areas of Brazil [56]. Minas Gerais is the Brazilian state that, since the emergence of vaccinia in Brazil in the late 1990s, has recorded the highest number of cases of bovine vaccinia in humans and animals [50,57,58]. The state has a significant dairy economy and artisanal cheeses of international cultural value, which could be affected by the impacts of bovine vaccinia. Despite this, Minas Gerais and Goiás are the only Brazilian states where notification of cases is mandatory [50]. Even so, the scenario of vaccinia virus circulation has many gaps.
In this context, the urban environment has a large population that has not been vaccinated against smallpox or mpox; therefore, it is susceptible to orthopoxvirus infections. As well, a portion of the urban population may be immunocompromised, which can present severe symptoms [49]. Also, the high density of pets in this environment and the greater proximity to humans highlight the need for further research into the circulation of vaccinia virus in these populations.
Therefore, the objective of this study was to retrospectively evaluate the circulation of orthopoxvirus in urban areas of Minas Gerais, Brazil, through owned dogs. To this end, we investigated the presence of neutralizing antibodies and the viral genome in serum samples from dogs living in urban areas in three municipalities of Minas Gerais.
2. Materials and Methods
2.1. Areas of Study and Companion Animal Samples
The convenience collections analyzed consisted of serum or plasma samples from 318 urban owned dogs, collected between 2008 and 2020 (Table 1). The samples of dogs included in the present study were obtained from animals living in urban areas. Blood samples were collected as described by Coura-Vital et al. [59] and Leal et al. [60]. Most individual animals were females (164/51.6%) and adults (228/71.7%). This study adopted two age categories: puppies, animals ≤12 months, and adults, >12 months.

Table 1.
Collections of owned dogs from urban areas of Minas Gerais, Brazil, were analyzed in this study.
The animals were sampled in three municipalities of Minas Gerais: Belo Horizonte, the state capital, Montes Claros and Governador Valadares. These municipalities were investigated in our study because they are in different state mesoregions (Figure 1A and Table 2) [61]. In addition, it has been documented that orthopoxviruses are present in the urban environment of four distinct regions within the city of Belo Horizonte (Figure 1B). Differently, data concerning orthopoxvirus circulation in Montes Claros and Governador Valadares are limited. The straight-line distance between Montes Claros and Belo Horizonte is 353.93 km and it is 242.24 km between Governador Valadares and Belo Horizonte [62].
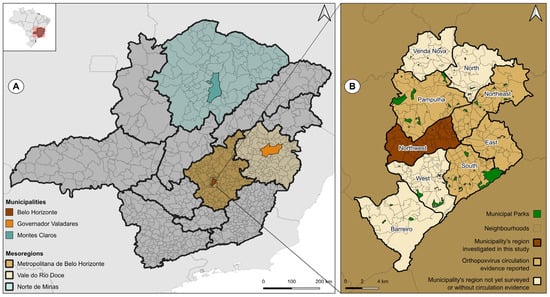
Figure 1.
Locations of municipalities where samples were obtained from owned dogs. (A) The map of Minas Gerais displays the municipalities investigated and their respective mesoregions. (B) The map of Belo Horizonte and its regions illustrates the data available on the urban circulation of orthopoxvirus in four of the nine regions. This study presents the first investigation of the northwest region. The maps were constructed with Universal Transverse Mercator (UTM 22S-24S), DATUM SIRGAS 2000, cartographic base: [57]. Created with QGIS Software, version 3.24.3.

Table 2.
Demographic, economic, and environmental characteristics of the municipalities included in this study. Data are from the Brazilian Institute of Geography and Statistics [61].
Belo Horizonte had a total population of 2,315,560 inhabitants in 2022 (Table 2) and is located in the Belo Horizonte Metropolitan mesoregion (Figure 1A) [61]. Approximately 82% of the city’s area consists of urbanized regions. The city is situated between two biomes: the Cerrado and the Atlantic Forest.
The city of Montes Claros is situated within the Norte de Minas mesoregion (Figure 1A) and registered a population of 414,240 in 2022 [61]. Only 2.04% of the total territory has been urbanized (Table 2) [61]. The city is situated between two distinct biomes, the Caatinga and the Cerrado, and thus represents a transition zone between these two ecological systems.
2.2. PRNT
A total of 314 serum/plasma samples from dogs were subjected to the plaque reduction neutralization test (PRNT) to investigate the presence of neutralizing anti-orthopoxvirus antibodies, which is considered the standard technique for this purpose. The followed protocol was described by Newman et al. [63], with modifications previously published by Kroon et al. [64]. Samples from 4 of the 318 dogs did not have enough volume for serological analysis. All these animals were from Montes Claros.
