Aphid Species in Citrus Orchards in Crete: Key Vectors of Citrus Tristeza Virus and Automated Monitoring Innovations for Alate Aphids
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid Samplings and Species Identification
2.2. Molecular Identification of Aphid Species
2.3. A Survey in CTV-Infected Fields, Virus Sources
2.4. RNA Isolation from Plants and Aphids
2.5. CTV Detection in Aphids by RT-PCR or Real-Time PCR
2.6. Sequence Analysis
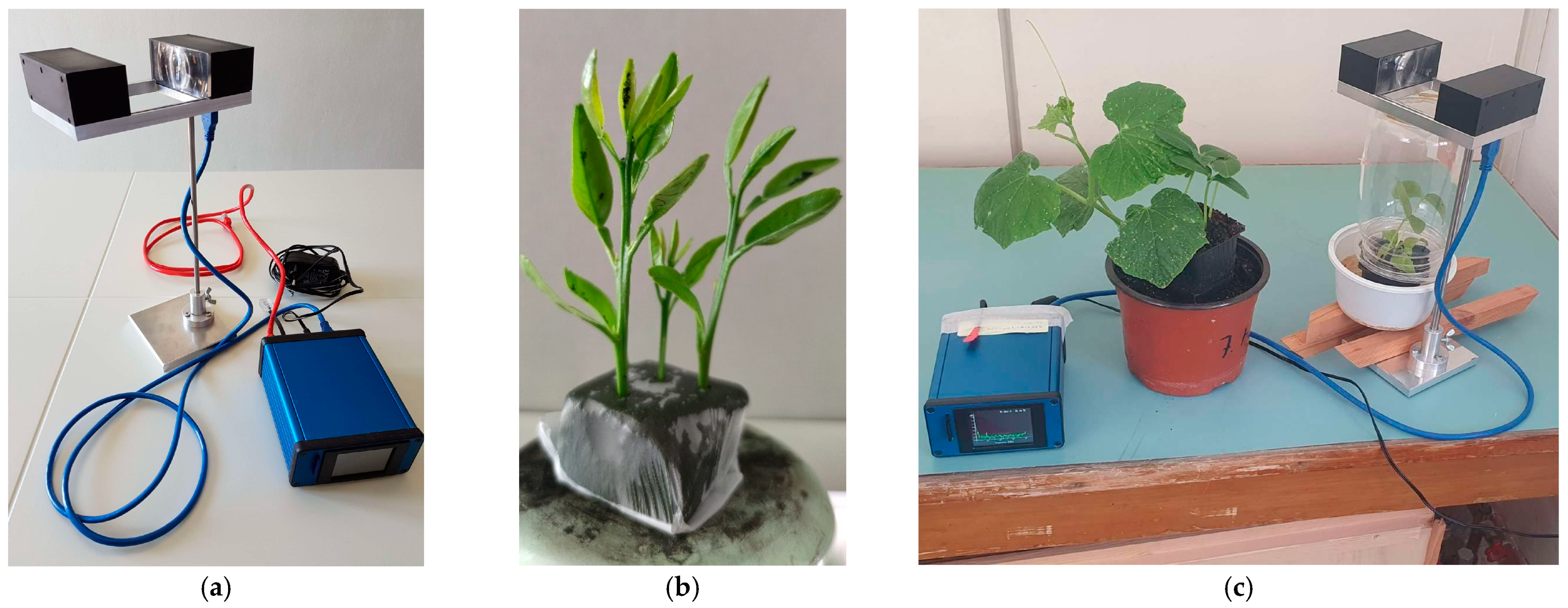
2.7. Wingbeat Analysis of Alate Adult Aphids
2.8. Statistical Analysis
3. Results
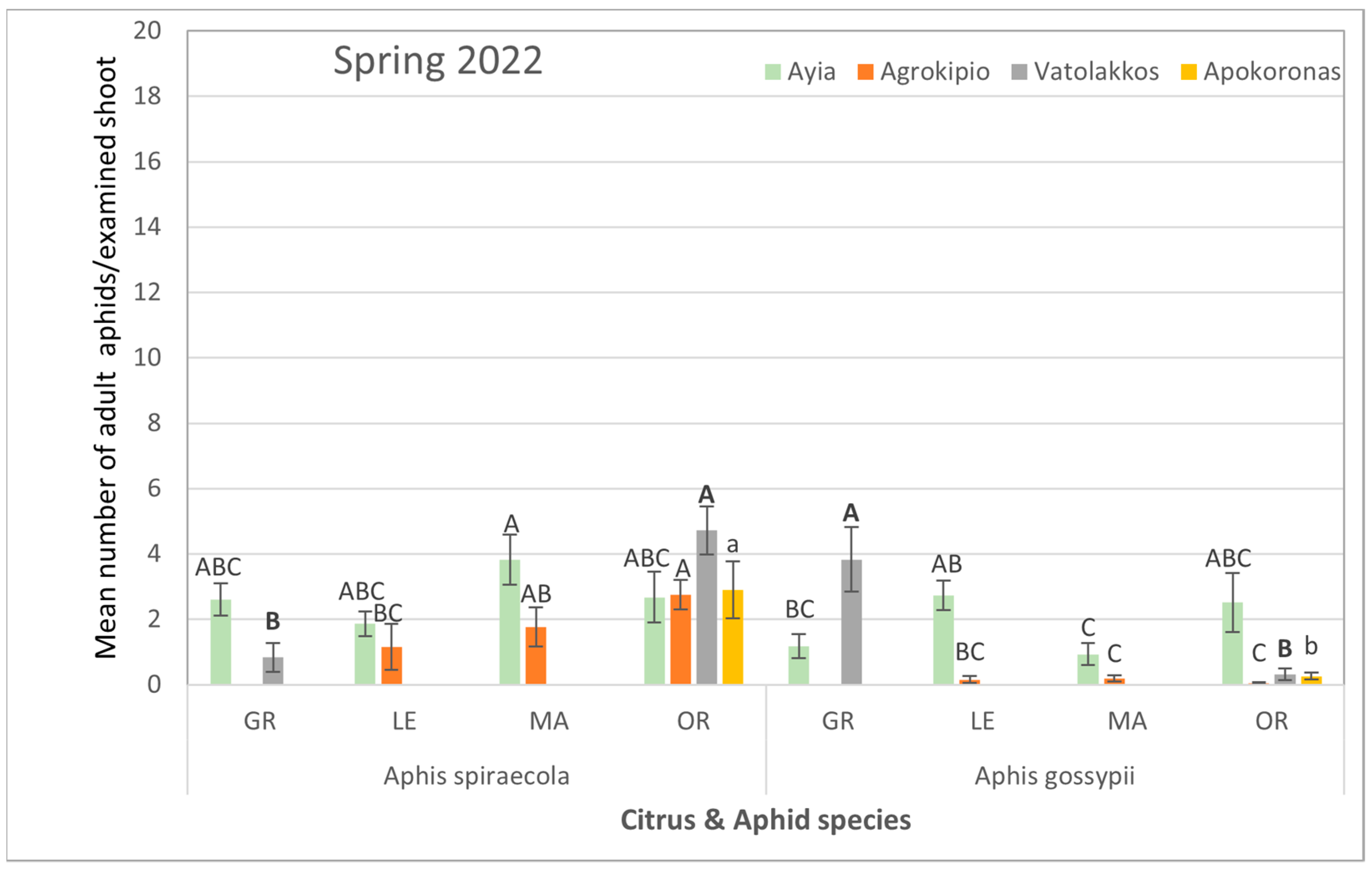
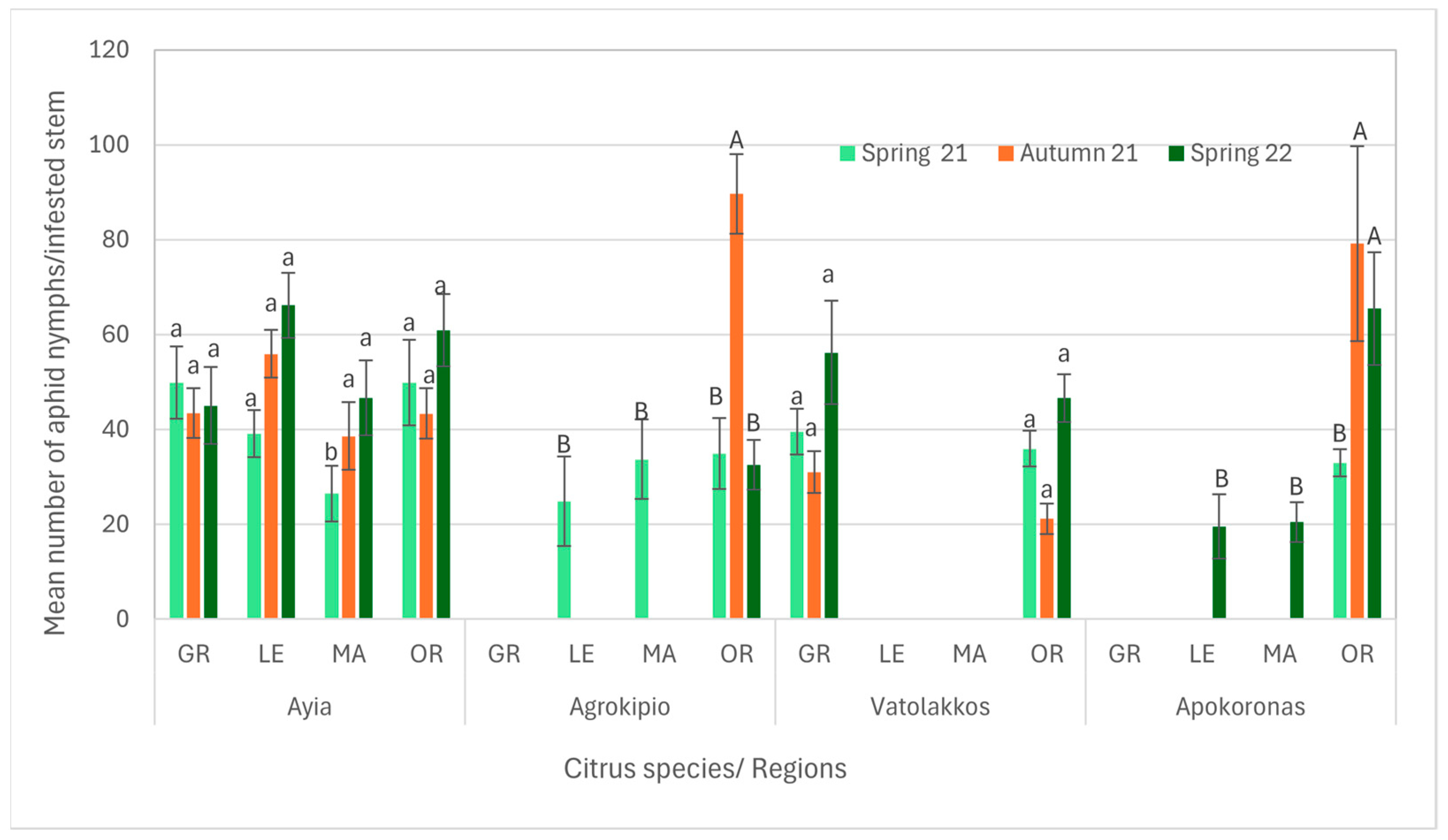
3.1. Aphids’ Composition and Population Distribution Among Citrus Species
3.2. Molecular Identification of Aphid Species
