Retrospective Study of Arbovirus Circulation in Northeast Brazil in 2019 and 2022: Insights into the Re-Emergence of DENV-3 and the Co-Infection of DENV-1 and CHIKV
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Sampling and Ethical Procedures
2.3. Collection of Biological Material
2.4. Viral RNA Extraction and RT-qPCR
2.5. Viral Isolation in C6/36 Cells
2.6. Nucleotide Sequencing
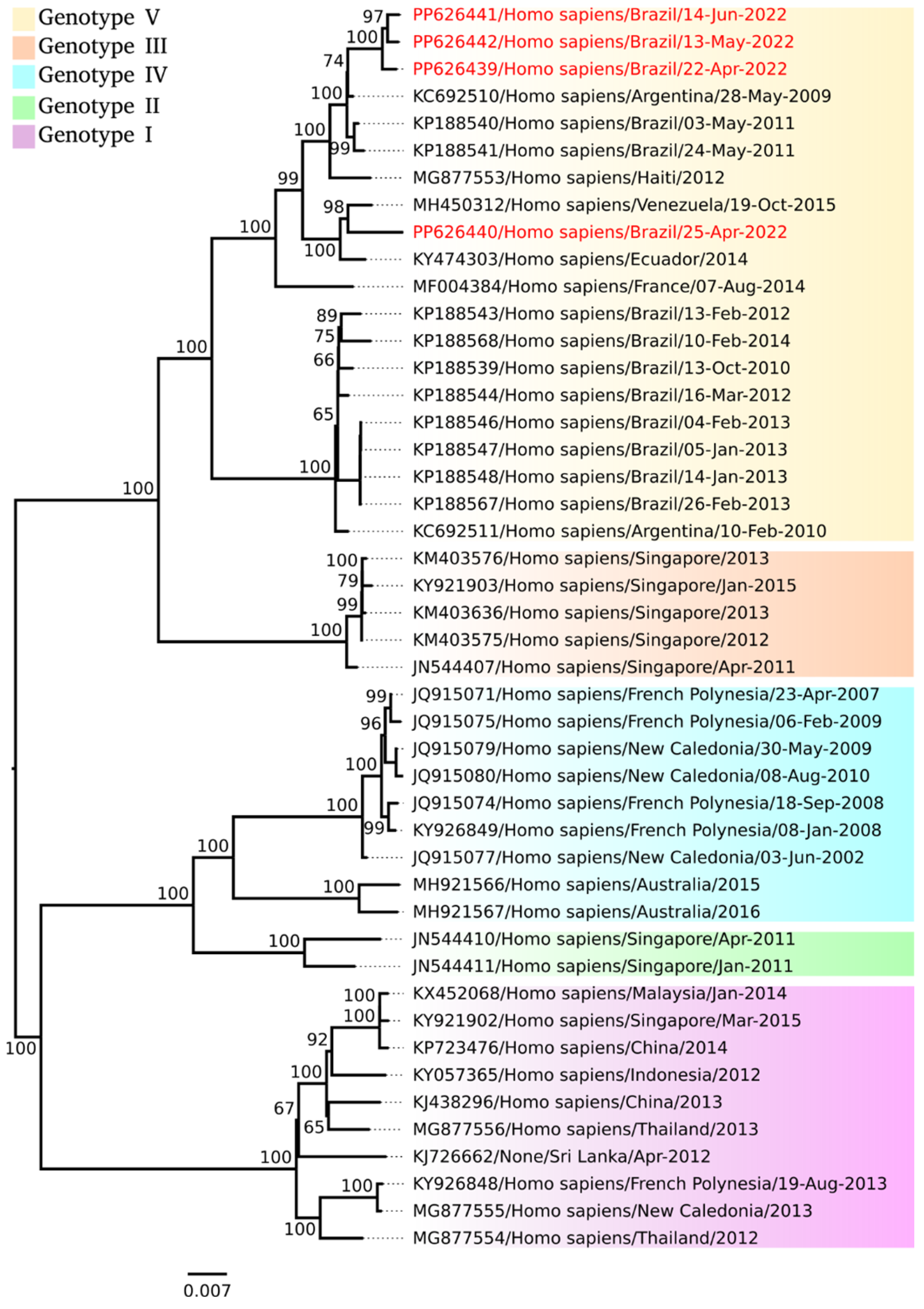
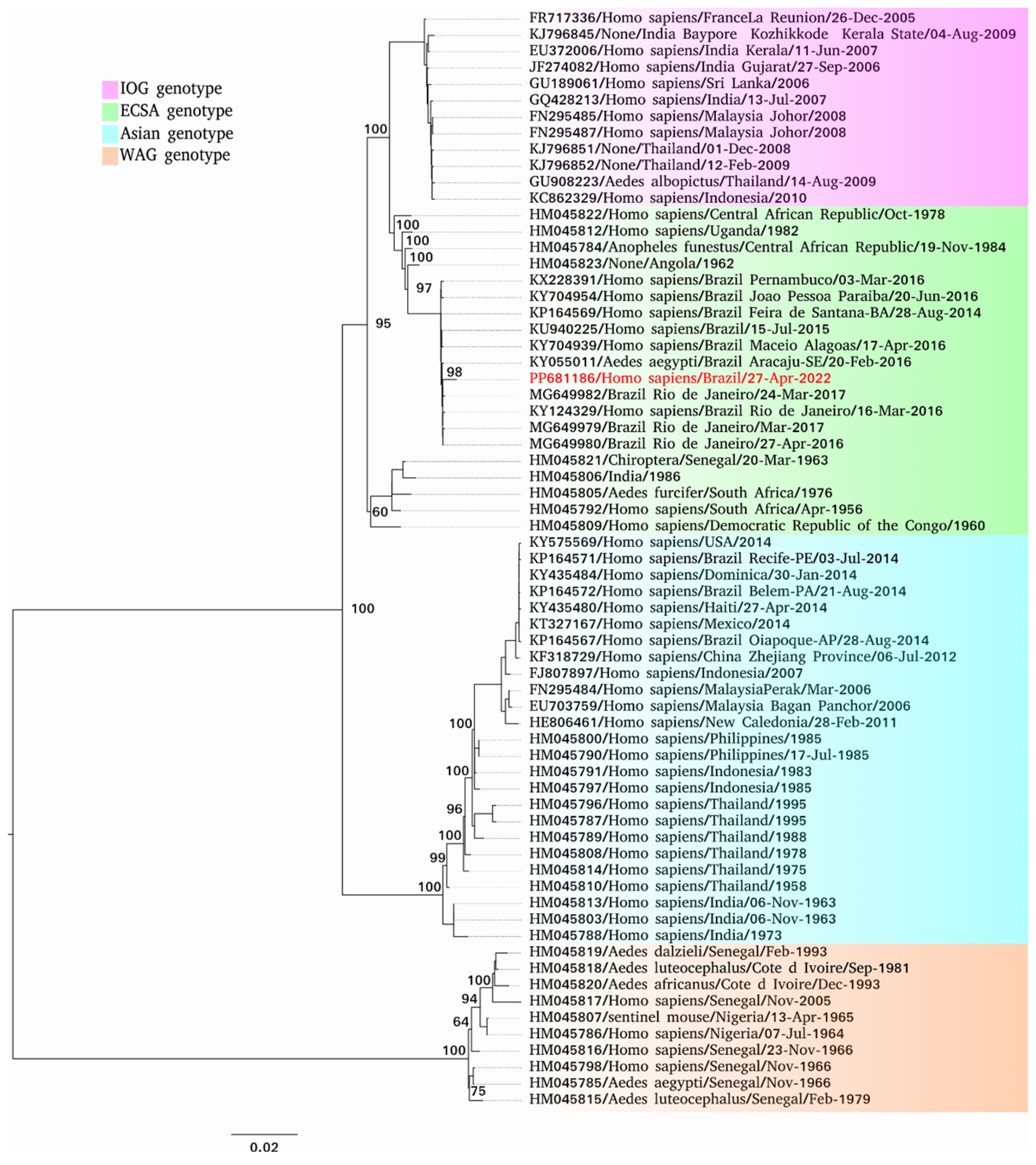
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation (WHO). Global Arbovirus Initiative: A Joint Initiative of the Health Emergencies Programme, the Department of Neglected Tropical Diseases Control, and the Department of Immunisations, Vaccines and Biological Products. 2024. Available online: https://www.who.int/initiatives/global-learning-opportunities-for-vaccine-quality/global-arbovirus-initiative (accessed on 21 February 2025).
