Chronic Hepatitis B in the Transplant Setting: A 30-Year Experience in a Single Tertiary Italian Center
Abstract
1. Introduction
- -
- to assess overall long-term patient and graft survival in HBV patients who underwent a LT over the past 30 years;
- -
- to analyze trends in waiting list (WL) inclusion and outcomes for HBV patients over the last 15 years in a tertiary center in Italy.
2. Materials and Methods
2.1. Data Collection and Study Design
- -
- HBV and non-HBV patients;
- -
- HBV-monoinfected and HDV-coinfected patients;
- -
- HBV-Dec and HBV-HCC patients.
- -
- HBV and non-HBV patients;
- -
- HBV-monoinfected and HDV-coinfected patients;
- -
- HBV-Dec and HBV-HCC patients;
- -
- Patients waitlisted between 2006–2013 and 2014–2020.
2.2. Patients’ Management After LT
2.3. Statistical Analysis
3. Results
3.1. Patients Undergoing Liver Transplantation Between 1991 and 2020
3.1.1. Pre- and Post-Transplant Patients’ Characteristics
3.1.2. Outcome After LT
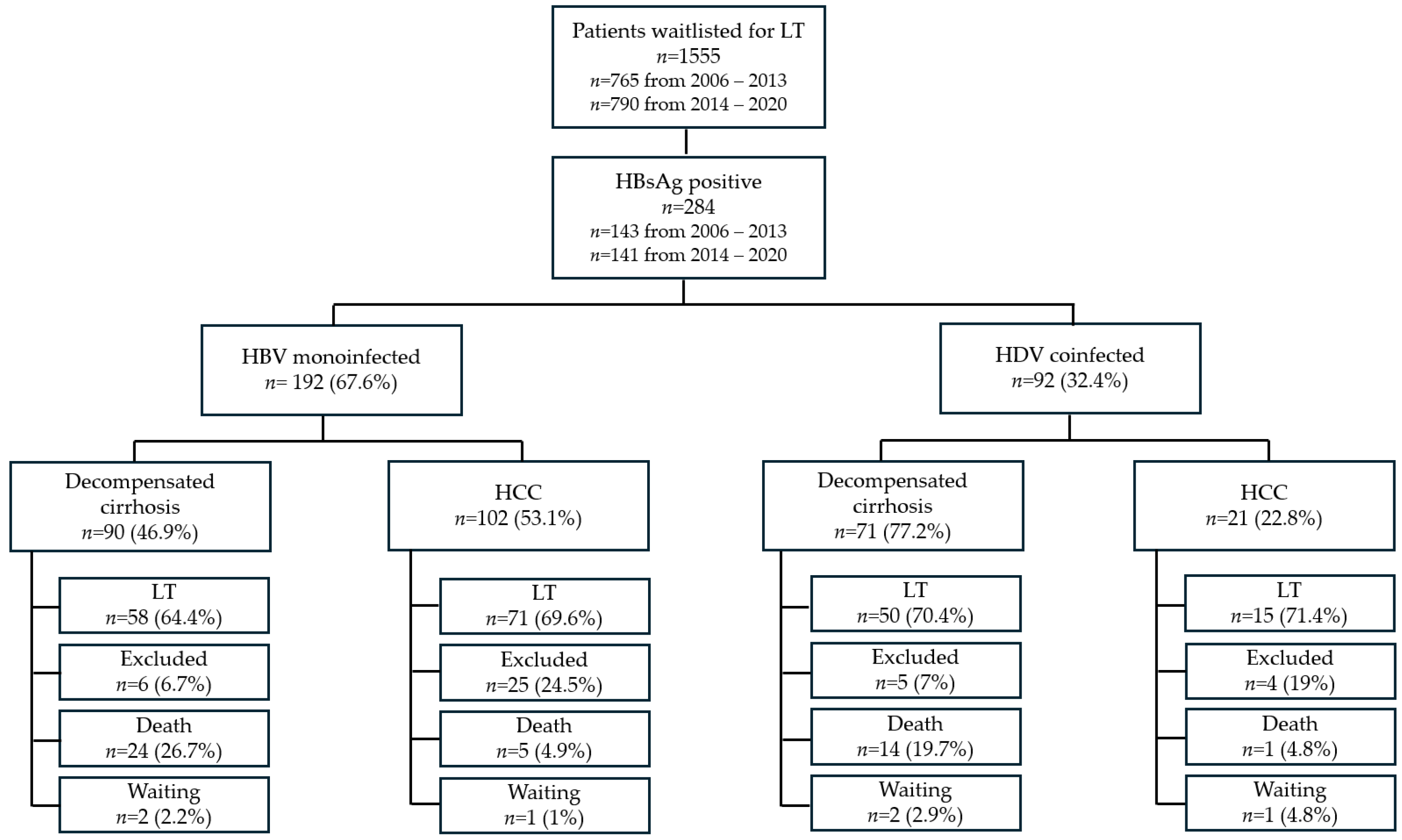
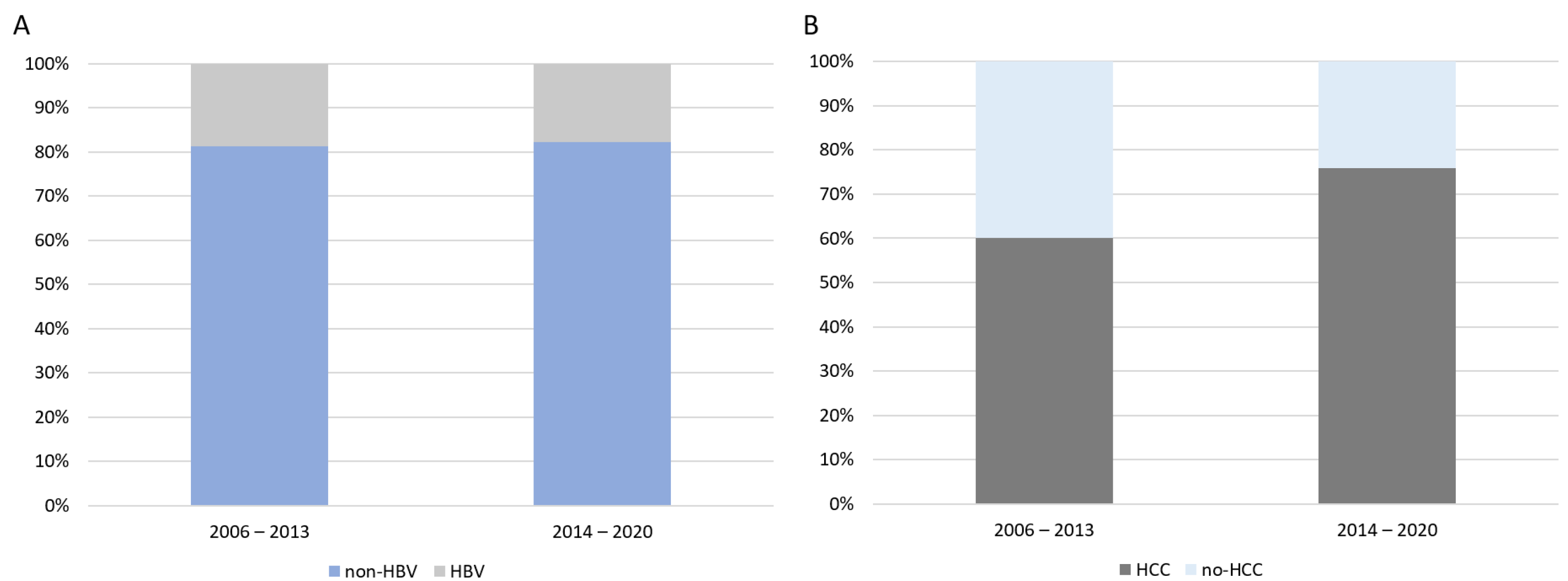
3.2. Analysis of the Waiting List Trends Between 2006 and 2020
3.2.1. Characteristics of HBV-Monoinfected Patients at WL Inclusion
3.2.2. Characteristics of HDV-Coinfected Patients at WL Inclusion
3.2.3. Trajectories of WL Inclusion
3.2.4. The Impact of hbNUCs on Patients Waitlisted for LT
3.2.5. Outcome of Patients After WL Inclusion
3.2.6. HBV-Monoinfected Patients
3.2.7. HDV-Coinfected Patients
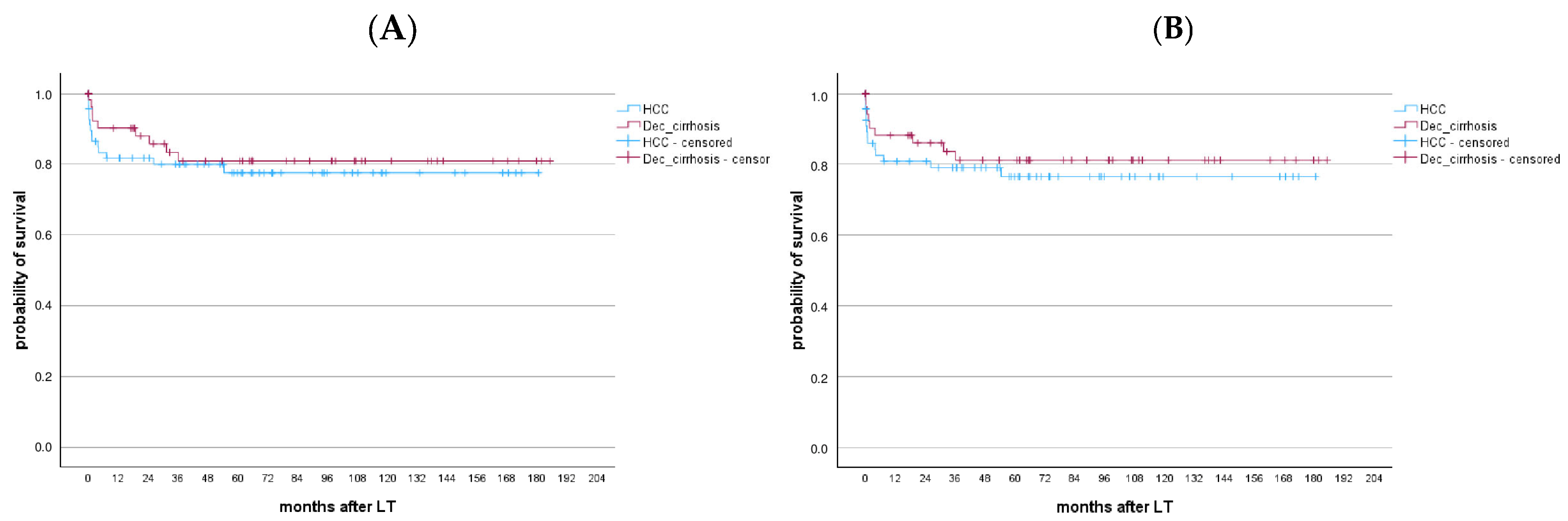
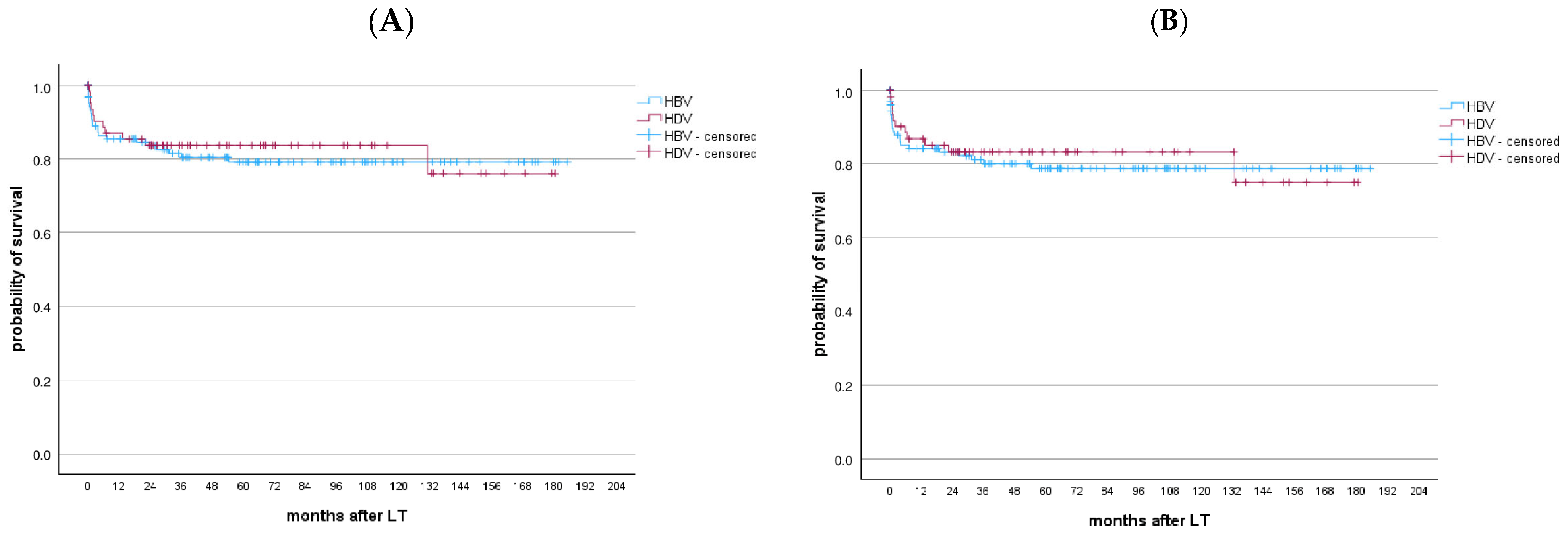
3.2.8. Long-Term Outcomes After LT
3.2.9. HBV-Monoinfected Patients
3.2.10. HDV-Coinfected Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HBV | hepatitis B virus |
| HDV | hepatitis delta virus |