3.3. Evaluation of the Total RNA Isolation from Aphids
3.4. Identification of Aphids as Putative Vectors of CTV in This Study
3.5. CTV Sequences from Putative Viruliferous Aphids
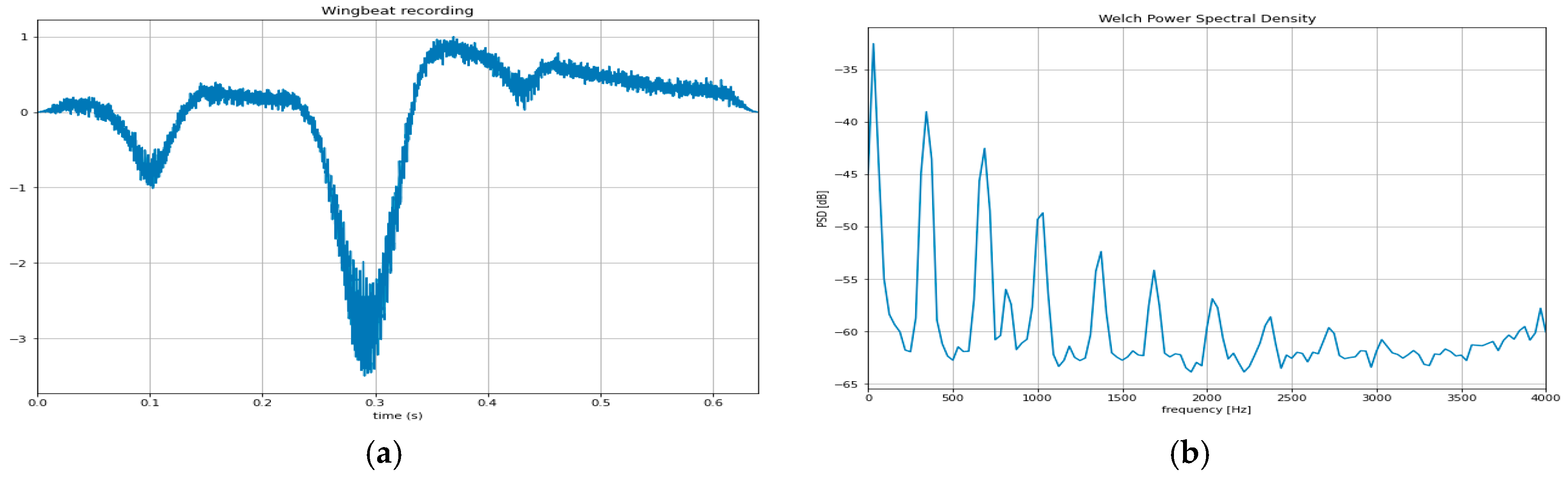
3.6. Wingbeat Analysis of Alate Adult Aphids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT Database. 2020. Available online: http://www.fao.org/faostat/en/#data (accessed on 20 December 2023).