- Organização Pan-Americana da Saúde (OPAS). OPAS Destaca aumento de Casos de Dengue, Oropouche e Influenza Aviária nas Américas e Recomenda Medidas de Controle. Available online: https://www.paho.org/pt/noticias/10-12-2024-opas-destaca-aumento-casos-dengue-oropouche-e-gripe-aviaria-nas-americas-e (accessed on 25 January 2025).
- Thompson, R.; Martin Del Campo, J.; Constenla, D. A review of the economic evidence of Aedes-borne arboviruses and Aedes-borne arboviral disease prevention and control strategies. Expert Rev. Vaccines 2020, 19, 143–162. [Google Scholar] [PubMed]
- Pan-American Health Organization-PAHO. Strategy for the prevention and control of arboviroses: Final Report, 58 Board of Directors. In Proceedings of the 72nd Session of the WHO Regional Committee for the Americas, Washington, DC, USA, 28–29 September 2020. [Google Scholar]
- Raghwani, J.; Rambaut, A.; Holmes, E.C.; Hang, V.T.; Hien, T.T.; Farrar, J.; Wills, B.; Lennon, N.J.; Birren, B.W.; Henn, M.R.; et al. Endemic dengue associated with co-circulation of multiple viral lineages and density-dependent localized transmission. PLoS Pathog. 2011, 7, e100. [Google Scholar] [CrossRef]
- Gubler, D.J. Arboviruses as agents of imported diseases: The need for greater awareness. Arch. Virol. Suppl. 1996, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Chang, G.J.; Lanciotti, R.S.; Kinney, R.M.; Mayer, L.W.; Trent, D.W. Phylogenetic relationships of dengue virus 2. Virologia 1993, 197, 216–224. [Google Scholar] [CrossRef]
- Chen, R.; Vasilakis, N. Dengue—Quo tu et quo vadis? Viruses 2011, 3, 1562–1608. [Google Scholar] [CrossRef]
- Marinho, R.D.S.S.; Duro, R.L.S.; Santos, G.L.; Hunter, J.; Teles, M.d.A.R.; Brustulin, R.; Milagres, F.A.d.P.; Sabino, E.C.; Diaz, R.S.; Komninakis, S.V. Detection of coinfection with Chikungunya virus and Dengue virus serotype 2 in serum samples of patients in State of Tocantins, Brazil. Infect. Public Health 2020, 13, 724–729. [Google Scholar] [CrossRef]
- Monitoramento dos Casos de Arboviroses até a Semana Epidemiológica 52 de 2022. Brasil. 2022. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2023/boletim-epidemiologico-volume-54-no-01/view (accessed on 4 March 2025).
- Ministério da Saúde. Painel Informativo de Arbovírus. Brasil. 2022. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/aedes-aegypti/monitoramento-das-arboviroses (accessed on 26 December 2023).
- Ministério da Saúde. Painel de Monitoramento das Arboviroses. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/a/arboviroses (accessed on 4 March 2025).
