Interspecies Differences in Cytomegalovirus Inhibition by Cardiac Glycosides—A Unique Role of the Alpha3 Isoform of the Na+/K+-ATPase Pump
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Viruses
2.3. Cell Culture, Virus Infection, and Antiviral Assays
2.4. Cytotoxicity Assay
2.5. Quantitative Reverse Transcriptase PCR (qRT-PCR)
2.6. SDS-Polyacrylamide Gel Electrophoresis and Immunoblot Analysis
2.7. Cellular Localization of α3
2.8. NLS Analysis of the α3 Isoform
2.9. Immunofluorescence Assay
2.10. Nuclear Coimmunoprecipitation and Immunoblotting
2.11. Statistical Analysis
3. Results
3.1. α3 mRNA Is Induced in GPCMV and HCMV-Infected Cells but Not in MCMV-Infected Cells
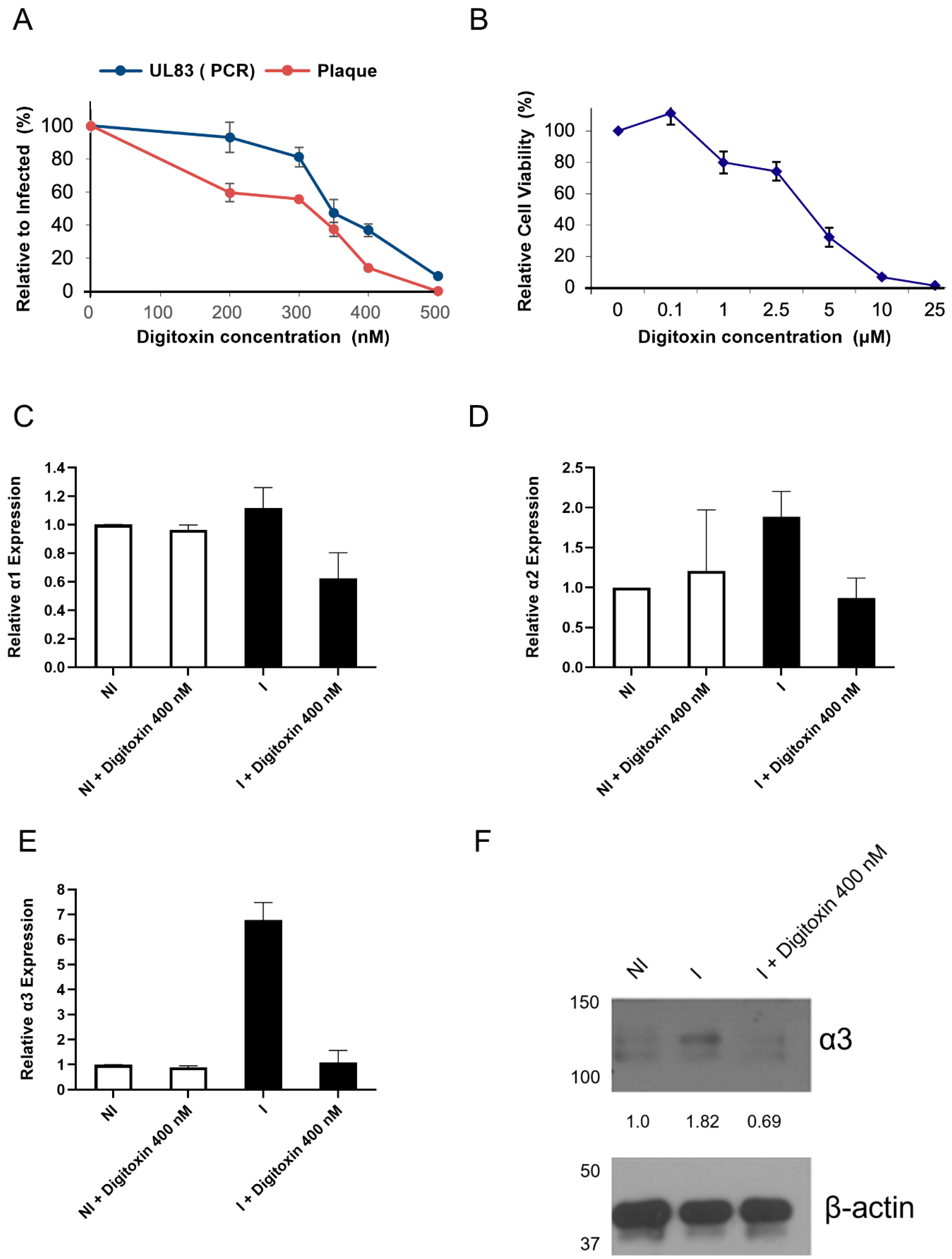
3.2. Inhibition of GPCMV Replication with Digitoxin Correlates with Reduced α3 Expression
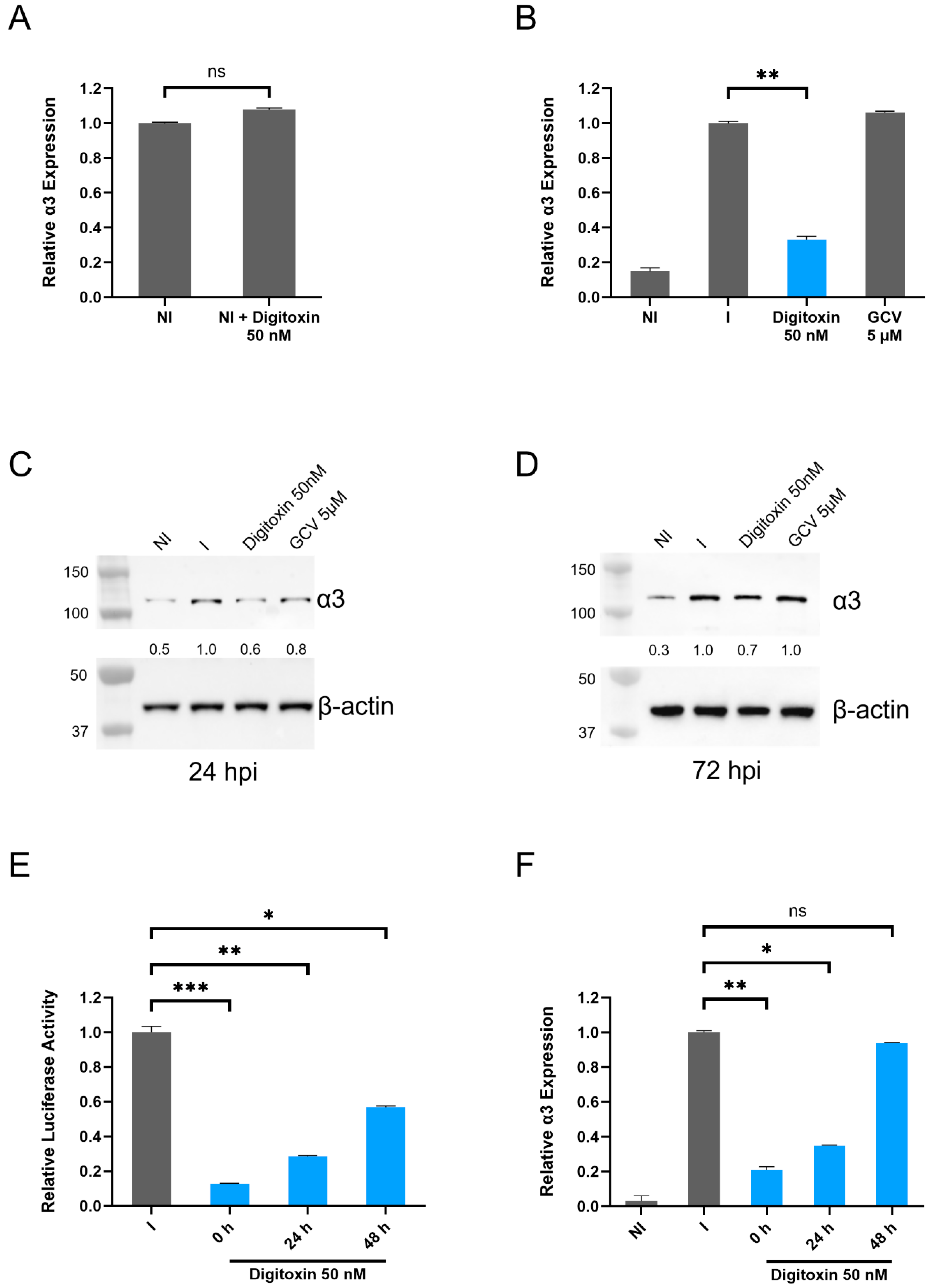
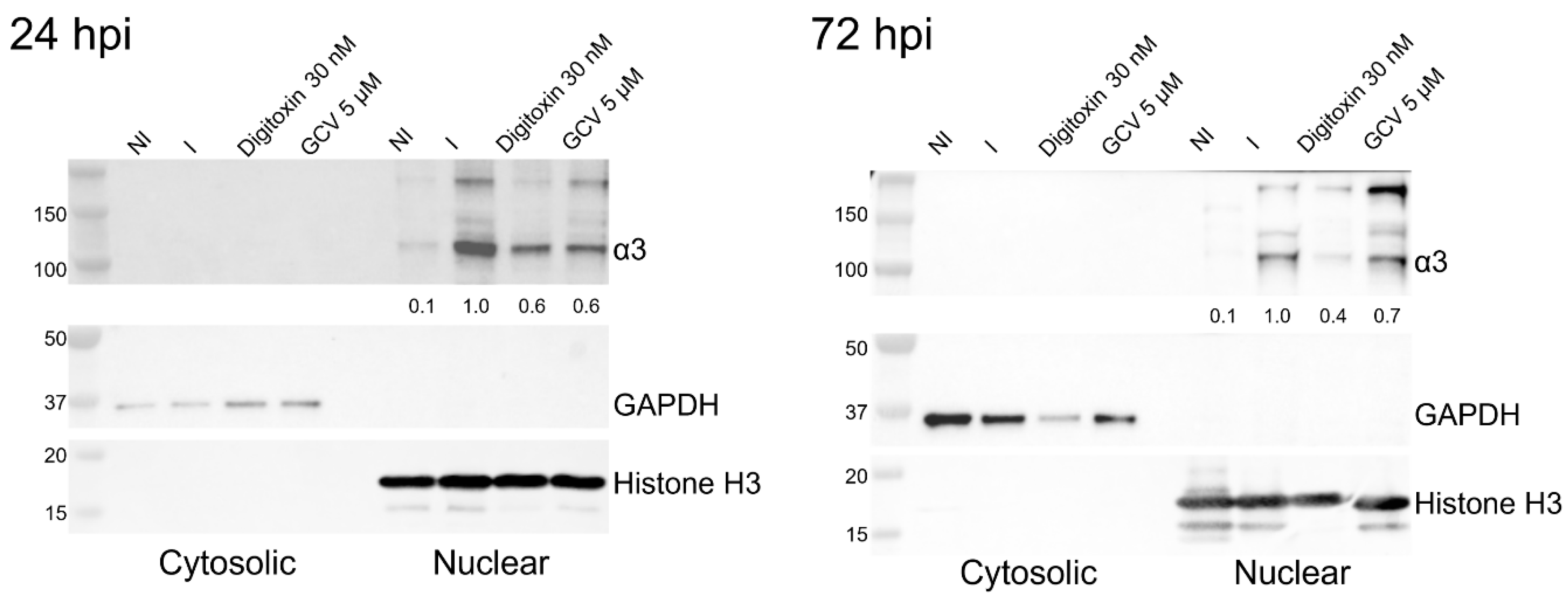
3.3. Levels of α3 mRNA and Protein Are Reduced in HCMV-Infected Digitoxin-Treated Cells
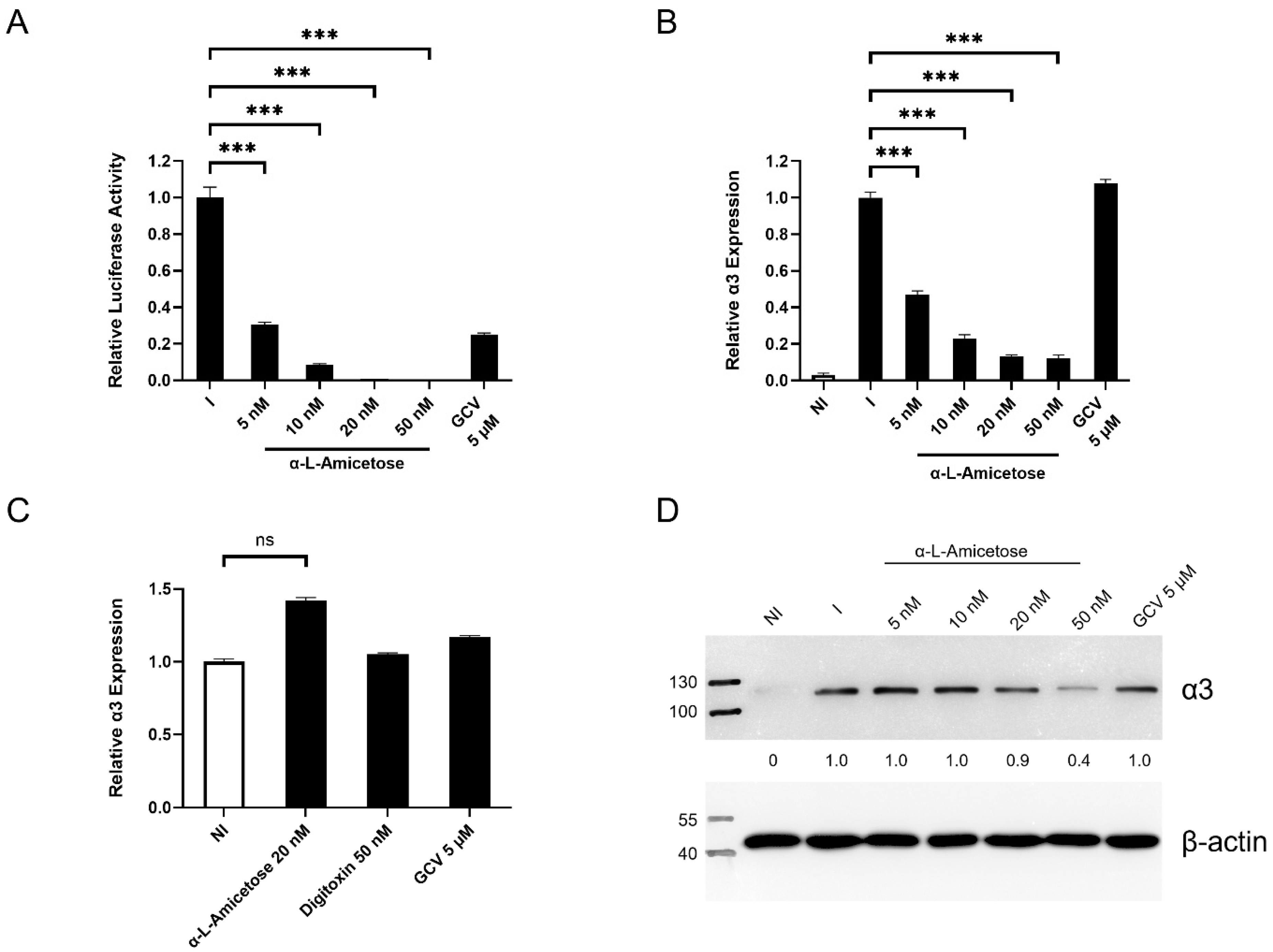
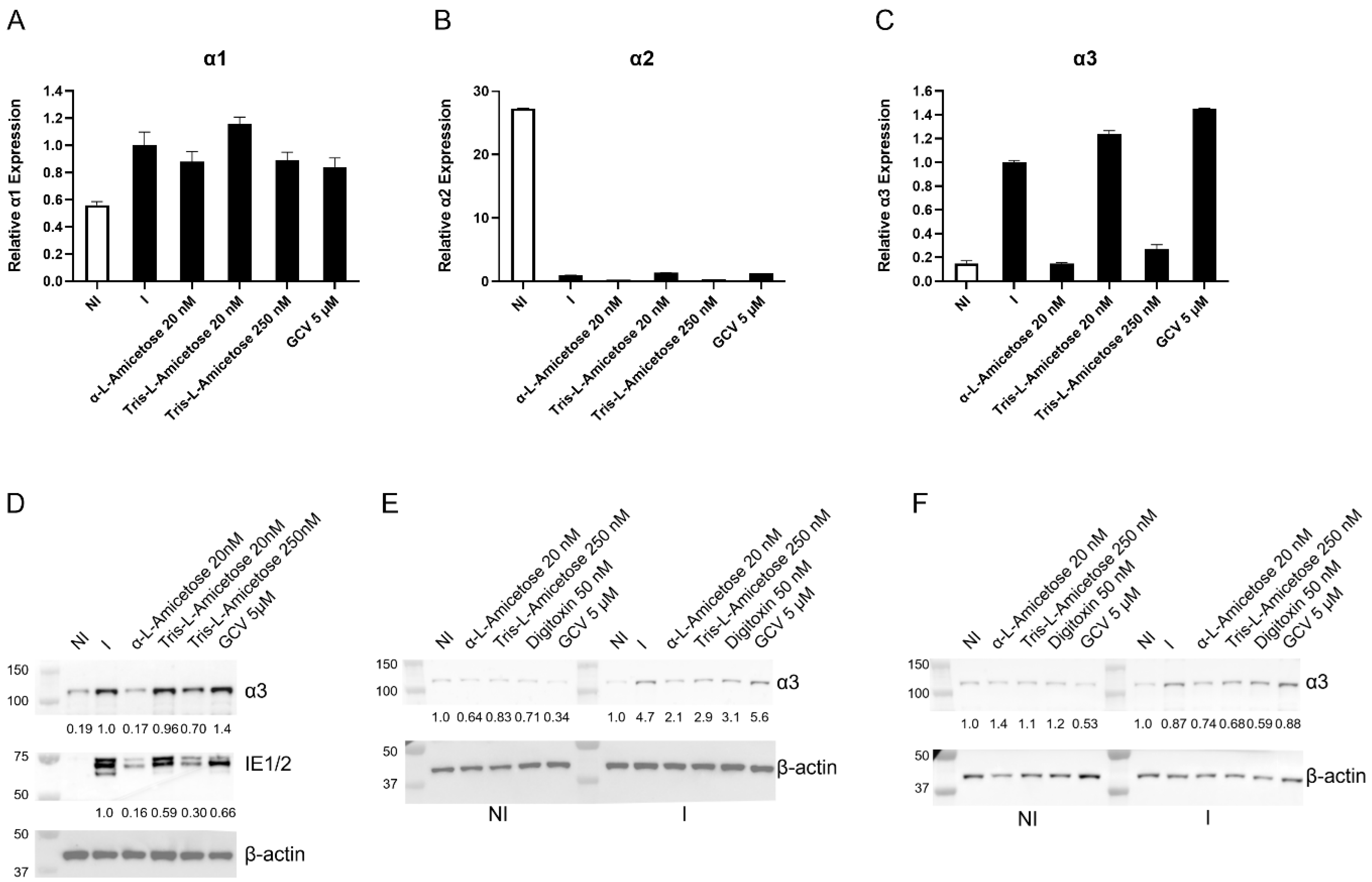
3.4. Concentration-Dependent Decrease in α3 Expression in HCMV-Infected HFFs Treated with Digitoxin Analogs
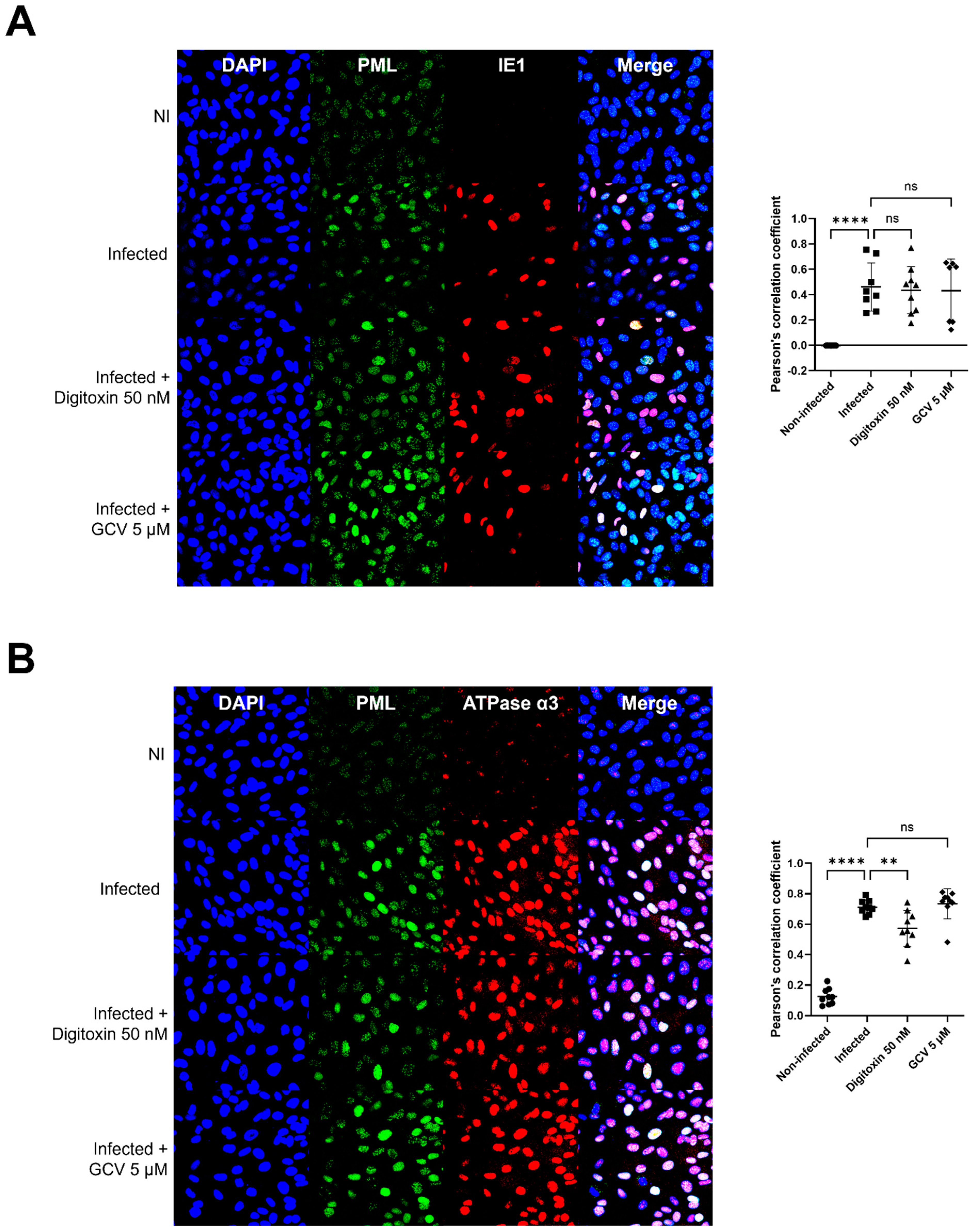
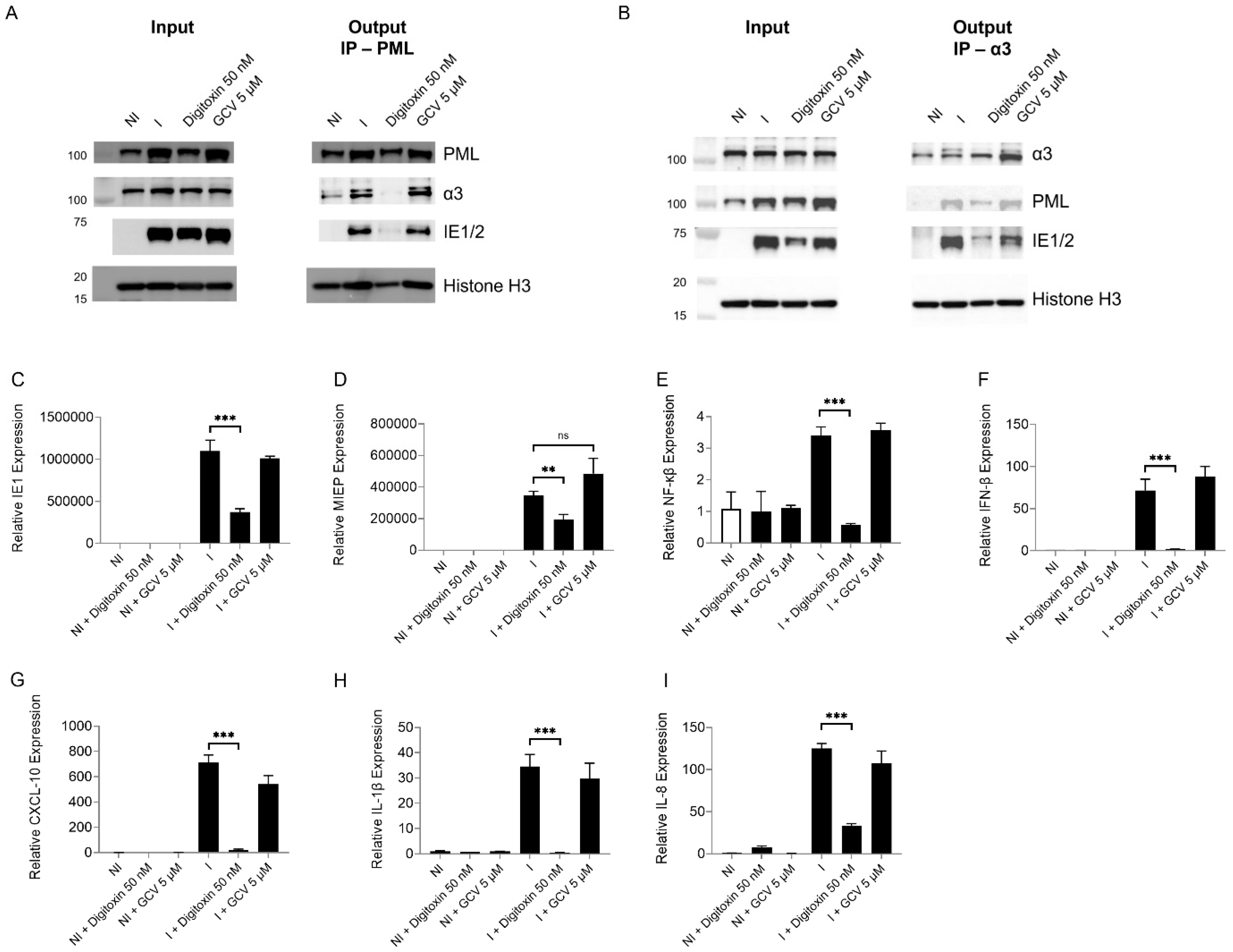
3.5. α3 Is Localized in the Nuclear Compartment and Interacts with PML and IE1 During Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kjeldsen, K.; Norgaard, A.; Gheorghiade, M. Myocardial Na,K-ATPase: The molecular basis for the hemodynamic effect of digoxin therapy in congestive heart failure. Cardiovasc. Res. 2002, 55, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Van, Q.E.; Delest, B.; Debeir, O.; Darro, F.; Kiss, R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim. Biophys. Acta 2007, 1776, 32–57. [Google Scholar] [CrossRef]
- Newman, R.A.; Yang, P.; Pawlus, A.D.; Block, K.I. Cardiac glycosides as novel cancer therapeutic agents. Mol. Interv. 2008, 8, 36–49. [Google Scholar] [CrossRef]
- Skubnik, J.; Bejcek, J.; Pavlickova, V.S.; Rimpelova, S. Repurposing Cardiac Glycosides: Drugs for Heart Failure Surmounting Viruses. Molecules 2021, 26, 5627. [Google Scholar] [CrossRef] [PubMed]
- Caohuy, H.; Eidelman, O.; Chen, T.; Liu, S.; Yang, Q.; Bera, A.; Walton, N.I.; Wang, T.T.; Pollard, H.B. Common cardiac medications potently inhibit ACE2 binding to the SARS-CoV-2 Spike, and block virus penetration and infectivity in human lung cells. Sci. Rep. 2021, 11, 22195. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Barh, D.; Azevedo, V.; Ghosh, P. Anticancer and Antiviral Properties of Cardiac Glycosides: A Review to Explore the Mechanism of Actions. Molecules 2020, 25, 3596. [Google Scholar] [CrossRef] [PubMed]