| CHB | chronic hepatitis B |
| LT | liver transplantation |
| HCC | hepatocellular carcinoma |
| NUCs | nucleos(t)ide analogues |
| hbNUCs | high-barrier nucleos(t)ide analogues |
| lbNUCs | low-barrier nucleos(t)ide analogues |
| WL | waiting list |
| HBV-Dec | HBV-related decompensated cirrhosis |
| HBV-HCC | HBV-related hepatocellular carcinoma |
| MELD | model for end-stage liver disease score |
| CTP | Child–Turcotte–Pugh score |
| BMI | body mass index |
| IS | immunosuppressive |
| IQR | interquartile ranges |
| LAM | lamivudine |
| ETV | entecavir |
| ADV | adefovir |
| TAF | tenofovir alafenamide |
| TDF | tenofovir disoproxil |
References
- Burra, P.; Germani, G.; Adam, R.; Karam, V.; Marzano, A.; Lampertico, P.; Salizzoni, M.; Filipponi, F.; Klempnauer, J.L.; Castaing, D.; et al. Liver transplantation for HBV-related cirrhosis in Europe: An ELTR study on evolution and outcomes. J. Hepatol. 2012, 58, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M. Hepatocellular Carcinoma: Epidemiology, Surveillance, and Diagnosis. Semin. Liver Dis. 2010, 30, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Raffetti, E.; Fattovich, G.; Donato, F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis. Liver Int. 2016, 36, 1239–1251. [Google Scholar] [CrossRef]
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016, 64, S84–S101. [Google Scholar] [CrossRef]
- Mederacke, I.; Filmann, N.; Yurdaydin, C.; Bremer, B.; Puls, F.; Zacher, B.J.; Heidrich, B.; Tillmann, H.L.; Rosenau, J.; Bock, C.-T.; et al. Rapid early HDV RNA decline in the peripheral blood but prolonged intrahepatic hepatitis delta antigen persistence after liver transplantation. J. Hepatol. 2012, 56, 115–122. [Google Scholar] [CrossRef]