BSC-40 cells were implanted into 6-well plates and maintained in Eagle’s minimum essential medium supplemented with 5% fetal bovine serum, 100 mg/mL streptomycin, 100 IU/mL penicillin, and 1 mg/mL amphotericin B. The BSC-40 cell was obtained from the Laboratório de Vírus cell bank. The serum was diluted at 1:20, tested in duplicate and incubated with vaccinia virus Western Reserve strain (VACV-WR). Samples with a volume of less than 100 µL available were tested at a dilution of 1:40. Samples considered positive were those with ≥50% reduction of lysis plates compared to the negative serum control (composed of fetal bovine serum).
2.3. qPCR
The orthopoxvirus genes investigated were C11R, which encodes the viral growth factor (VGF), and A56R, which encodes the viral hemagglutinin (HA) [60,61]. The primers sequences were, respectively: VGF-F 5′-CGCTACAACAGATATTCCAGCTATCAG-3′ and VGF-R 5′-AGCGTGGATACAGTCACCGTGTAA-3′; HA-gen F 5′-CATCATCTGGAATTGTCACTACTAAA-3′ and HA-gen R 5′-ACGGCCGACAATATAATTAATGC-3′. The chemical and physical conditions employed in the reactions were based on those described by Trindade et al. [65] and Kroon et al. [64], with modifications. Serum or plasma samples were diluted in a proportion of 1:10 in phosphate-buffered saline 1X and tested directly via qPCR. The qPCRs were carried out on Step One™ and QuantStudio™ 3 and 6 equipment (Applied Biosystems, Thermo Fisher Scientific, São Paulo, Brazil).
A total of 318 samples from owned dogs were tested in duplicate and each reaction was conducted in a final volume of 10 µL. The cycling conditions for amplification of the C11R and A56R genes were 95 °C for 10 min for initial denaturation, 40 cycles of denaturation at 95 °C for 10 s, and pairing and extension at 58 °C for 40 s. To construct the denaturation curve, heating at 95 °C for 15 s, cooling at 58 °C for 15 s, and heating again at 95 °C for 15 s were performed. The detection system was SYBR® Green I. Samples considered positive would meet the following criteria: amplification in both duplicates, amplification in duplicates during repetition, mean Cq < 38, and mean denaturation temperature (Tm) range ± 1 °C to the positive control [64]. The VACV-WR strain was used as a positive control.
3. Results
Of the 314 serum or plasma samples subjected to the PRNT, 45 samples (14.3%) were positive for the presence of neutralizing anti-orthopoxvirus antibodies (Table 3). The percentage reduction observed ranged from 50 to 88.2%, an average of 62.9%. As for gender, 60% of the seropositive animals were females. Moreover, 80% of seropositive individuals were adults. The mean age of the seropositive individuals was of 4.2 years (0.5–12 years). As the vaccinia virus was the orthopoxvirus circulating in Brazil prior to the arrival of the monkeypox virus in 2022, these results can indicate exposure to the vaccinia virus.

Table 3.
Urban owned dogs that were positive for anti-orthopoxvirus neutralizing antibodies in the serum or plasma samples.
Belo Horizonte was the municipality with the highest number of seropositive animals, 31.7% (40/126), followed by Montes Claros with 7.9% (3/38) and Governador Valadares with 1.3% (2/150). Regarding the detection of the orthopoxvirus DNA, none of the 318 samples were positive for the presence of the C11R or A56R gene.
4. Discussion
Zoonotic orthopoxviruses are relevant to the global epidemiological scenario, as recently seen in the emergence of the monkeypox virus. A decrease in vaccination coverage against orthopoxviruses after the end of mass vaccination against smallpox is one factor pointing to this potential risk of the emergence of this viral group [56,66]. Also, anthropogenic factors, such as growing urbanization, increase the frequency of contact between the human population and domestic and wildlife populations [15,67,68]. The findings of our study indicate that owned dogs in urban regions of Minas Gerais, Brazil, are exposed to orthopoxviruses.
The changes in the epidemiology of the cowpox virus and monkeypox virus have some points in common. Cowpox virus infections in humans have historically been associated with direct contact with cattle, mainly in rural areas. Since 1970, no outbreaks caused by cowpox virus in cattle have been reported. The most affected and the main source of human infection in urban areas are domesticated or stray cats, as well as wild animals in zoos [28,42,69] As for the monkeypox virus, human cases have increased in urbanized areas of the African continent, as reported in the 2017 outbreak in Nigeria [32,34,35]. In addition, the majority of mpox cases worldwide have been detected in urban areas [70,71].