- Amarelle, L.; Lecuona, E. The Antiviral Effects of Na,K-ATPase Inhibition: A Minireview. Int. J. Mol. Sci. 2018, 19, 2154. [Google Scholar] [CrossRef]
- Cho, J.; Lee, Y.J.; Kim, J.H.; Kim, S.I.; Kim, S.S.; Choi, B.S.; Choi, J.H. Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19. Sci. Rep. 2020, 10, 16200. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Venkatadri, R.; Katsnelson, J.; Arav-Boger, R. Digitoxin Suppresses Human Cytomegalovirus Replication via Na(+), K(+)/ATPase alpha1 Subunit-Dependent AMP-Activated Protein Kinase and Autophagy Activation. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Souza, E.S.K.F.C.; Moraes, B.P.T.; Paixao, I.; Burth, P.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Na(+)/K(+)-ATPase as a Target of Cardiac Glycosides for the Treatment of SARS-CoV-2 Infection. Front. Pharmacol. 2021, 12, 624704. [Google Scholar] [CrossRef]
- Cohen, T.; Williams, J.D.; Opperman, T.J.; Sanchez, R.; Lurain, N.S.; Tortorella, D. Convallatoxin-Induced Reduction of Methionine Import Effectively Inhibits Human Cytomegalovirus Infection and Replication. J. Virol. 2016, 90, 10715–10727. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, S.; Lin, X.; Yang, S.; Ba, X.; Xiong, D.; Xiao, L.; Li, R. Lanatoside C inhibits herpes simplex virus 1 replication by regulating NRF2 distribution within cells. Phytomedicine 2024, 124, 155308. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.F.Z.; Cerella, C.; Simoes, C.M.O.; Diederich, M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22, 1932. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.; Hartley, M.; Pardoe, I.; Knight, A. Ionic Contra-Viral Therapy (ICVT); a new approach to the treatment of DNA virus infections. Arch. Virol. 2006, 151, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.J.; Cohen, T.; Redmann, V.; Lau, Z.; Felsenfeld, D.; Tortorella, D. Development of a high-content screen for the identification of inhibitors directed against the early steps of the cytomegalovirus infectious cycle. Antiviral Res. 2015, 113, 49–61. [Google Scholar] [CrossRef]