- ELSTAT. 2019. Available online: http://www.statistics.gr/el/statistics/agr (accessed on 15 January 2024).
- Tennant, P.F.; Robinson, D.; Fisher, L.; Bennett, S.M.; Hutton, D.; Coates-Beckford, P.; McLaughlin, W. Diseases and pests of citrus (Citrus spp.). Tree For. Sci. Biotechnol. 2009, 3, 81–107. [Google Scholar]
- Barbagallo, S.; Cocuzza, G.; CravediI, P.; Komazaki, S. IPM case studies: Tropical and subtropical fruit trees. In Aphids as Crop Pests, 2nd ed.; CABI: Wallingford, UK, 2007; pp. 643–654. [Google Scholar]
- Komazaki, S. Studies on the biology of the spirea aphid, Aphis spiraecola Patch, with special reference to biotypic differences. Bull. Fruit Tree Res. Stn. 1991, 2, 1–60. [Google Scholar]
- Roistacher, M.; Bar-Joseph, C.N. Aphid transmission of citrus tristeza virus: A review. Phytophylactica 1987, 19, 163–168. [Google Scholar]
- Yokomi, R.K.; Garnsey, S.M.; Civerolo, E.L.; Gumpf, D.J. Transmission of exotic citrus tristeza virus isolates by a Florida colony of Aphis gossypii. Plant Dis. 1989, 73, 552–556. [Google Scholar] [CrossRef]
- Yokomi, R.K.; Lastra, R.; Stoetzel, M.B.; Damsteegt, V.D.; Lee, R.F.; Garnsey, S.M.; Gottwald, T.R.; Rocha-Pena, M.A.; Niblett, C.L. Establishment of the brown citrus aphid (Homoptera: Aphididae) in Central America and the Caribbean Basin and transmission of citrus tristeza virus. J. Econ. Entomol. 1994, 87, 1078–1085. [Google Scholar] [CrossRef]
- Cambra, M.; Gorris, M.T.; Marroquín, C.; Román, M.P.; Olmos, A.; Martínez, P.C.; Hermoso de Mendoza, A.H.; López, A.; Navarro, L. Incidence and epidemiology of Citrus tristeza virus in the Valencian Community of Spain. Virus Res. 2000, 71, 85–95. [Google Scholar] [CrossRef]
- Marroquín, C.; Olmos, A.; Teresa Gorris, M.; Bertolini, E.; Carmen Martínez, M.; Carbonell, E.A.; Hermoso de Mendoza, A.; Cambra, M. Estimation of the number of aphids carrying Citrus tristeza virus that visit adult citrus trees. Virus Res. 2004, 100, 101–108. [Google Scholar] [CrossRef]
- Yokomi, R.K.; DeBorde, R.L. Incidence, transmissibility, and genotype analysis of Citrus tristeza virus (CTV) isolates from CTV eradicative and non-eradicative districts in central California. Plant Dis. 2005, 89, 859–866. [Google Scholar] [CrossRef]
- Norman, P.A.; Grant, T.J. Transmission of tristeza virus by aphids. Proc. Fla. State Hortic. Soc. 1956, 69, 38–42. [Google Scholar]
- Raccah, B.; Loebenstein, G.; Bar-Joseph, M.; Oren, Y. Transmission of tristeza by aphids prevalent on citrus, and operation of the tristeza suppression program in Israel. In Proceedings of the Seventh Conference of the International Organization of Citrus Virologists; University of California Press: Riverside, CA, USA, 1976; pp. 47–49. [Google Scholar]
- Moreno, P.; Ambrós, S.; Albiach-Martí, M.R.; Guerri, J.; Peña, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Mol. Plant Pathol. 2008, 9, 251–268. [Google Scholar] [CrossRef]
- Argyriou, L.C. Biological control of citrus in Greece. In Proceedings of the 1st International Citrus Symposium, Riverside, CA, USA, 16–26 March 1968; UC Riverside: Riverside, CA, USA, 1969; pp. 817–822. [Google Scholar]
- Kavallieratos, N.; Lykouresis, D. Parasitoids (Hymenoptera: Braconidae) emerged from aphids (Homoptera: Aphidoidea) on citrus and their frequency in Greece. Boll. Lab. Entomol. Agrar. Filippo Silvestri 1999, 55, 93–104. [Google Scholar]