Given this scenario of other orthopoxviruses, evidence has been accumulating on the circulation of vaccinia virus in the Brazilian urban environment [51,52,53,54,56,72]. In 2012, an analysis of capybara feces found in a green area in Belo Horizonte detected the presence of vaccinia virus in Minas Gerais’s urbanized areas. Our findings suggest that the vaccinia virus may have been present there previously. Following this chronology, the data suggest urban circulation in other municipalities in Minas Gerais, such as Governador Valadares in 2014–2015 and Montes Claros in 2020, despite the low rates of seropositivity detected in these municipalities in our study (1.3 and 7.9%, respectively). This study marks the first time the vaccinia virus has been detected in Governador Valadares and Montes Claros, as well as a new region of Belo Horizonte, the northwest [54,72].
Furthermore, dogs and cats have been investigated in the context of vaccinia virus circulation in rural areas of Brazil [73,74,75]. Peres et al. [73,75] investigated the presence of vaccinia virus in farms with and without reported outbreaks in the state of São Paulo. They found antibodies against orthopoxvirus in 22.8% of the dogs sampled and in one cat. The presence of C11R was not detected in any of these animals. Peres et al. [74] found the presence of the A56R gene in three dogs from two farms with reported cases of bovine vaccine. Nonetheless, no clinical signs were observed in the animals.
Anti-orthopoxvirus neutralizing antibodies were detected in 14.3% of dogs tested in our study. Costa et al. [51] found seropositivity of 19% (35/184) in owned dogs in Vila Marçola, Belo Horizonte, but a cut-off point of ≥70% was adopted. Vila Marçola is close to Mangabeiras Park, a large natural green space in the city. The authors also found coatis positive for the presence of vaccinia virus DNA and anti-orthopoxvirus neutralizing antibodies (14.4%, 13/90) within Mangabeiras Park. These findings emphasize that green spaces such as parks can be an important contact interface between owned free-roaming cats and dogs, wild species that host the virus, and humans [25,68]. Among the seropositive dogs in Vila Marçola, 20% were positive for the presence of the C11R and A56R genes in the serum. Additionally, DNA vaccinia virus was detected in an anal swab sample from one individual, which may be another route of excretion of the virus into the environment [51].
Costa et al. [54] analyzed serum samples from 277 urban owned cats from five Brazilian states, but only the animals from Belo Horizonte had evidence of exposure to vaccinia virus. The authors found a seropositivity rate of 5.8%. This finding aligns with our own, in which Belo Horizonte exhibited the highest seropositivity rate. The authors also found vaccinia virus DNA in 4.7% of the animals analyzed.
There is no robust evidence for the role of domestic animals in the circulation and maintenance of vaccinia virus in urban areas, nor for how infection occurs in these animals. Therefore, further studies should be conducted to assess whether dogs could be a source of infection for humans. Our findings strengthen the potential of dogs as sentinels for zoonotic diseases [4,15]. Dogs as sentinels have already been proposed for some flaviviruses [27,76,77], alphaviruses and orthobunyaviruses [26]. Surveillance of sentinel populations can be an important tool for incorporating the One Health concept into research. In addition, sentinels can indicate viruses spread over time and space, which could be a valuable addition to monitoring wild populations [78].
According to Halliday et al. [79], three features define a potential sentinel: first, the sentinel population must develop a detectable response to the pathogen, such as the production of antibodies or the detection of pathogen presence; second, an epidemiological, spatial or ecological connection between the sentinel population and target population must exist; and third, there must be a route of transmission of the pathogen to these populations. Within the context of Minas Gerais, dogs have the potential to act as sentinels for orthopoxvirus in urban settings. Domestic dogs residing in urban areas have developed detectable responses to orthopoxvirus, as evidenced by the seroconversion in our study. Moreover, these animals have proximity to humans, who may be infected with orthopoxviruses. However, the transmission route of orthopoxvirus to these animals remains unclear. It is noteworthy that our study utilized convenience samples, which have certain limitations. No data were collected that could indicate how and where these animals were exposed to orthopoxviruses in urban areas, nor the frequency of their outside access. Conversely, the human population in each of the municipalities studied indicates that more animals could be sampled and tested. Additionally, stray pets may be an appropriate population to investigate the orthopoxvirus circulation in urban settings, as they may have more opportunities for virus exposure due to roaming freely.