- Cai, H.; Wang, H.Y.; Venkatadri, R.; Fu, D.X.; Forman, M.; Bajaj, S.O.; Li, H.; O’Doherty, G.A.; Arav-Boger, R. Digitoxin Analogues with Improved Anticytomegalovirus Activity. In ACS Medicinal Chemistry Letters; American Chemical Society: Washington, DC, USA, 2014; Volume 5, pp. 395–399. [Google Scholar]
- Lopez-Lazaro, M.; Pastor, N.; Azrak, S.S.; Ayuso, M.J.; Austin, C.A.; Cortes, F. Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients. J. Nat. Prod. 2005, 68, 1642–1645. [Google Scholar] [CrossRef]
- Kapoor, A.; Cai, H.; Forman, M.; He, R.; Shamay, M.; Arav-Boger, R. Human cytomegalovirus inhibition by cardiac glycosides: Evidence for involvement of the HERG gene. Antimicrob. Agents Chemother. 2012, 56, 4891–4899. [Google Scholar] [CrossRef]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Brashear, A.; Dobyns, W.B.; de Carvalho Aguiar, P.; Borg, M.; Frijns, C.J.; Gollamudi, S.; Green, A.; Guimaraes, J.; Haake, B.C.; Klein, C.; et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain 2007, 130 Pt 3, 828–835. [Google Scholar] [CrossRef]
- Heinzen, E.L.; Swoboda, K.J.; Hitomi, Y.; Gurrieri, F.; Nicole, S.; de Vries, B.; Tiziano, F.D.; Fontaine, B.; Walley, N.M.; Heavin, S.; et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat. Genet. 2012, 44, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Paciorkowski, A.R.; McDaniel, S.S.; Jansen, L.A.; Tully, H.; Tuttle, E.; Ghoneim, D.H.; Tupal, S.; Gunter, S.A.; Vasta, V.; Zhang, Q.; et al. Novel mutations in ATP1A3 associated with catastrophic early life epilepsy, episodic prolonged apnea, and postnatal microcephaly. Epilepsia 2015, 56, 422–430. [Google Scholar] [CrossRef]
- Sweadner, K.J. Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta 1989, 988, 185–220. [Google Scholar] [CrossRef]
- Raghavendra, P.B.; Sreenivasan, Y.; Manna, S.K. Oleandrin induces apoptosis in human, but not in murine cells: Dephosphorylation of Akt, expression of FasL, and alteration of membrane fluidity. Mol. Immunol. 2007, 44, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; O’Doherty, G. The de novo synthesis of oligosaccharides: Application to the medicinal chemistry SAR-study of digitoxin. Curr. Top. Med. Chem. 2008, 8, 114–125. [Google Scholar] [PubMed]
- Wang, H.Y.; Rojanasakul, Y.; O’Doherty, G.A. Synthesis and Evaluation of the alpha-D-/alpha-L-Rhamnosyl and Amicetosyl Digitoxigenin Oligomers as Anti-tumor Agents. ACS Med. Chem. Lett. 2011, 2, 264–269. [Google Scholar] [CrossRef]