- Kalaitzaki, A.; Awad, S.; Malandraki, E.; Papapetrou, P.D.; Livieratos, I.; Margaritopoulos, J.T. Aphid species composition in populations from citrus orchards in a region of the island of Crete. Bull. Insectol. 2019, 72, 143–149. [Google Scholar]
- Karasev, A.; Boyko, V.; Gowda, S.; Nikolaeva, O.; Hilf, M.; Koonin, E.; Niblett, C.; Cline, K.; Gumpf, D.; Lee, R.; et al. Complete Sequence of the Citrus Tristeza Virus RNA Genome. Virology 1995, 208, 511–520. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Marcus, R.; Lee, R.F. The continuous challenge of Citrus tristeza virus control. Annu. Rev. Phytopathol. 1989, 27, 291–316. [Google Scholar] [CrossRef]
- Sabade, A.; López, C.; Rubio, L.; Flores, R.; Guerri, J.; Moreno, P. Polymorphism of a specific region in gene p23 of Citrus tristeza virus allows discrimination between mild and severe isolates. Arch. Virol. 2003, 148, 2325–2340. [Google Scholar] [CrossRef]
- Sagheer, A.; Zhou, C.Y.; Zhou, Y.; Cao, M.J.; Wang, X.F. Distribution and research advances in Citrus tristeza virus. J. Integr. Agric. 2012, 11, 346–358. [Google Scholar]
- Bester, R.; Cook, G.; Maree, H.J. Citrus Tristeza Virus Genotype Detection Using High-Throughput Sequencing. Viruses 2021, 13, 168. [Google Scholar] [CrossRef]
- Katsarou, K.; Chiumenti, M.; Kalantidis, K.; Mathioudakis, M.M. First report of citrus viroids infecting Persian (Tahiti) lime in Greece. Plant Dis. 2020, 104, 998. [Google Scholar] [CrossRef]
- Beris, D.; Malandraki, I.; Vassilakos, N.; Theologidis, I.; Rampou, A.; Kektsidou, O.; Massart, S.; Varveri, C. Association of Citrus Virus A to Citrus Impietratura Disease Symptoms. Phytopathology 2021, 111, 1782–1789. [Google Scholar] [CrossRef]
- Mathioudakis, M.M.; Tektonidis, N.; Karagianni, A.; Mikalef, L.; Gómez, P.; Hasiów-Jaroszewska, B. Incidence and Epidemiology of Citrus Viroids in Greece: Role of Host and Cultivar in Epidemiological Characteristics. Viruses 2023, 22, 605. [Google Scholar] [CrossRef]
- Dimou, D.; Drossopoulou, J.; Moschos, E.; Varveri, C.; BEM, F. First report of Citrus tristeza virus in Greece. Plant Dis. 2002, 86, 329. [Google Scholar] [CrossRef]
- Owen, C.; Mathioudakis, M.; Gazivoda, A.; Gal, P.; Nol, N.; Kalliampakou, K.; Figas, A.; Bellan, A.; Iparaguirre, A.; Rubio, L.; et al. Evolution and molecular epidemiology of Citrus tristeza virus on Crete: Recent introduction of a severe strain. J. Phytopathol. 2014, 162, 839–843. [Google Scholar] [CrossRef]
- Chatzivassiliou, E.K.; Nolasco, G. Detection of a new variant of Citrus tristeza virus in Greek citrus crops. Phytopathol. Mediterr. 2015, 53, 140–147. [Google Scholar]
- Varveri, C.; Olmos, A.; Pina, J.A.; Marroquín, C.; Cambra, M. Biological and molecular characterization of a distinct Citrus tristeza virus isolate originating from a lemon tree in Greece. Plant Pathol. 2015, 64, 792–798. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Vidakis, N.; Petousis, M.; Weber, M. Affordable Bimodal Optical Sensors to Spread the Use of Automated Insect Monitoring. J. Sens. 2018, 3949415. [Google Scholar] [CrossRef]
- Kirkeby, C.; Rydhmer, K.; Cook, S.M.; Strand, A.; Torrance, M.T.; Swain, J.L.; Prangsma, J.; Johnen, A.; Jensen, M.; Brydegaard, M.; et al. Advances in automatic identification of flying insects using optical sensors and machine learning. Sci. Rep. 2021, 11, 1555. [Google Scholar] [CrossRef]