Author Contributions
Conceptualization, D.d.M., A.G.S.-D. and G.d.S.T.; investigation, D.d.M., A.G.S.-D., V.S.B., I.M.d.A., K.L.D., I.J.d.S.D. and G.P.R.; resources, W.C.-V., A.B.R., T.M.V. and G.d.S.T.; data curation, D.d.M.; writing—original draft preparation, D.d.M. and G.d.S.T.; writing—review and editing, D.d.M., A.G.S.-D., I.J.d.S.D., W.C.-V. and G.d.S.T.; visualization, D.d.M. and G.d.S.T.; supervision, G.d.S.T.; project administration, D.d.M. and G.d.S.T.; funding acquisition, G.d.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Câmara Pox–Ministério da Ciência, Tecnologia e Inovações, grant number MCTI/405249/2022-5; Instituto Nacional de Ciência e Tecnologia em Poxvírus, INCT-POX/406441/2022-7; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, process 88882.348380/2010-1; Fundação de Amparo à Pesquisa do Estado de Minas Gerais—FAPEMIG, APQ-04005-23; and Pró-Reitoria de Pesquisa/UFMG—PRPq. W.C.V, A.B.R. and G.S.T. are CNPq researcher. SISGEN A7BAA6B/A19E6BB.
Institutional Review Board Statement
This study was approved by the Committee of Ethics in Animal Experimentation of the Universidade Federal de Minas Gerais (protocol no. 348/2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We would like to thank Laboratório de Vírus (ICB-UFMG) for their technical support and assistance. Additionally, we are grateful to Raphael Costa for his kind English review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morens, D.M.; Fauci, A.S. Emerging Pandemic Diseases: How We Got to COVID-19. Cell 2020, 182, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Olival, K.; Hosseini, P.; Zambrana-Torrelio, C.; Ross, N.; Bogich, T.L.; Daszak, P. Host and viral traits predict zoonotic spillover from mammals. Nature 2017, 546, 646–650. [Google Scholar] [CrossRef]
- Tomori, O.; Oluwayelu, D.O. Domestic animals as potential reservoirs of zoonotic viral diseases. Annu. Rev. Anim. Biosci. 2023, 11, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Ujvari, S.C. The History of the Dissemination of Microorganisms. Estud. Av. 2008, 22, 171–182. [Google Scholar] [CrossRef]
- Pearce-Duvet, J.M. The origin of human pathogens: Evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol. Rev. 2006, 81, 369–382. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; Boeckel, T.P.; Vanwambeke, S.O.; Wint, G.R.; Robinson, T.P. Data Descriptor: Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci. Data 2018, 5, 180227. [Google Scholar] [CrossRef]
- Morand, S.; Mcintyre, K.M.; Baylis, M. Domesticated Animals And human infectious diseases of zoonotic origins: Domestication time matters. Infect. Genet. Evol. 2014, 24, 76–81. [Google Scholar] [CrossRef]
- Johnson, C.K.; Hitchens, P.L.; Pandit, P.S.; Rushmore, J.; Evans, T.S.; Young, C.C.; Doyle, M.M. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc. R. Soc. B 2020, 287, 20192736. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.; Morand, S.; Wardeh, M.; Baylis, M. Distinct spread of DNA and RNA viruses among mammals amid prominent role of domestic species. Glob. Ecol. Biogeogr. 2020, 29, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Desvars-Larrive, A.; Vogl, A.E.; Puspitarani, G.A.; Yang, L.; Joachim, A.; Käsbohrer, A. A One Health framework for exploring zoonotic interactions demonstrated through a case study. Nat. Commun. 2024, 15, 5650. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Ostfeld, R.S. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023540118. [Google Scholar] [CrossRef] [PubMed]
- Gamble, A.; Olarte-Castillo, X.A.; Whittaker, G.R. Backyard zoonoses: The roles of companion animals and peri-domestic wildlife. Sci. Transl. Med. 2023, 15, eadj0037. [Google Scholar] [CrossRef]
- Chomel, B.B.; Sun, A.B. Zoonoses in the Bedroom. Emerg. Infect. Dis. 2011, 17, 167. [Google Scholar] [CrossRef]
- Reperant, L.A.; Brown, I.H.; Haenen, O.L.; de Jong, M.D.; Osterhaus, A.D.M.E.; Papa, A.; Rimstad, E.; Valarcher, J.F.; Kuiken, T. Companion Animals as a Source of Viruses for Human Beings and Food Production Animals. J. Comp. Pathol. 2016, 155, S41eS53. [Google Scholar] [CrossRef]
- Forman, R.T. Urban Ecology: Science of Cities; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-0-521-18824-1. [Google Scholar]
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Orozco, L.; López-Pérez, A.M.; Zarza, H.; Suzán, G.; List, R. Dog demography and husbandry practices facilitate dog-wildlife conflict in a suburban-forest interface. Urban Ecosyst. 2022, 25, 1725–1734. [Google Scholar] [CrossRef]
- Chomel, B.B. Emerging and re-emerging zoonoses of dogs and cats. Animals 2014, 4, 434–445. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Taufiquer Rahman, A.M.M.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Associação Brasileira da Indústria de Produtos Para Animais de Estimação (ABINPET). 2023. Available online: https://abinpet.org.br/wp-content/uploads/2023/07/abinpet_folder_dados_mercado_2023_draft5.pdf (accessed on 29 February 2024). (In Portuguese).