- Niro, G.A.; Smedile, A.; Ippolito, A.M.; Ciancio, A.; Fontana, R.; Olivero, A.; Valvano, M.R.; Abate, M.L.; Gioffreda, D.; Caviglia, G.P.; et al. Outcome of chronic delta hepatitis in Italy: A long-term cohort study. J. Hepatol. 2010, 53, 834–840. [Google Scholar] [CrossRef]
- Lok, A.S.; McMahon, B.J.; Brown, R.S.; Wong, J.B.; Ahmed, A.T.; Farah, W.; Almasri, J.; Alahdab, F.; Benkhadra, K.; Mouchli, M.A.; et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 2015, 63, 284–306. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Chien, R.-N.; Liaw, Y.-F. Hepatitis B virus-related decompensated liver cirrhosis: Benefits of antiviral therapy. J. Hepatol. 2012, 57, 442–450. [Google Scholar] [CrossRef]
- Jang, J.W.; Choi, J.Y.; Kim, Y.S.; Woo, H.Y.; Choi, S.K.; Lee, C.H.; Kim, T.Y.; Sohn, J.H.; Tak, W.Y.; Han, K. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus–related cirrhosis. Hepatology 2015, 61, 1809–1820. [Google Scholar] [CrossRef]
- Katz, L.H.; Paul, M.; Guy, D.G.; Tur-Kaspa, R. Prevention of recurrent hepatitis B virus infection after liver transplantation: Hepatitis B immunoglobulin, antiviral drugs, or both? Systematic review and meta-analysis: Post liver transplant hepatitis B prophylaxis. Transpl. Infect. Dis. 2010, 12, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Battistella, S.; Zanetto, A.; Gambato, M.; Germani, G.; Senzolo, M.; Burra, P.; Russo, F.P. The Role of Antiviral Prophylaxis in Preventing HBV and HDV Recurrence in the Setting of Liver Transplantation. Viruses 2023, 15, 1037. [Google Scholar] [CrossRef]