- Saha, T.; Genoud, A.P.; Williams, G.M.; Thomas, B.P. Monitoring the Abundance of Flying Insects and Atmospheric Conditions during a 9-Month Campaign Using an Entomological Optical Sensor. Sci. Rep. 2023, 13, 15606. [Google Scholar] [CrossRef]
- Rigakis, I.; Potamitis, I.; Tatlas, N.A.; Livadaras, I.; Ntalampiras, S.A. Multispectral Backscattered Light Recorder of Insects’ Wingbeats. Electronics 2019, 8, 277. [Google Scholar] [CrossRef]
- Hassall, K.L.; Dye, A.; Potamitis, I.; Bell, J.R. Resolving the identification of weak-flying insects during flight: A coupling between rigorous data processing and biology. Agric. For. Entomol. 2021, 23, 489–505. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2000; p. 466. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Mathioudakis, Μ.Μ.; Saponari, M.; Hasiów-Jaroszewska, B.; Elbeaino, T.; Koubouris, G. Detection of viruses in olive cultivars in Greece, using a rapid and effective RNA extraction method, for certification of virus-tested propagation material. Phytopathol. Mediterr. 2020, 59, 203–211. [Google Scholar] [CrossRef]
- Campolo, O.; Chiera, E.; Malacrino, A.; Laudani, F.; Fontana, A.; Albanese, G.R.; Palmeri, V. Acquisition and transmission of selected CTV isolates by Aphis gossypii. J. Asia Pac. Entomol. 2014, 17, 493–498. [Google Scholar] [CrossRef]
- Galetto, L.; Bosco, D.; Marzachì, C. Selection of reference genes from two leafhopper species challenged by phytoplasma infection, for gene expression studies by RT-qPCR. BMC Res. Notes 2013, 6, 409. [Google Scholar] [CrossRef]
- Nolasco, G.; Sequeira, Z.; Soares, C.; Mansinho, A.; Bailey, A.M.; Niblett, C.L. Asymmetric PCR ELISA: Increased sensitivity and reduced costs for the detection of plant viruses. Eur. J. Plant Pathol. 2002, 108, 293–298. [Google Scholar] [CrossRef]
- Argyriou, A.V.; Tektonidis, N.; Alevizos, E.; Ferentinos, K.P.; Kourgialas, N.N.; Mathioudakis, M.M. Precision Farming Multimodal Technologies Using Optical Sensors for the Detection of Citrus Tristeza Virus Endemics. Sustainability 2024, 16, 5748. [Google Scholar] [CrossRef]
- Shegani, M.; Tsikou, D.; Velimirovic, A.; Afifi, H.; Karayianni, A.; Gazivoda, A.; Manevska, K.; Manakos, I.; Livieratos, I.C. Citrus tristeza virus on the island of Crete: A survey and detection protocol applications. J. Plant Pathol. 2012, 94, 71–78. [Google Scholar]
- SAS Institute. JMP: A User’s Guide to Statistical and Data Analysis; Version 9.4; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Yahiaoui, D.; Addante, R.; Djelouah, K.; D’Onghia, A.M. Preliminary monitoring of Citrus tristeza virus (CTV) vectors in Apulia region. Options Méditerranéennes 2009, 65, 173–175. [Google Scholar]
- Paiva, P.E.B.; Neto, L.M.; Marques, N.T.; Duarte, B.Z.; Duarte, A.M. Citrus Aphids in Algarve Region (Portugal): Species, Hosts, and Biological Control. Ecologies 2024, 5, 101–115. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, A.; Lepcha, R.; Majumdar, K.; Baranwal, V. Identification and distribution of aphid vectors spreading citrus tristeza virus in Darjeeling hills and Dooars of India. J. Asia Pac. Entomol. 2015, 18, 601–605. [Google Scholar] [CrossRef]
- Braham, M.; Boulahia-Kheder, S.; Kahia, M.; Nouira, S. Aphids and citrus responses to nitrogen fertilization. J. Saudi Soc. Agric. Sci. 2023, 22, 374–383. [Google Scholar] [CrossRef]
- EPPO Datasheet: Citrus tristeza Virus (Closterovirus tristezae). Last Updated: 19 June 2020. Available online: https://gd.eppo.int/taxon/CTV000/datasheet (accessed on 27 February 2025).