- Barros, M.; Pons, D.J.; Moreno, A.; Vianna, J.; Ramos, B.; Dueñas, F.; Coccia, C.; Saavedra-Rodríguez, R.; Santibañez, A.; Medina-Vogel, G. Domestic dog and alien North American mink as reservoirs of infectious diseases in the endangered Southern river otter. Austral J. Vet. Sci. 2022, 54, 65–75. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. The triad “dogs, conservation and zoonotic diseases”—An old and still neglected problem in Brazil. Perspect. Ecol. Conserv. 2019, 17, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.G.; Familiar-Macedo, D.; Garrido, I.O.; Dos Santos, F.B.; Pauvolid-Corrêa, A. Exposure of domestic animals to Mayaro and Oropouche viruses in urban and peri-urban areas of West-Central Brazil. One Health Outlook 2024, 6, 12. [Google Scholar] [CrossRef]
- Davila, E.; Fernández-Santos, N.A.; Estrada-Franco, J.G.; Wei, L.; Aguilar-Durán, J.A.; López-López, M.D.J.; Solís-Hernández, R.; García-Miranda, R.; Velázquez-Ramírez, D.D.; Torres-Romero, J.; et al. Domestic dogs as sentinels for West Nile virus but not Aedes-borne flaviviruses, Mexico. Emerg. Infect. Dis. 2022, 28, 1071. [Google Scholar] [CrossRef]
- Tack, D.M.; Reynolds, M.G. Zoonotic poxviruses associated with companion animals. Animals 2011, 1, 377–395. [Google Scholar] [CrossRef]
- Silva, N.I.; Oliveira, J.S.; Kroon, E.G.; Trindade, G.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses. Viruses 2021, 13, 43. [Google Scholar] [CrossRef]
- MacNeill, A.L. Comparative pathology of zoonotic orthopoxviruses. Pathogens 2022, 11, 892. [Google Scholar] [CrossRef]
- Douglass, N. Borealpox (Alaskapox) virus: Will there be more emerging zoonotic orthopoxviruses? Lancet Microbe 2024, 5, 100883. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Ogoina, D.; Aworabhi, N.; Eteng, W.; Badaru, S.; Mohammed, A.; Agenyi, J.; Etebu, E.N.; Numbere, T.M. Reemergence of human monkeypox in Nigeria, 2017. Emerg. Infect. Dis. 2018, 24, 1149. [Google Scholar] [CrossRef] [PubMed]
- Ogoina, D.; Iroezindu, M.; James, H.I.; Oladokun, R.; Yinka-Ogunleye, A.; Wakama, P.; Otike-odibi, B.; Usman, L.M.; Obazee, E.; Aruna, O.; et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020, 7, e210–e214. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Y.; Ajisegiri, W.S.; Costantino, V.; Chughtai, A.A.; MacIntyre, C.R. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021, 27, 1007. [Google Scholar] [CrossRef]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; Pluart, D.L.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of human-to-dog transmission of monkeypox virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
- Secretaria de Estado de Saúde (SES-MG). Detecção de Monkeypox em animal em Minas Gerais. Available online: https://www.saude.mg.gov.br/lme/story/17178-nota-informativa-sobre-deteccao-de-monkeypox-em-animal-em-minas-gerais-23-8-2022 (accessed on 29 February 2024). (In Portuguese)
- Morgan, C.N.; Wendling, N.M.; Baird, N.; Kling, C.; Lopez, L.; Navarra, T.; Fischer, G.; Wynn, N.; Ayuk-Takor, L.; Darby, B.; et al. One Health Investigation into Mpox and Pets, United States. Emerg. Infect. Dis. 2024, 30, 2025–2032. [Google Scholar] [CrossRef]