- He, R.; Sandford, G.; Hayward, G.S.; Burns, W.H.; Posner, G.H.; Forman, M.; Arav-Boger, R. Recombinant Luciferase-Expressing Human Cytomegalovirus (CMV) for evaluation of CMV inhibitors. Virol. J. 2011, 8, 40. [Google Scholar] [CrossRef]
- Schleiss, M.R.; Bourne, N.; Bravo, F.J.; Jensen, N.J.; Bernstein, D.I. Quantitative-competitive PCR monitoring of viral load following experimental guinea pig cytomegalovirus infection. J. Virol. Methods 2003, 108, 103–110. [Google Scholar] [CrossRef]
- Mason, R.; Groves, I.J.; Wills, M.R.; Sinclair, J.H.; Reeves, M.B. Human cytomegalovirus major immediate early transcripts arise predominantly from the canonical major immediate early promoter in reactivating progenitor-derived dendritic cells. J. Gen. Virol. 2020, 101, 635–644. [Google Scholar] [CrossRef]
- Collins-McMillen, D.; Rak, M.; Buehler, J.C.; Igarashi-Hayes, S.; Kamil, J.P.; Moorman, N.J.; Goodrum, F. Alternative promoters drive human cytomegalovirus reactivation from latency. Proc. Natl. Acad. Sci. USA 2019, 116, 17492–17497. [Google Scholar] [CrossRef]
- Kumar, M.; Michael, S.; Alvarado-Valverde, J.; Zeke, A.; Lazar, T.; Glavina, J.; Nagy-Kanta, E.; Donagh, J.M.; Kalman, Z.E.; Pascarelli, S.; et al. ELM-the Eukaryotic Linear Motif resource-2024 update. Nucleic Acids Res. 2024, 52, D442–D455. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Ba, A.N.; Pogoutse, A.; Provart, N.; Moses, A.M. NLStradamus: A simple Hidden Markov Model for nuclear localization signal prediction. BMC Bioinform. 2009, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelieres, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224 Pt 3, 213–232. [Google Scholar] [CrossRef]
- Bernardi, R.; Papa, A.; Pandolfi, P.P. Regulation of apoptosis by PML and the PML-NBs. Oncogene 2008, 27, 6299–6312. [Google Scholar] [CrossRef]
- Cheng, X.; Kao, H.Y. Post-translational modifications of PML: Consequences and implications. Front. Oncol. 2012, 2, 210. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Stamminger, T. Emerging Role of PML Nuclear Bodies in Innate Immune Signaling. J. Virol. 2016, 90, 5850–5854. [Google Scholar] [CrossRef]
- Maarifi, G.; Chelbi-Alix, M.K.; Nisole, S. PML control of cytokine signaling. Cytokine Growth Factor. Rev. 2014, 25, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wright, J.; Meng, X.; Leppard, K.N. Promyelocytic Leukemia Protein Isoform II Promotes Transcription Factor Recruitment to Activate Interferon Beta and Interferon-Responsive Gene Expression. Mol. Cell Biol. 2015, 35, 1660–1672. [Google Scholar] [CrossRef]