- Saponari, M.; Manjunath, K.; Yokomi, R.K. Quantitative detection of Citrus tristeza virus in citrus and aphids by real-time reverse transcription-PCR (TaqMan®). J. Virol. Methods 2008, 147, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Elhaddad, A.; ElAmrani, A.; Fereres, A.; Moreno, A. Spatial and temporal spread of Citrus tristeza virus and its aphid vectors in the North western area of Morocco. Insect Sci. 2015, 23, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Camps, R.; Fiore, N.; Riquelme, N.; Barros-Parada, W.; Besoain, X. Genotype variation of citrus tristeza virus after passage on different hosts, and changes in the virus genotype populations by the vector Aphis gossypii. Phytopathol. Mediterr. 2022, 61, 55–63. [Google Scholar] [CrossRef]
- Vanella, D.; Consoli, S.; Ramírez-Cuesta, J.M.; Tessitori, M. Suitability of the MODIS-NDVI Time-Series for a Posteriori Evaluation of the Citrus Tristeza Virus Epidemic. Remote Sens. 2020, 12, 1965. [Google Scholar] [CrossRef]
- Chen, Y.; Why, A.; Batista, G.; Mafra-Neto, A.; Keogh, E. Flying insect classification with inexpensive sensors. J. Insect Behav. 2014, 27, 657–677. [Google Scholar] [CrossRef]
- Kiskin, I.; Zilli, D.; Li, Y.; Sinka, M.; Willis, K.; Roberts, S. Bioacoustic detection with wavelet-conditioned convolutional neuralnetworks. Neural Comput. Appl. 2020, 32, 915–927. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. Insect biometrics: Optoacoustic signal processing and its applications to remote monitoring of McPhail type traps. PLoS ONE 2015, 10, e0140474. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Månefjord, H.; Travers, P.; Salvador, J.; Müller, L.; Dreyer, D.; Alison, J.; Høye, T.T.; Hu, G.; et al. Lidar as a potential tool for monitoring migratory insects. iScience 2024, 27, 109588. [Google Scholar] [CrossRef]
- Bell, J.R.; Shephard, G. How aphids fly: Take-off, free flight and implications for short and long distance migration. Agric. Forest Entomol. 2025, 27, 90–99. [Google Scholar] [CrossRef]
- Moore, A.; Miller, R.H. Automated identification of optically sensed aphid (Homoptera: Aphidae) wingbeat waveforms. Ann. Entomol. Soc. Am. 2002, 95, 1–8. [Google Scholar] [CrossRef]
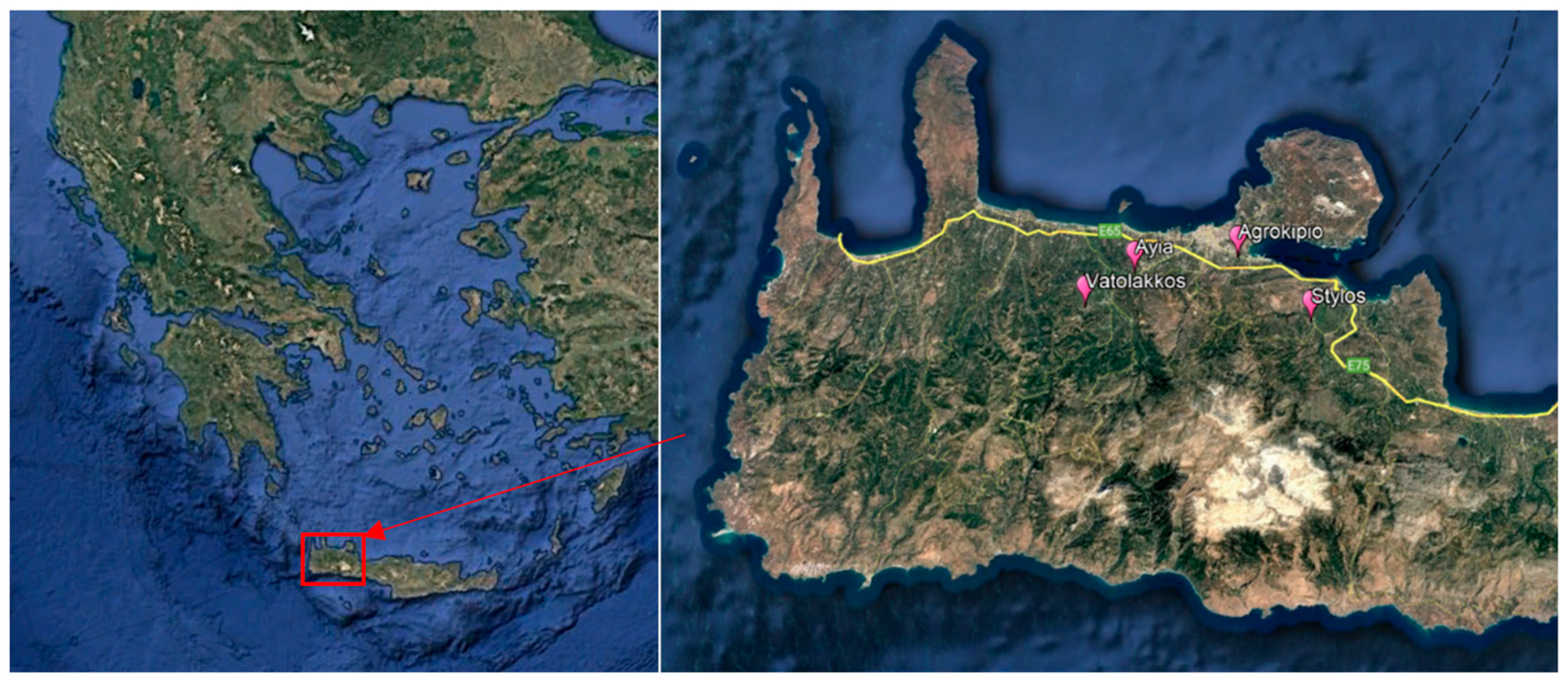


| Area | Co-Ordinates | No of Orchards | Citrus Species | Date of Sampling During Autumn 2020, Spring and Autumn 2021, Spring 2022 |
|---|---|---|---|---|
| Ayia | 35.4678223, 23.9202723 | 4 | Grapefruit, Lemon, Mandarin, Orange | 2020: 5.11 |
| 35.4763655, 23.9419056 35.4715441, 23.9257644 | 2021: 7.4, 19.4, 4.5, 14.5, 27.5, 7.6, 22.6, 10.9, 22.9, 4.10, 15.10, 27.10, 8.11 | |||
