A Spatiotemporal Analysis of a High-Resolution Molecular Network Reveals Shifts of HIV-1 Transmission Hotspots in Guangzhou, China
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Molecular Network Analysis
2.3. Geographic Assortativity
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
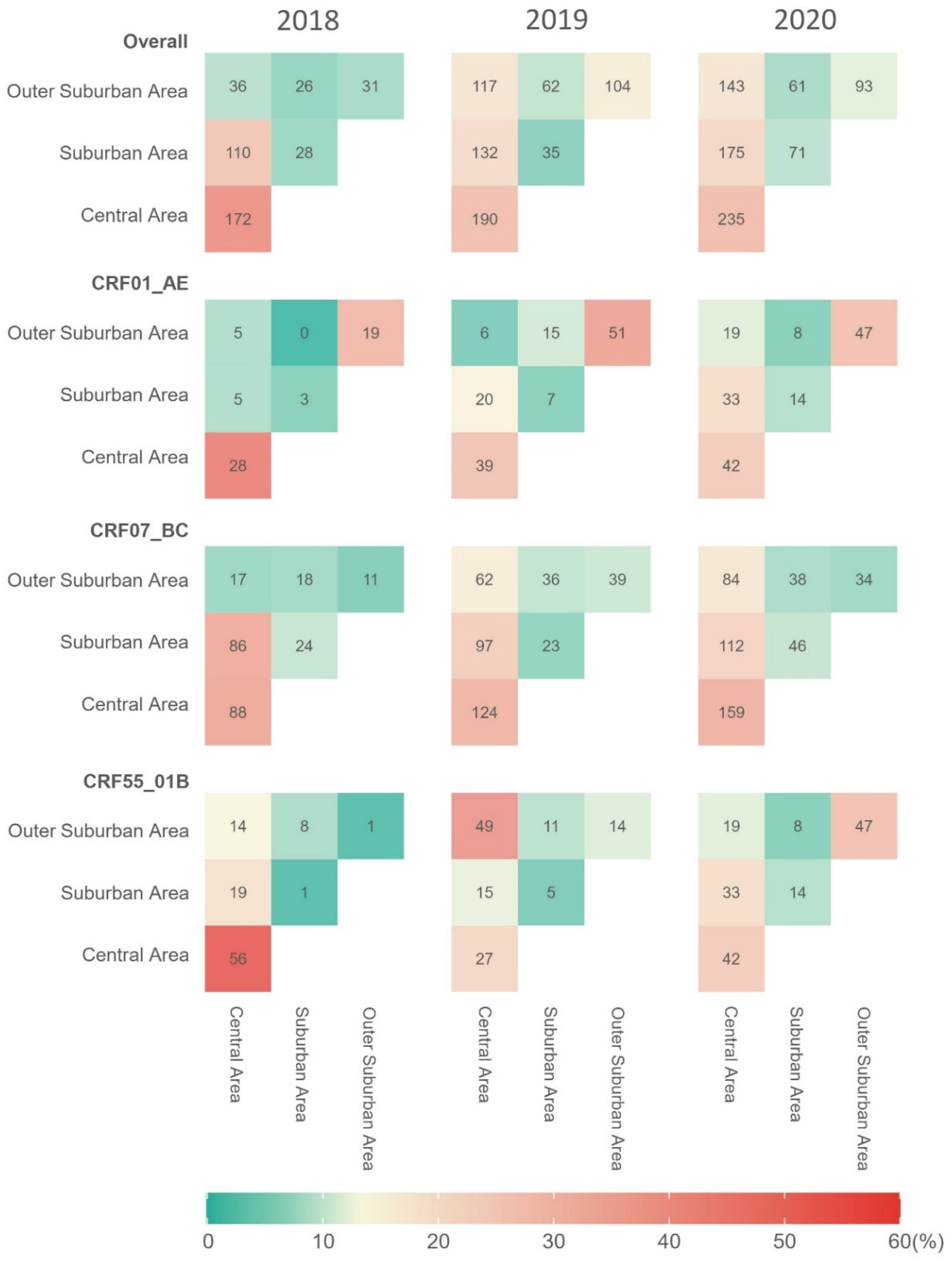
3.2. Spatiotemporal Patterns of Clustering Rates
3.3. Intra-Area and Inter-Area Transmission Links
3.4. Factors Associated with Inter-Area Transmission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet 2024. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 7 July 2024).