- Russo, F.P.; Viganò, M.; Stock, P.; Ferrarese, A.; Pugliese, N.; Burra, P.; Aghemo, A. HBV-positive and HIV-positive organs in transplantation: A clinical guide for the hepatologist. J. Hepatol. 2022, 77, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Belli, L.S.; Berenguer, M.; Cortesi, P.A.; Strazzabosco, M.; Rockenschaub, S.-R.; Martini, S.; Morelli, C.; Donato, F.; Volpes, R.; Pageaux, G.-P.; et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J. Hepatol. 2016, 65, 524–531. [Google Scholar] [CrossRef]
- Farinati, F.; Vitale, A.; Spolverato, G.; Pawlik, T.M.; Huo, T.; Lee, Y.-H.; Frigo, A.C.; Giacomin, A.; Giannini, E.G.; Ciccarese, F.; et al. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med. 2016, 13, e1002006. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Cailliez, V.; O’Grady, J.G.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Flemming, J.A.; Kim, W.R.; Brosgart, C.L.; Terrault, N.A. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2017, 65, 804–812. [Google Scholar] [CrossRef]
- European Association for The Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Charlton, M.; Levitsky, J.; Aqel, B.; O’Grady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Filmann, N.; Adam, R.; Bachellier, P.; Bechstein, W.O.; Becker, T.; Bhoori, S.; Bilbao, I.; Brockmann, J.; Burra, P.; et al. mTOR Inhibition Is Most Beneficial After Liver Transplantation for Hepatocellular Carcinoma in Patients with Active Tumors. Ann. Surg. 2020, 272, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Terrault, N.A.; Francoz, C.; Berenguer, M.; Charlton, M.; Heimbach, J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin. Gastroenterol. Hepatol. 2023, 21, 2150–2166. [Google Scholar]
- Ferraro, D.; Urone, N.; Pizzillo, P.; Gussio, M.; Magliocco, S.; Cacopardo, B.; Craxì, A.; Di Marco, V.; Di Stefano, R. Phylogenetic analysis of isolates from new cases of HBV infection in Southern Italy. Infect. Genet. Evol. 2012, 12, 1591–1596. [Google Scholar] [CrossRef][Green Version]