- Aragão, C.F.; Pinheiro, V.C.S.; Neto, J.P.N.; da Silva, E.V.P.; Pereira, G.J.G.; Nascimento, B.L.S.D.; Castro, K.d.S.; Maia, A.M.; Catete, C.P.; Martins, L.C.; et al. Natural Infection of Aedes aegypti by Chikungunya and Dengue type 2 Virus in a Transition Area of North-Northeast Brazil. Viruses 2019, 11, 1126. [Google Scholar] [CrossRef]
- da Silva Sousa, S.S.; da Silva, B.P.; Tadei, W.P.; da Silva, J.S.; Bezerra JM, T.; Pinheiro VC, S. Perfil reprodutivo de Aedes aegypti e Aedes albopictus de uma área urbana endêmica para arboviroses da região Nordeste do Brasil. Res. Soc. Dev. 2021, 10, e6310917631. [Google Scholar]
- Sousa, M.M.F.; Melo, B.d.O.d.; Costa, A.K.S.; Nunes, J.P.P.; Monteiro, S.G.; Carvalho, A.C.P.; Pinto, C.d.M.F.d.S.; Cosme, L.M.S.S.; Turri, R.d.J.G.; Teixeira, M.M.; et al. Detection and differentiation of dengue virus serotypes by one-step multiplex reverse transcription PCR assays. Braz. J. Dev. 2020, 6, 227–246. [Google Scholar] [CrossRef]
- Mehta, N.; Perrais, B.; Martin, K.; Kumar, A.; Hobman, T.C.; Cabalfin-Chua, M.N.; Donaldo, M.E.; Painaga, M.S.S.; Gaite, J.Y.; Tran, V.; et al. A direct from blood/plasma reverse transcription–polymerase chain reaction for dengue virus detection in point-of-care settings. Am. J. Trop. Med. Hyg. 2019, 100, 1534–1540. [Google Scholar] [CrossRef]
- Nunes, J.P.P.; Melo, B.d.O.d.; Monteiro, S.G.; Almeida, V.d.S.S.; Monteiro, A.d.S.; Cosme, L.M.S.S.; Pinto, C.d.M.F.d.S.; Silva, L.M.S.; Turri, R.d.J.G.; Mendes, M.d.F.C.; et al. One-Step reverse transcriptase PCR for detection of arboviruses in serum samples of patients assisted in Basic health Units in the State of Maranhão, Brazil. Braz. J. Dev. 2019, 5, 16620–16644. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística—IBGE. Estados: Maranhão, Brasil. 2022. Available online: https://cidades.ibge.gov.br/ (accessed on 10 November 2022).
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Secretaria Estadual de Saúde do Maranhão. 52° Boletim Epidemiológico de Arboviroses. Brasil. 2022. Available online: https://www.saude.ma.gov.br/boletins-epidemiologicos-arboviroses/ (accessed on 4 March 2025).
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya virus in us travelers returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef]
- Faye, O.; Faye, O.; Diallo, D.; Diallo, M.; Weidmann, M.; Sall, A.A. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught Mosquitoes. Virol. J. 2013, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Travassos da Rosa, A.P.A.; Travassos da Rosa, E.S.; Travassos da Rosa, J.F.S.; Dégallier, N.; Vasconcelos, P.D.C.; Rodrigues, S.G.; Cruz, A.C.R. Os arbovírus no Brasil: Generalidades, Métodos e Técnicas de Estudo: Documento Técnico no 2 Instituto Evandro Chagas, Fundação Nacional de Saúde. Ministério da Saúde: Belém, Brazil, 1998; p. 46. [Google Scholar]
- Bankevich, A.; Kolmogorov, M.; Antipov, D.; Pevzner, P.A. Montagem de leituras longas e precisas usando gráficos multiplex de Bruijn. bioRxiv 2020, 420448. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar]
- Myung, I.J. Tutorial on maximum likelihood estimation. J. Math. Psychol. 2003, 47, 90–100. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Barreto, F.A.; França, J.M.S.d.F.; Miro, V.H.; Santos, A. Pós Pandemia, Extrema Pobreza cai à Metade no Brasil, e NE é 50% da redução. Blog do IBRE. 24 June 2024. Available online: https://blogdoibre.fgv.br/posts/pos-pandemia-extrema-pobreza-cai-metade-no-brasil-e-ne-e-50-da-reducao#:~:text=Economia%20Regional-,P%C3%B3s%20pandemia%2C%20extrema%20pobreza%20cai%20%C3%A0%20metade%20no%20Brasil%2C%20e,NE%20%C3%A9%20 (accessed on 10 August 2024).