- Kim, Y.E.; Ahn, J.H. Positive role of promyelocytic leukemia protein in type I interferon response and its regulation by human cytomegalovirus. PLoS Pathog. 2015, 11, e1004785. [Google Scholar] [CrossRef]
- Sakai, H.; Suzuki, T.; Maeda, M.; Takahashi, Y.; Horikawa, N.; Minamimura, T.; Tsukada, K.; Takeguchi, N. Up-regulation of Na(+),K(+)-ATPase alpha 3-isoform and down-regulation of the alpha1-isoform in human colorectal cancer. FEBS Lett. 2004, 563, 151–154. [Google Scholar] [CrossRef]
- Yang, P.; Menter, D.G.; Cartwright, C.; Chan, D.; Dixon, S.; Suraokar, M.; Mendoza, G.; Llansa, N.; Newman, R.A. Oleandrin-mediated inhibition of human tumor cell proliferation: Importance of Na,K-ATPase alpha subunits as drug targets. Mol. Cancer Ther. 2009, 8, 2319–2328. [Google Scholar] [CrossRef]
- Lin, Y.; Ho, D.H.; Newman, R.A. Human tumor cell sensitivity to oleandrin is dependent on relative expression of Na+, K+ -ATPase subunitst. J. Exp. Ther. Oncol. 2010, 8, 271–286. [Google Scholar]
- O’Brien, W.J.; Lingrel, J.B.; Wallick, E.T. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch. Biochem. Biophys. 1994, 310, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Lifshitz, Y.; Bab-Dinitz, E.; Kapri-Pardes, E.; Goldshleger, R.; Tal, D.M.; Karlish, S.J. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J. Biol. Chem. 2010, 285, 19582–19592. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cartwright, C.; Efuet, E.; Hamilton, S.R.; Wistuba, II; Menter, D.; Addington, C.; Shureiqi, I.; Newman, R.A. Cellular location and expression of Na+, K+ -ATPase alpha subunits affect the anti-proliferative activity of oleandrin. Mol. Carcinog. 2014, 53, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, S.; Heynen-Genel, S.; Chao, E.; Dewing, A.; Solano, R.; Milan, L.; Barron, N.; He, M.; Diaz, P.W.; Matsuzawa, S.; et al. Cardiac Glycosides Activate the Tumor Suppressor and Viral Restriction Factor Promyelocytic Leukemia Protein (PML). PLoS ONE 2016, 11, e0152692. [Google Scholar] [CrossRef]
- Takahashi, Y.; Lallemand-Breitenbach, V.; Zhu, J.; de The, H. PML nuclear bodies and apoptosis. Oncogene 2004, 23, 2819–2824. [Google Scholar] [CrossRef]
- Ivanschitz, L.; De The, H.; Le Bras, M. PML, SUMOylation, and Senescence. Front. Oncol. 2013, 3, 171. [Google Scholar] [CrossRef]