- Study Group; Zuccaro, O.; Romanò, L.; Mele, A.; Mariano, A.; Clementi, M.; Tosti, M.E.; Taliani, G.; Galli, C.; Zanetti, A.R.; et al. Clinical, epidemiological and virological features of acute hepatitis B in Italy. Infection 2015, 43, 431–441. [Google Scholar] [CrossRef]
- Sagnelli, C.; Ciccozzi, M.; Coppola, N.; Minichini, C.; Presti, A.L.; Starace, M.; Alessio, L.; Macera, M.; Cella, E.; Gualdieri, L.; et al. Molecular diversity in irregular or refugee immigrant patients with HBV-genotype-E infection living in the metropolitan area of Naples. J. Med. Virol. 2016, 89, 1015–1024. [Google Scholar] [CrossRef]
- Battistella, S.; Grasso, M.; Catanzaro, E.; D’arcangelo, F.; Corrà, G.; Germani, G.; Senzolo, M.; Zanetto, A.; Ferrarese, A.; Gambato, M.; et al. Evolution of Liver Transplantation Indications: Expanding Horizons. Medicina 2024, 60, 412. [Google Scholar] [CrossRef]
- Asselah, T.; Rizzetto, M. Hepatitis D Virus Infection. N. Engl. J. Med. 2023, 389, 58–70. [Google Scholar]
- Rizzetto, M.; Hamid, S.; Negro, F. The changing context of hepatitis D. J. Hepatol. 2021, 74, 1200–1211. [Google Scholar] [CrossRef]
- Fung, J.; Wong, T.; Chok, K.; Chan, A.; Cheung, T.; Dai, J.W.; Sin, S.; Ma, K.; Ng, K.; Ng, K.T.; et al. Long-term outcomes of entecavir monotherapy for chronic hepatitis B after liver transplantation: Results up to 8 years. Hepatology 2017, 66, 1036–1044. [Google Scholar] [CrossRef]
- Villeret, F.; Lebossé, F.; Radenne, S.; Samuel, D.; Roche, B.; Mabrut, J.-Y.; Leroy, V.; Pageaux, G.-P.; Anty, R.; Thevenon, S.; et al. Early intrahepatic recurrence of HBV infection in liver transplant recipients despite antiviral prophylaxis. JHEP Rep. 2023, 5, 100728. [Google Scholar] [CrossRef] [PubMed]