- Araújo, J.M.G.D.; Bello, G.; Romero, H.; Nogueira, R.M.R. Origin and evolution of dengue virus type 3 in brazil. PLoS Negl. Trop. Dis. 2012, 6, e1784. [Google Scholar] [CrossRef]
- de Bruycker-Nogueira, F.; Souza, T.M.A.; Chouin-Carneiro, T.; Faria, N.R.d.C.; Santos, J.B.; Torres, M.C.; Ramalho, I.L.C.; de Aguiar, S.F.; Nogueira, R.M.R.; de Filippis, A.M.B.; et al. DENV-1 Genotype V in Brazil: Spatiotemporal dispersion pattern reveals continuous co-circulation of distinct lineages until 2016. Sci. Rep. 2018, 8, 17160. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, H.; Moreno, K.; Lima, I.A.B.; Santos, C.S.; Costa, B.G.G.; de Almeida, B.L.; dos Santos, R.A.; Francisco, M.V.L.d.O.; Sampaio, M.P.S.; de Lima, M.M.; et al. Phylogenetic Reconstructions Reveal the Circulation of a Novel Dengue Virus-1v Clade and the Persistence of a Dengue Virus-2 iii Genotype in Northeast Brazil. Viruses 2023, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Santiago, G.A.; Maito, R.M.; Meneses, C.A.R.; Nascimento, V.A.D.; de Souza, V.C.; Nascimento, F.O.D.; Silva, D.; Mejía, M.; Gonçalves, L.; et al. Reemergence of Dengue Virus Serotype 3, Brazil, 2023. Emerg. Infect. Dis 2023, 29, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.T.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.; de Oliveira, L.F.; Azevedo, R.D.S.d.S.; da Silva, D.E.A.; da Silva, E.V.P.; da Silva, S.P.; et al. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef]
- Tanabe, E.L.d.L.; Tanabe, I.S.B.; dos Santos, E.C.; Marques, J.P.d.S.; Borges, A.A.; de Lima, M.C.; Anderson, L.; Bassi, Ê.J. Report of East-Central South African Chikungunya virus genotype during the 2016 outbreak in the Alagoas State, Brazil. Rev. Inst. Med. Trop. São Paulo 2018, 60, e19. [Google Scholar] [CrossRef]
- Cardoso, F.D.; de Rezende, I.M.; Barros, E.L.T.; Sacchetto, L.; Garcês, T.C.d.C.S.; Silva, N.I.O.; Alves, P.A.; Soares, J.O.; Kroon, E.G.; Pereira, A.C.T.d.C.; et al. Circulation of chikungunya virus East-Central-South Africa genotype during an outbreak in 2016-17 in Piaui State, Northeast Brazil. Rev. Inst. Med. Trop. São Paulo 2019, 61, e57. [Google Scholar] [CrossRef]
- Xavier, J.; Giovanetti, M.; Fonseca, V.; Thézé, J.; Gräf, T.; Fabri, A.; de Jesus, J.G.; de Mendonça, M.C.L.; Rodrigues, C.D.d.S.; Mares-Guia, M.A.; et al. Circulation of chikungunya virus East/Central/South African lineage in Rio de Janeiro, Brazil. PLoS ONE 2019, 14, e0217871. [Google Scholar] [CrossRef]
- Cruz, A.C.R.; Neto, J.P.N.; da Silva, S.P.; da Silva, E.V.P.; Pereira, G.J.G.; Santos, M.M.; Monteiro, H.A.d.O.; dos Santos, F.B.; Guimarães, R.J.d.P.S.e.; Aragão, C.F.; et al. Chikungunya virus detection in Aedes aegypti and Culex quinquefasciatus during an outbreak in the amazon region. Viruses 2020, 12, 853. [Google Scholar] [CrossRef]