- Corpet, A.; Olbrich, T.; Gwerder, M.; Fink, D.; Stucki, M. Dynamics of histone H3.3 deposition in proliferating and senescent cells reveals a DAXX-dependent targeting to PML-NBs important for pericentromeric heterochromatin organization. Cell Cycle 2014, 13, 249–267. [Google Scholar] [CrossRef]
- Tashiro, S.; Muto, A.; Tanimoto, K.; Tsuchiya, H.; Suzuki, H.; Hoshino, H.; Yoshida, M.; Walter, J.; Igarashi, K. Repression of PML nuclear body-associated transcription by oxidative stress-activated Bach2. Mol. Cell Biol. 2004, 24, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, A.; von Mikecz, A.; Hemmerich, P. Cell cycle-dependent association of PML bodies with sites of active transcription in nuclei of mammalian cells. J. Struct. Biol. 2002, 140, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Luciani, J.J.; Depetris, D.; Usson, Y.; Metzler-Guillemain, C.; Mignon-Ravix, C.; Mitchell, M.J.; Megarbane, A.; Sarda, P.; Sirma, H.; Moncla, A.; et al. PML nuclear bodies are highly organised DNA-protein structures with a function in heterochromatin remodelling at the G2 phase. J. Cell Sci. 2006, 119 Pt 12, 2518–2531. [Google Scholar] [CrossRef]
- Dellaire, G.; Ching, R.W.; Dehghani, H.; Ren, Y.; Bazett-Jones, D.P. The number of PML nuclear bodies increases in early S phase by a fission mechanism. J. Cell Sci. 2006, 119 Pt 6, 1026–1033. [Google Scholar] [CrossRef]
- Dellaire, G.; Eskiw, C.H.; Dehghani, H.; Ching, R.W.; Bazett-Jones, D.P. Mitotic accumulations of PML protein contribute to the re-establishment of PML nuclear bodies in G1. J. Cell Sci. 2006, 119 Pt 6, 1034–1042. [Google Scholar] [CrossRef]
- Everett, R.D.; Chelbi-Alix, M.K. PML and PML nuclear bodies: Implications in antiviral defence. Biochimie 2007, 89, 819–830. [Google Scholar] [CrossRef]
- Sahin, U.; Lallemand-Breitenbach, V.; de The, H. PML nuclear bodies: Regulation, function and therapeutic perspectives. J. Pathol. 2014, 234, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Schilling, E.M.; Stamminger, T. The Human CMV IE1 Protein: An Offender of PML Nuclear Bodies. Adv. Anat. Embryol. Cell Biol. 2017, 223, 77–94. [Google Scholar]
- Nisole, S.; Maroui, M.A.; Mascle, X.H.; Aubry, M.; Chelbi-Alix, M.K. Differential Roles of PML Isoforms. Front. Oncol. 2013, 3, 125. [Google Scholar] [CrossRef]