| Variables at WL Inclusion | HBV-Monoinfected Patients (n = 192) N (%), Median (IQR) | HDV-Coinfected Patients (n = 92) N (%), Median (IQR) | p Value |
|---|---|---|---|
| Sex, male | (85.9) | (57.6) | <0.001 |
| Age | 58 (52.25–63) | 54 (47–58) | <0.001 |
Indication for LT
| <0.001 | ||
| 102 (53.1) | 21 (22.8) | ||
| 90 (46.9) | 71 (77.2) | ||
| HCC, yes | 129 (67.2) | 36 (39.1) | <0.001 |
| Portal vein thrombosis | 30 (15.6) | 8 (8.7) | 0.223 |
| Refractory ascites | 32 (16.7) | 22 (23.9) | 0.145 |
| MELD (Dec_cirrhosis) | 18 (15–24) | 18 (15–23) | 0.485 |
| CTP (Dec_cirrhosis) | 10 (8–11) | 9 (9–11) | 0.905 |
| HBV DNA detectable | 42 (21.9%) | 26 (28.3) | 0.977 |
Antiviral therapy before LT, yes
| 171 (89) | 86 (93.5) | 0.249 |
| 44 (22.9) | 20 (21.7) | ||
| 82 (42.7) | 47 (51.1) | ||
| 38 (19.8) | 19 (20.7) | ||
| 7 (3.7) | 0 (0) |
| Variables at WL Inclusion | No Therapy (n = 26) N (%), Median (IQR) | lb-NUCs (n = 71) N (%), Median (IQR) | hb-NUCs (186) N (%), Median (IQR) | p Value |
|---|---|---|---|---|
| Sex, male | 22 (84.6) | 56 (78.9) | 139 (74.7) | 0.472 |
| Age | 56.5 (48.75–63.25) | 55 (48–60) | 57 (51–62) | 0.405 |
| HDV coinfection | 6 (23.1) | 20 (28.2) | 66 (35.5) | 0.299 |
| HBV DNA detectable | 8 (30.8) | 15 (21.1) | 45 (24.3) | 0.613 |
Indication for LT
| 8 (30.8) | 30 (42.3) | 84 (45.2) | 0.376 |
| 18 (69.2) | 41 (57.7) | 102 (54.8) | ||
| HCC, yes | 10 (38.5) | 41 (57.7) | 113 (60.8) | 0.098 |
| Portal vein thrombosis | 2 (7.7) | 10 (14.1) | 26 (14) | 0.139 |
| Refractory ascites | 5 (19.2) | 16 (22.5) | 33 (17.7) | 0.682 |