- Jia, P.; Yang, S. Time to Spatialise Epidemiology in China. Lancet Glob. Health 2020, 8, e764–e765. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Yu, B.; Liang, S.; Fei, T.; Tang, H.; Kang, R.; Li, Y.; Ye, L.; Jia, P.; Yang, S. HIV-1 Genetic Transmission Networks among People Living with HIV/AIDS in Sichuan, China: A Genomic and Spatial Epidemiological Analysis. Lancet Reg. Health West Pac. 2022, 18, 100318. [Google Scholar] [CrossRef]
- Wirtz, A.L.; Trapence, G.; Kamba, D.; Gama, V.; Chalera, R.; Jumbe, V.; Kumwenda, R.; Mangochi, M.; Helleringer, S.; Beyrer, C.; et al. Geographical Disparities in HIV Prevalence and Care Among Men Who Have Sex with Men in Malawi: Results from a Multisite Cross-sectional Survey. Lancet HIV 2017, 4, e260–e269. [Google Scholar] [CrossRef]
- Anderson, S.J.; Cherutich, P.; Kilonzo, N.; Cremin, I.; Fecht, D.; Kimanga, D.; Harper, M.; Masha, R.L.; Ngongo, P.B.; Maina, W.; et al. Maximising the Effect of Combination HIV Prevention through Prioritisation of the People and Places in Greatest Need: A Modelling Study. Lancet 2014, 384, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Kosakovsky Pond, S.L.; Forgione, L.A.; Mehta, S.R.; Murrell, B.; Shah, S.; Smith, D.M.; Scheffler, K.; Torian, L.V. Social and Genetic Networks of HIV-1 Transmission in New York City. PLoS Pathog. 2017, 13, e1006000. [Google Scholar] [CrossRef]
- Oster, A.M.; France, A.M.; Mermin, J. Molecular Epidemiology and the Transformation of HIV Prevention. JAMA 2018, 319, 1657–1658. [Google Scholar] [CrossRef]
- Gibney, K.B.; Cheng, A.C.; Hall, R.; Leder, K. Sociodemographic and Geographical Inequalities in Notifiable Infectious Diseases in Australia: A Retrospective Analysis of 21 Years of National Disease Surveillance Data. Lancet Infect. Dis. 2017, 17, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Ratmann, O.; Kagaayi, J.; Hall, M.; Golubchick, T.; Kigozi, G.; Xi, X.; Wymant, C.; Nakigozi, G.; Abeler-Dörner, L.; Bonsall, D.; et al. Quantifying HIV Transmission Flow Between High-prevalence Hotspots and Surrounding Communities: A Population-based Study in Rakai, Uganda. Lancet HIV 2020, 7, e173–e183. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, T.; Jiang, L.; Shi, H.; Li, X.; Qiao, M.; Wu, S.; Wu, R.; Yuan, X.; Wang, J.; et al. Combining Molecular Transmission Network Analysis and Spatial Epidemiology to Reveal HIV-1 Transmission Pattern among the Older People in Nanjing, China. Virol. J. 2024, 21, 218. [Google Scholar] [CrossRef]
- Yuan, R.; Cheng, H.; Chen, L.S.; Zhang, X.; Wang, B. Prevalence of Different HIV-1 Subtypes in Sexual Transmission in China: A Systematic Review and Meta-analysis. Epidemiol. Infect. 2016, 144, 2144–2153. [Google Scholar] [CrossRef]
- Xiao, P.; Li, J.; Fu, G.; Zhou, Y.; Huan, X.; Yang, H. Geographic Distribution and Temporal Trends of HIV-1 Subtypes through Heterosexual Transmission in China: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2017, 14, 830. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, J.; Scott, S.R.; McGoogan, J.M. History of the HIV Epidemic in China. Curr. HIV/AIDS Rep. 2019, 16, 458–466. [Google Scholar] [CrossRef]