- Schilling, E.M.; Scherer, M.; Reuter, N.; Schweininger, J.; Muller, Y.A.; Stamminger, T. The Human Cytomegalovirus IE1 Protein Antagonizes PML Nuclear Body-Mediated Intrinsic Immunity via the Inhibition of PML De Novo SUMOylation. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Paulus, C.; Harwardt, T.; Walter, B.; Marxreiter, A.; Zenger, M.; Reuschel, E.; Nevels, M.M. Revisiting promyelocytic leukemia protein targeting by human cytomegalovirus immediate-early protein 1. PLoS Pathog. 2020, 16, e1008537. [Google Scholar] [CrossRef] [PubMed]
- Benfante, R.; Antonini, R.A.; Vaccari, M.; Flora, A.; Chen, F.; Clementi, F.; Fornasari, D. The expression of the human neuronal alpha3 Na+,K+-ATPase subunit gene is regulated by the activity of the Sp1 and NF-Y transcription factors. Biochem. J. 2005, 386 Pt 1, 63–72. [Google Scholar] [CrossRef]
- Yurochko, A.D.; Mayo, M.W.; Poma, E.E.; Baldwin, A.S., Jr.; Huang, E.S. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J. Virol. 1997, 71, 4638–4648. [Google Scholar] [CrossRef] [PubMed]
- Karas, K.; Salkowska, A.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. Cardiac glycosides with target at direct and indirect interactions with nuclear receptors. Biomed. Pharmacother. 2020, 127, 110106. [Google Scholar] [CrossRef] [PubMed]
- Adamson, C.S.; Nevels, M.M. Bright and Early: Inhibiting Human Cytomegalovirus by Targeting Major Immediate-Early Gene Expression or Protein Function. Viruses 2020, 12, 110. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, W.; Jozwik, C.; Lin, Y.; Glasman, M.; Caohuy, H.; Srivastava, M.; Esposito, D.; Gillette, W.; Hartley, J.; et al. Cardiac glycosides inhibit TNF-alpha/NF-kappaB signaling by blocking recruitment of TNF receptor-associated death domain to the TNF receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 9631–9636. [Google Scholar] [CrossRef]


| Gene | Primer Sequence |
|---|---|
| Human α1—Fwd | 5′-CCAAGCTGCTACAGAAGAGGAACC-3′ |
| Human α1—Rev | 5′-TTGAAGGATTCCATGATCTTTGAAC-3′ |
| Human α2—Fwd | 5′-CGCTCGACAAGGAGATGCAAGA-3′ |
| Human α2—Rev | 5′-CCGAGGAAACTTTCCAGATGGC-3′ |
| Human α3—Fwd | 5′-TTC GGG GGC TTC TCC ATC CT-3′ |
| Human α3—Rev | 5’-GCA CTC GGT CTC CAC CCT TGA-3’ |
| Mouse α1—Fwd | 5’-CCGTGGATAACCTCTGCTTCGT-3’ |
| Mouse α1—Rev | 5’-CGCTGTGATTGGATGGTCTCCT-3’ |
| Mouse α2—Fwd | 5’-CTGTCCTTGGATGAGCTGGGC-3’ |
| Mouse α2—Rev | 5’-ACTTGACCCACTCAGGAGTTGTGG-3’ |
| Mouse α3—Fwd | 5’-GGTGTGGGTATCATCTCTGAGG-3’ |
| Mouse α3—Rev | 5’-CGTCAATCTGCTCCGAGGTGAA-3’ |
| GP α1—Fwd | 5’-CAGGGATATGGATGAACTGAAGAA-3’ |
| GP α1—Rev | 5’-GTGTGAGGGAATTAGGACCATCTC-3’ |
| GP α3—Fwd | 5’-GGAGTGGGCATCATTTCTGAGG-3’ |
| GP α3—Rev | 5′-CGTCGATTTGCTCTGAGGTGAA-3′ |
| GP α2- Fwd | 5′-CTGTCCTTGGATGAGCTGGGT-3′ |