- dos Santos Andrade, A.T.; Bezerra, J.M.T.; Pinheiro, V.C.S. Characterization of the Proliferation Sites of Aedes aegypti (Diptera: Culicidae) in the Artificial Breeding Sites of Caxias, Maranhão, Brazil. In Life Cycle and Development of Diptera; IntechOpen: London, UK, 2019. [Google Scholar]
- Neto, J.P.N.; Reis, L.A.M.; Freitas, M.N.O.; Nascimento, B.L.S.D.; das Chagas, L.L.; da Costa, H.H.M.; Rodrigues, J.C.P.; Braga, C.M.; da Silva, E.V.P.; Silva, S.P.; et al. First Isolation and Genome Sequence Analysis of West Nile Virus in Mosquitoes in Brazil. Trop. Med. Infect. Dis 2023, 8, 237. [Google Scholar] [CrossRef]
- Ramos, B.A.; Das Chagas, L.L.; Silva, F.d.A.e.; dos Santos, E.B.; Chiang, J.O.; Neto, J.P.N.; Vieira, D.B.R.; Junior, J.W.R.; da Silva, E.V.P.; Freitas, M.N.O.; et al. Arboviruses in Free-Ranging Birds and Hematophagous Arthropods (Diptera, Nematocera) from Forest Remnants and Urbanized Areas of an Environmental Protection Area in the Amazon Biome. Viruses 2022, 14, 2101. [Google Scholar] [CrossRef]
- Vieira, M.A.C.S.; Romano, A.P.M.; Borba, A.S.; Silva, E.V.P.; Chiang, J.O.; Eulálio, K.D.; Azevedo, R.S.S.; Rodrigues, S.G.; Almeida-Neto, W.S.; Vasconcelos, P.F.C. West Nile Virus encephalitis: The first human case recorded in Brazil. Am. J. Trop. Med. Hyg. 2015, 93, 377. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Lee, S.; Lana, R.M.; Codeço, C.T.; Castro, M.C.; Pascual, M. Emerging arboviruses in the urbanized Amazon rainforest. BMJ 2020, 371, m4385. [Google Scholar] [PubMed]
- Departamento Nacional de Infraestrutura de Transportes (DNIT). Pavimentação da BR-316 no Estado de Alagoas Prevê Sete Novas Pontses. Available online: https://www.gov.br/dnit/pt-br/assuntos/noticias/pavimentacao-da-br-316-no-estado-de-alagoas-preve-sete-novas-pontes (accessed on 15 August 2024).
| Viral Serotype | Nucleotide Sequence (5′–3′) | Frequency | Genomic Position |
|---|---|---|---|
| DENV-1 F | CAAAAGGAAGTCGYGCAATA | 48/48 | 8936–8955 |
| DENV-1 R | CTGAGTGAATTCTCTCTGCTRAAC | 48/48 | 9023–9047 |
| Probe DENV-1 | FAM-CATGTGGYTGGGAGCRCGC-BHQ1 | 48/48 | 8961–8979 |
| DENV-2 F | CAGGCTATGGCACYGTCACGAT | 40/50 | 1426–1447 |
| DENV-2 R | CCATYTGCAGCARCACCATCTC | 48/50 | 1482–1504 |
| Probe DENV-2 | VIC—CTCYCCRAGAACGGGCCTCGACTTCAA-BHQ1 | 43/50 | 1454–1480 |
| DENV-3 F | GGACTRGACACACGCACCCA | 50/51 | 701–720 |
| DENV-3 R | CATGTCTCTACCTTCTCGACTTGYCT | 51/51 | 749–775 |
| Probe DENV-3 | Texas Red–ACCTGGATGTCGGCTGAAGGAGCTTG-BHQ2 | 50/51 | 722–747 |
| DENV-4 F | TTGTCCTAATGATGCTRGTCG | 17/18 | 884–904 |