- Eder, I.; Vollmar, P.; Pfeffer, M.; Naether, P.; Rodloff, A.C.; Meyer, H. Two Distinct Clinical Courses of Human Cowpox, Germany, 2015. Viruses 2017, 9, 375. [Google Scholar] [CrossRef]
- Haddadeen, C.; Ouwerkerk, M.V.; Vicek, T.; Fityan, A. A case of cowpox virus infection in the UK occurring in a domestic cat and transmitted to the adult male owner. Br. J. Dermatol. 2020, 183, e190. [Google Scholar] [CrossRef]
- Krankowska, D.C.; Wozniak, P.A.; Cybula, A.; Izdebska, J.; Suchacz, M.; Samelska, K.; Wiercińska-Drapało, A.; Szaflik, J.P. Cowpox: How dangerous could it be for humans? Case report. Int. J. Infect. Dis. 2021, 104, 239–241. [Google Scholar] [CrossRef]
- Costa, T.; Stidworthy, M.F.; Ehmann, R.; Denk, D.; Ashpole, I.; Drake, G.; Maciuca, I.; Zoeller, G.; Meyer, H.; Chantrey, J. Cowpox in zoo and wild animals in the United Kingdom. J. Comp. Pathol. 2023, 204, 39–46. [Google Scholar] [CrossRef]
- Smith, K.C.; Bennett, M.; Garret, A.D. Skin lesions caused by orthopoxvirus infection in a dog. J. Small Anim. Pract. 1999, 40, 495–497. [Google Scholar] [CrossRef]
- Pelkonen, P.M.; Tarvainen, K.; Hynninen, A.; Kallio, E.R.; Henttonen, H.; Palva, A.; Vaheri, A.; Vapalahti, O. Cowpox with Severe Generalized Eruption, Finland. Emerg. Infect. Dis. 2003, 9, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- von Bomhard, W.; Mauldin, E.A.; Breuer, W.; Pfleghaar, S.; Nitsche, A. Localized cowpox infection in a 5-month-old Rottweiler. Vet. Dermatol. 2010, 22, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Stagegaard, J.; Kurth, A.; Stern, D.; Dabrowski, P.W.; Pocknell, A.; Nitsche, A.; Schrick, L. Seasonal recurrence of cowpox virus outbreaks incaptive cheetahs (Acinonyx jubatus). PLoS ONE 2017, 12, e0187089. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.S.; Guedes, M.I.C.; Drumond, B.P.; Mota, B.E.F.; Abrahao, J.S.; Lobato, Z.I.P.; Gomes, J.A.S.; Corrêa-Oliveira, R.; Nogueira, M.L.; Kroon, E.G.; et al. Zoonotic vaccinia virus: Clinical and immunological characteristics in a naturally infected patient. Clin. Infect. Dis. 2009, 48, e37–e40. [Google Scholar] [CrossRef]
- Megid, J.; Borges, I.A.; Abrahão, J.S.; Trindade, G.S.; Appolinário, C.M.; Ribeiro, M.G.; Allendorf, S.D.; Antunes, J.M.A.P.; Silva-Fernandes, A.T.; Kroon, E.G. Vaccinia virus zoonotic infection, Sao Paulo State, Brazil. Emerg. Infect. Dis. 2012, 18, 189. [Google Scholar] [CrossRef]
- Laiton-Donato, K.; Ávila-Robayo, P.; Páez-Martinez, A.; Benjumea-Nieto, P.; Usme-Ciro, J.A.; Pinzón-Nariño, N.; Giraldo, I.; Torres-Castellanos, D.; Nakazawa, Y.; Patel, N.; et al. Progressive Vaccinia Acquired through Zoonotic Transmission in a Patient with HIV/AIDS, Colombia. Emerg. Infect. Dis. 2020, 26, 601–605. [Google Scholar] [CrossRef]
- Domingos, I.J.; Oliveira, J.S.; Oliveira, D.B.; Kroon, E.G.; Costa, G.B.; Trindade, G.S. Twenty Years after Bovine Vaccinia in Brazil: Where We Are and Where Are We Going? Pathogens 2021, 10, 406. [Google Scholar] [CrossRef]