| GP and mouse α2—Rev | 5′-ACTTGACCCACTCAGGAGTTGTGG-3′ |
| UL83 F6 | 5′-CGACGACGACGATGACGAAAAC-3′ |
| UL83 B11 | 5′-TCCTCGGTCTCAACGAAGGGTC-3′ |
| GP-GAPDH—Fwd | 5′-GGGCAAGGTCATCCCAGAG-3′ |
| GP-GAPDH—Rev | 5′-TGGAAGAATGGCTGTCACTGTT-3′ |
| Gene | Primer Sequence |
|---|---|
| HCMV IE1—Fwd | 5′-CTTAATACAAGCCATCCACA-3′ |
| HCMV IE1—Rev | 5′-TAGATAAGGTTCATGAGCCT-3′ |
| HCMV MIEP-derived IE—Fwd | 5′-TTGACCTCCATAGAAGACAC-3′ |
| HCMV MIEP-derived IE—Rev | 5′-AGGACTCCATCGTGTCAAGG-3′ |
| Human CXCL-10—Fwd | 5′-TTACTGAAAGCAGTTAGCAAGGAA-3’ |
| Human CXCL-10—Rev | 5’-AGCTGATTTGGTGACCATCATTG-3’ |
| Human IFN-β—Fwd | 5’-GATTCATCTAGCACTGGCTGG-3’ |
| Human IFN-β—Rev | 5’-CTTCAGGTAATGCAGAATCC-3’ |
| Human IL-8—Fwd | 5’-TGCAGCTCTGTGTGAAGGTGCAGT-3’ |
| Human IL-1β—Fwd | 5’-GCTCGCCAGTGAAATGATGGCTT-3’ |
| Human IL-1β—Rev | 5’-CAGAGGTCCAGGTCCTGGAAGG -3’ |
| Human IL-8—Rev | 5’-CAGTGTGGTCCACTCTCAATCACTC-3’ |
| Human GAPDH—Fwd | 5’-TTGGTATCGTGGAAGGACTC-3’ |
| Human GAPDH—Rev | 5’-ACAGTCTTCTGGGTGGCAGT-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, H.; Cai, H.; Liu, F.; Venkatadri, R.; Miller, H.E.; Mathison, A.J.; Wang, H.-Y.L.; Silva, S.C.; O’Doherty, G.A.; Arav-Boger, R. Interspecies Differences in Cytomegalovirus Inhibition by Cardiac Glycosides—A Unique Role of the Alpha3 Isoform of the Na+/K+-ATPase Pump. Viruses 2025, 17, 398. https://doi.org/10.3390/v17030398
Mei H, Cai H, Liu F, Venkatadri R, Miller HE, Mathison AJ, Wang H-YL, Silva SC, O’Doherty GA, Arav-Boger R. Interspecies Differences in Cytomegalovirus Inhibition by Cardiac Glycosides—A Unique Role of the Alpha3 Isoform of the Na+/K+-ATPase Pump. Viruses. 2025; 17(3):398. https://doi.org/10.3390/v17030398
Chicago/Turabian StyleMei, Hong, Hongyi Cai, Fengjie Liu, Rajkumar Venkatadri, Halli E. Miller, Angela J. Mathison, Hua-Yu Leo Wang, Simone C. Silva, George A. O’Doherty, and Ravit Arav-Boger. 2025. "Interspecies Differences in Cytomegalovirus Inhibition by Cardiac Glycosides—A Unique Role of the Alpha3 Isoform of the Na+/K+-ATPase Pump" Viruses 17, no. 3: 398. https://doi.org/10.3390/v17030398
APA StyleMei, H., Cai, H., Liu, F., Venkatadri, R., Miller, H. E., Mathison, A. J., Wang, H.-Y. L., Silva, S. C., O’Doherty, G. A., & Arav-Boger, R. (2025). Interspecies Differences in Cytomegalovirus Inhibition by Cardiac Glycosides—A Unique Role of the Alpha3 Isoform of the Na+/K+-ATPase Pump. Viruses, 17(3), 398. https://doi.org/10.3390/v17030398