| 35.4680203, 23.9196292 | 2022: 19.4, 3.5, 16.5, 26.5, 7.6 | |||
| Vatolakkos | 35.4564375, 23.8971727 35.4519540, 23.8864000 | 2 | Grapefruit, Orange | 2020: 5.11 2021: 14.5, 27.5, 7.6, 22.6, 10.9, 22.9, 4.10, 15.10, 27.10, 8.11 2022: 19.4, 3.5, 16.5, 26.5, 7.6 |
| Apokoronas (Stylos) | 35.4479499, 24.1353730 | 3 | Orange | 2021: 7.4, 9.4, 4.5, 14.5, 27.5, 7.6, 22.6, 10.9, 22.9, 4.10, 15.10, 27.10, 8.11 |
| 35.4464094, 24.1386021 | ||||
| 35.4383835, 24.1322942 | 2022: 19.4, 3.5, 16.5, 26.5, 7.6 | |||
| Agrokipio | 35.4863107, 24.0485113 | 1 | Lemon, Mandarin, Orange | 2021: 14.5, 27.5, 7.6, 22.6 10.9, 22.9, 4.10, 15.10, 27.10, 8.11 2022: 19.4, 3.5, 16.5, 26.5 |
| Model | Features | (%) Accuracy |
|---|---|---|
| DenseNet121 | Spectrogram | 95.6 |
| 5-layer CNN | PSD | 93.3 |
| InceptionV3 | Spectrogram | 95.3 |
| MobileNet | Spectrogram | 95.2 |
| Xception | Spectrogram | 93.9 |
| NASNetMobile | Spectrogram | 94.1 |
| Shallow Learners | ||
| XGBoost | PSD | 87.1 |
| LightGBM | PSD | 86.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathioudakis, M.M.; Varikou, K.; Karagianni, A.; Psirofonia, P.; Tektonidis, N.; Kapantaidaki, D.; Evangelou, V.; Economou, L.; Hasiów-Jaroszewska, B.; Potamitis, I. Aphid Species in Citrus Orchards in Crete: Key Vectors of Citrus Tristeza Virus and Automated Monitoring Innovations for Alate Aphids. Viruses 2025, 17, 395. https://doi.org/10.3390/v17030395
Mathioudakis MM, Varikou K, Karagianni A, Psirofonia P, Tektonidis N, Kapantaidaki D, Evangelou V, Economou L, Hasiów-Jaroszewska B, Potamitis I. Aphid Species in Citrus Orchards in Crete: Key Vectors of Citrus Tristeza Virus and Automated Monitoring Innovations for Alate Aphids. Viruses. 2025; 17(3):395. https://doi.org/10.3390/v17030395
Chicago/Turabian StyleMathioudakis, Matthaios M., Kyriaki Varikou, Antonia Karagianni, Panagiota Psirofonia, Nikolaos Tektonidis, Despoina Kapantaidaki, Vasiliki Evangelou, Leonidas Economou, Beata Hasiów-Jaroszewska, and Ilyas Potamitis. 2025. "Aphid Species in Citrus Orchards in Crete: Key Vectors of Citrus Tristeza Virus and Automated Monitoring Innovations for Alate Aphids" Viruses 17, no. 3: 395. https://doi.org/10.3390/v17030395
APA StyleMathioudakis, M. M., Varikou, K., Karagianni, A., Psirofonia, P., Tektonidis, N., Kapantaidaki, D., Evangelou, V., Economou, L., Hasiów-Jaroszewska, B., & Potamitis, I. (2025). Aphid Species in Citrus Orchards in Crete: Key Vectors of Citrus Tristeza Virus and Automated Monitoring Innovations for Alate Aphids. Viruses, 17(3), 395. https://doi.org/10.3390/v17030395