- Costa, G.B.; Almeida, L.R.A.; Cerqueira, G.R.; Mesquita, W.U.; Oliveira, J.S.; Miranda, J.B.; Saraiva-Silva, A.T.; Abrahão, J.S.; Drumond, B.P.; Kroon, E.G.; et al. Vaccinia Virus among Domestic Dogs and Wild Coatis, Brazil, 2013–2015. Emerg. Infect. Dis. 2018, 24, 2338. [Google Scholar] [CrossRef]
- Dutra, L.A.; Almeida, G.M.; Oliveira, G.P.; Abrahão, J.S.; Kroon, E.G.; Trindade, G.S. Molecular evidence of Orthopoxvirus DNA in capybara (Hydrochoerus hydrochaeris) stool samples. Arch. Virol. 2017, 162, 439–448. [Google Scholar] [CrossRef]
- Antunes, J.M.; Borges, I.A.; Trindade, G.D.; Kroon, E.G.; Cruvinel, T.M.; Peres, M.G.; Megid, J. Exposure of free-ranging capybaras (Hydrochoerus hydrochaeris) to the vaccinia virus. Transbound. Emerg. Dis. 2019, 67, 481–485. [Google Scholar] [CrossRef]
- Costa, G.B.; Miranda, J.B.; Almeida, G.G.; Oliveira, J.S.; Pinheiro, M.S.; Gonçalves, S.A.; Reis, J.K.P.; Gonçalves, R.; Ferreira, P.C.P.; Bonjardim, C.A.; et al. Detection of Vaccinia Virus in Urban Domestic Cats, Brazil. Emerg. Infect. Dis. 2017, 23, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Babolin, L.; Almeida-Silva, M.J.; Potenza, M.R.; Fava, C.D.; Castro, V.; Harakava, R.; Okuda, L.H.; Rebouças, M.M.; Campos, A.E. Zoonosis associated to Rattus rattus and the impacts of the public actions to control the species. Arq. Inst. Biol. 2016, 83, e0832014. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Costa, G.B.; Dutra, A.G.; Domingos, I.J.; Costa, P.S.; Silva, P.H.; Kroon, E.G.; Oliveira, D.B.; Trindade, G.S. Low prevalence of anti-Orthopoxvirus neutralizing antibodies in an urban population of Brazil. J. Med. Virol. 2023, 95, e28859. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.; da Fonseca, F.G.; dos Santos, J.R.; Madureira, M.C.; Guedes, M.I.; Ferreira, J.M.; Bonjardim, C.A.; et al. Passatempo virus, a vaccinia virus strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1938. [Google Scholar] [CrossRef]
- Trindade, G.S.; Lobato, Z.I.P.; Drumond, B.P.; Leite, J.A.; Trigueiro, R.C.; Guedes, M.I.M.C.; da Fonseca, F.G.; dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Short report: Isolation of two vaccinia virus strains from a single bovine vaccinia outbreak in rural area from Brazil: Implications on the emergence of zoonotic orthopoxviruses. Am. J. Trop. Med. Hyg. 2006, 75, 486–490. [Google Scholar] [CrossRef]
- Leal, G.G.A.; Carneiro, M.; Pinheiro, A.C.; Marques, L.A.; Ker, H.G.; Reis, A.B.; Coura-Vital, W. Risk profile for Leishmania infection in dogs coming from an area of visceral leishmaniasis reemergence. Prev. Vet. Med. 2018, 150, 1–7. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Ker, H.G.; Roatt, B.M.; Aguiar-Soares, R.D.O.; Leal, G.G.D.A.; Moreira, N.D.D.; Oliveira, L.A.M.; Machado, E.M.M.; Morais, M.H.F.; Correa-Oliveira, R.; et al. Evaluation of change in canine diagnosis protocol adopted by the visceral leishmaniasis control program in Brazil and a new proposal for diagnosis. PLoS ONE 2014, 9, e91009. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Cidades e Estados do Brasil. 2022. Available online: https://cidades.ibge.gov.br/ (accessed on 12 March 2024). (In Portuguese)
- Distância Entre Cidades. 2024. Available online: https://distanciacidades.net/ (accessed on 12 March 2024). (In Portuguese).