| MELD (Dec_cirrhosis) | 19 (17.25–28.5) | 16 (13–21) | 19 (16–24) | 0.043 |
| CTP (Dec_cirrhosis) | 9 (8–11.25) | 9 (8–11) | 10 (9–11) | 0.743 |
Outcome in WL
| 0.255 | |||
| 16 (61.5) | 48 (67.6) | 130 (69.9) | ||
| 7 (26.9) | 14 (19.7) | 23 (12.4) | ||
| 3 (11.5) | 9 (12.7) | 27 (14.5) | ||
| - | - | 6 (3.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, F.P.; Battistella, S.; Zanetto, A.; Gambato, M.; Ferrarese, A.; Germani, G.; Senzolo, M.; Mescoli, C.; Piano, S.; D’Amico, F.E.; et al. Chronic Hepatitis B in the Transplant Setting: A 30-Year Experience in a Single Tertiary Italian Center. Viruses 2025, 17, 454. https://doi.org/10.3390/v17040454
Russo FP, Battistella S, Zanetto A, Gambato M, Ferrarese A, Germani G, Senzolo M, Mescoli C, Piano S, D’Amico FE, et al. Chronic Hepatitis B in the Transplant Setting: A 30-Year Experience in a Single Tertiary Italian Center. Viruses. 2025; 17(4):454. https://doi.org/10.3390/v17040454
Chicago/Turabian StyleRusso, Francesco Paolo, Sara Battistella, Alberto Zanetto, Martina Gambato, Alberto Ferrarese, Giacomo Germani, Marco Senzolo, Claudia Mescoli, Salvatore Piano, Francesco Enrico D’Amico, and et al. 2025. "Chronic Hepatitis B in the Transplant Setting: A 30-Year Experience in a Single Tertiary Italian Center" Viruses 17, no. 4: 454. https://doi.org/10.3390/v17040454
APA StyleRusso, F. P., Battistella, S., Zanetto, A., Gambato, M., Ferrarese, A., Germani, G., Senzolo, M., Mescoli, C., Piano, S., D’Amico, F. E., Vitale, A., Gringeri, E., Feltracco, P., Angeli, P., Cillo, U., & Burra, P. (2025). Chronic Hepatitis B in the Transplant Setting: A 30-Year Experience in a Single Tertiary Italian Center. Viruses, 17(4), 454. https://doi.org/10.3390/v17040454