| DENV-4 R | TCCACCYGAGACTCCTTCCA | 18/18 | 953–973 |
| Probe DENV-4 | Cy5–TYCCTACYCCTACGCATCGCATTCCG-BHQ2 | 18/18 | 939–965 |
| Name | Nucleotide Sequence (5′–3′) | Genomic Position |
| CHIKV NSP1 874F | AAAGGGCAAACTCAGCTTCAC | 874–894 |
| CHIKV NSP1 961R | GCCTGGGCTCATCGTTATTC | 961–942 |
| CHIKV NSP1 899p | FAM-CGCTGTGATACAGTGGTTTCGTGTG | 899–923 |
| Name | Nucleotide Sequence (5′–3′) | Genomic Position |
| ZIKV NS5 F | AARTACACATACCARAACAAAGTG G | 9271–9297 |
| ZIKV NS5 R | TCCRCTCCCYCTYTGGTCTTG | 9352–9373 |
| Probe ZIKV | FAM—CTYAGACCAGCTGAAR-BBQ | 9304–9320 |
| Municipality | DENV-1 (N/%) | DENV-2 (N/%) | DENV-3 (N/%) | CHIKV (N/%) | Samples Positive |
|---|---|---|---|---|---|
| Caxias | 57 (79.1%) | 4 (5.5%) | 1 (1.4%) | 6 (8.4%) | 68 (94.4%) |
| Codó | 2 (2.8%) | 0 | 0 | 0 | 2 (2.8%) |
| Peritoró | 1 (1.4%) | 0 | 0 | 0 | 1 (1.4%) |
| S. Mateus | 1 (1.4%) | 0 | 0 | 0 | 1 (1.4%) |
| Total | 61 (84.7%) | 4 (5.5%) | 1 (1.4%) | 6 (8.4%) | 72 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.S.d.S.; Cruz, A.C.R.; Aragão, C.F.; Cereja, G.J.G.P.; Silva, S.P.d.; Sousa, R.M.M.d.; Amorim, M.T.; da Silva, E.V.P.; Nunes, B.T.D.; Pinheiro, V.C.S. Retrospective Study of Arbovirus Circulation in Northeast Brazil in 2019 and 2022: Insights into the Re-Emergence of DENV-3 and the Co-Infection of DENV-1 and CHIKV. Viruses 2025, 17, 475. https://doi.org/10.3390/v17040475
Sousa SSdS, Cruz ACR, Aragão CF, Cereja GJGP, Silva SPd, Sousa RMMd, Amorim MT, da Silva EVP, Nunes BTD, Pinheiro VCS. Retrospective Study of Arbovirus Circulation in Northeast Brazil in 2019 and 2022: Insights into the Re-Emergence of DENV-3 and the Co-Infection of DENV-1 and CHIKV. Viruses. 2025; 17(4):475. https://doi.org/10.3390/v17040475
Chicago/Turabian StyleSousa, Sêmilly Suélen da Silva, Ana Cecília Ribeiro Cruz, Carine Fortes Aragão, Glennda Juscely Galvão Pereira Cereja, Sandro Patroca da Silva, Raira Maria Morais de Sousa, Murilo Tavares Amorim, Eliana Vieira Pinto da Silva, Bruno Tardelli Diniz Nunes, and Valéria Cristina Soares Pinheiro. 2025. "Retrospective Study of Arbovirus Circulation in Northeast Brazil in 2019 and 2022: Insights into the Re-Emergence of DENV-3 and the Co-Infection of DENV-1 and CHIKV" Viruses 17, no. 4: 475. https://doi.org/10.3390/v17040475
APA StyleSousa, S. S. d. S., Cruz, A. C. R., Aragão, C. F., Cereja, G. J. G. P., Silva, S. P. d., Sousa, R. M. M. d., Amorim, M. T., da Silva, E. V. P., Nunes, B. T. D., & Pinheiro, V. C. S. (2025). Retrospective Study of Arbovirus Circulation in Northeast Brazil in 2019 and 2022: Insights into the Re-Emergence of DENV-3 and the Co-Infection of DENV-1 and CHIKV. Viruses, 17(4), 475. https://doi.org/10.3390/v17040475