- Newman, F.K.; Frey, S.E.; Blevins, T.P.; Mandava, M.; Bonifacio, A., Jr.; Yan, L.; Belshe, R.B. Improved Assay To Detect Neutralizing Antibody following Vaccination with Diluted or Undiluted Vaccinia (Dryvax) Vaccine. J. Clin. Microbiol. 2003, 41, 3154–3157. [Google Scholar] [CrossRef]
- Kroon, E.G.; Abrahãao, J.S.; Trindade, G.S.; Oliveira, G.P.; Luiz, A.P.M.F.; Costa, G.B.; Lima, M.T.; Calixto, R.S.; Oliveira, D.B.; Drumond, B.P. Natural Vaccinia Virus Infection: Diagnosis, Isolation, and Characterization. Curr. Protoc. Microbiol. 2016, 42, 14A.5.1–14A.5.43. [Google Scholar] [CrossRef]
- Trindade, G.D.S.; Li, Y.; Olson, V.A.; Emerson, G.; Regnery, R.L.; da Fonseca, F.G.; Kroon, E.G.; Damon, I. Real-time PCR assay to identify variants of Vaccinia virus: Implications for the diagnosis of bovine vaccinia in Brazil. J. Virol. Methods 2008, 152, 63–71. [Google Scholar] [CrossRef]
- Taube, J.C.; Rest, E.C.; Lloyd-Smith, J.O.; Bansal, S. The global landscape of smallpox vaccination history and implications for current and future orthopoxvirus susceptibility: A modelling study. Lancet Infect. Dis. 2023, 23, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Marco, M.D.; Breit, N.; Olival, K.J.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife–Livestock–Human Interface. TREE 2017, 32, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Baxby, D.; Bennett, M.; Getty, B. Human cowpox 1969-93: A review based on 54 cases. Br. J. Dermatol. 1994, 131, 598–607. [Google Scholar] [CrossRef]
- Zelaya, C.E.; Smith, B.P.; Riser, A.P.; Hong, J.; Distler, S.; O’Connor, S.; Belay, E.; Shoeb, M.; Waltenburg, M.A.; Negron, M.E.; et al. Urban and rural mpox incidence among persons aged 15–64 years—United States, May 10–December 31, 2022. MMWR 2023, 72, 574–578. [Google Scholar] [CrossRef]
- Thornhill, J.; Gandhi, M.; Orkin, C. Mpox: The Reemergence of an Old Disease and Inequities. Annu. Rev. Med. 2024, 75, 159–175. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Figueiredo, P.O.; Costa, G.B.; Assis, F.L.; Drumond, B.P.; Fonseca, F.G.; Nogueira, M.L.; Kroon, E.G.; Trindade, G.S. Vaccinia Virus Natural Infections in Brazil: The Good, the Bad, and the Ugly. Viruses 2017, 9, 340. [Google Scholar] [CrossRef]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Allendorf, S.D.; Antunes, J.M.A.P.; Moreira, S.A.; Legatti, E.; Fonseca, C.R.; Pituco, E.M.; et al. Serological study of Vaccinia virus reservoirs in areas with and without official reports of outbreaks in cattle and humans in São Paulo, Brazil. Arch. Virol. 2013, 158, 2433–2441. [Google Scholar] [CrossRef]
- Peres, M.G.; Barros, C.B.; Appolinário, C.M.; Antunes, J.M.; Mioni, M.S.; Bacchiega, T.S.; Allendorf, S.D.; Vicente, A.F.; Fonseca, C.R.; Megid, J. Dogs and Opossums Positive for Vaccinia Virus during Outbreak Affecting Cattle and Humans, São Paulo State, Brazil. Emerg. Infect. Dis. 2016, 22, 271–273. [Google Scholar] [CrossRef]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Mioni, M.S.R.; Ribeiro, B.L.D.; Fonseca, C.R.S.; Pelícia, V.C.; Ferreira, F.; Oliveira, G.P.; et al. Vaccinia Virus in Blood Samples of Humans, Domestic and Wild Mammals in Brazil. Viruses 2018, 10, 42. [Google Scholar] [CrossRef]
- Kile, J.C.; Panella, N.A.; Komar, N.; Chow, C.C.; MacNeil, A.; Robbins, B.; Bunning, M.L. Serologic survey of cats and dogs during an epidemic of West Nile virus infection in humans. J. Am. Veter. Med. Assoc. 2005, 226, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Pham-Thanh, L.; Nguyen-Tien, T.; Magnusson, U.; Bui-Nghia, V.; Bui-Ngoc, A.; Le-Thanh, D.; Lundkvist, A.; Can-Xuan, M.; Thu, T.N.T.; Bich, A.B.N.; et al. Dogs as sentinels for flavivirus exposure in urban, peri-urban and rural Hanoi, Vietnam. Viruses 2021, 13, 507. [Google Scholar] [CrossRef]
- Bowser, N.H.; Anderson, N.E. Dogs (Canis familiaris) as sentinels for human infectious disease and application to Canadian populations: A systematic review. Vet. Sci. 2018, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J.E.; Meredith, A.L.; Knobel, D.L.; Shaw, D.J.; Bronsvoort, B.M.D.C.; Cleaveland, S. A framework for evaluating animals as sentinels for infectious disease surveillance. J. R. Soc. Interface 2007, 4, 973–984. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).