- Yan, H.; Luo, Y.; Wu, H.; Chen, M.; Li, S.; Tian, Z.; Zou, G.; Tang, S.; Bible, P.W.; Hao, Y.; et al. Evolving Molecular HIV Clusters Revealed Genotype-specific Dynamics in Guangzhou, China (2008–2020). Int. J. Infect. Dis. 2024, 148, 107218. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E.J. Mixing Patterns in Networks. Phys. Rev. E 2003, 67, 026126. [Google Scholar] [CrossRef]
- Skaathun, B.; Ragonnet-Cronin, M.; Poortinga, K.; Sheng, Z.; Hu, Y.W.; Wertheim, J.O. Interplay Between Geography and HIV Transmission Clusters in Los Angeles County. Open Forum Infect. Dis. 2021, 8, ofab211. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, K.; Jiang, J.; Fan, Q.; Ding, X.; Zhong, P.; Xing, H.; Chai, C.; Pan, X. Combining Molecular Network Analysis and Field Epidemiology to Quantify Local HIV Transmission and Highlight Ongoing Epidemics. Int. J. Infect. Dis. 2023, 128, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Stecher, M.; Chaillon, A.; Eis-Hübinger, A.M.; Lehmann, C.; Fätkenheuer, G.; Wasmuth, J.C.; Knops, E.; Vehreschild, J.J.; Mehta, S.; Hoenigl, M. Pretreatment Human Immunodeficiency Virus Type 1 (HIV-1) Drug Resistance in Transmission Clusters of the Cologne-Bonn region, Germany. Clin. Microbiol. Infect. 2019, 25, 253.e1–253.e4. [Google Scholar] [CrossRef]
- Gan, M.; Zheng, S.; Hao, J.; Ruan, Y.; Liao, L.; Shao, Y.; Feng, Y.; Xing, H. The Prevalence of CRF55_01B among HIV-1 Strain and Its Connection with Traffic Development in China. Emerg. Microbes Infect. 2021, 10, 256–265. [Google Scholar] [CrossRef]
- Luo, R.; Xie, Z.; Silenzio, V.M.B.; Kuang, Y.; Luo, D. Gay App Use, Sexuality Traits, and High-Risk Sexual Behaviors among Men Who Have Sex with Men in China: Mediation Analysis. J. Med. Internet Res. 2023, 25, e49137. [Google Scholar] [CrossRef]
- Dai, Z.; Mi, G.; Yu, F.; Chen, G.; Wang, X.; He, Q. Using a Geosocial Networking App to Investigate New HIV Infections and Related Risk Factors among Student and Nonstudent Men Who Have Sex with Men in Chengdu, China: Open Cohort Study. J. Med. Internet Res. 2023, 25, e43493. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xie, B.; He, Q.; Xie, X.; Huang, J.; Tang, K.; Fang, N.; Xie, H.; Ma, J.; Ge, X.; et al. Distinct Rates and Transmission Patterns of Major HIV-1 Subtypes among Men who Have Sex with Men in Guangxi, China. Front. Microbiol. 2023, 14, 1339240. [Google Scholar] [CrossRef] [PubMed]
- Ratmann, O.; Grabowski, M.K.; Hall, M.; Golubchik, T.; Wymant, C.; Abeler-Dorner, L.; Bonsall, D.; Hoppe, A.; Brown, A.L.; de Oliveira, T.; et al. Inferring HIV-1 Transmission Networks and Sources of Epidemic Spread in Africa with Deep-sequence Phylogenetic Analysis. Nat. Commun. 2019, 10, 1411. [Google Scholar] [CrossRef] [PubMed]

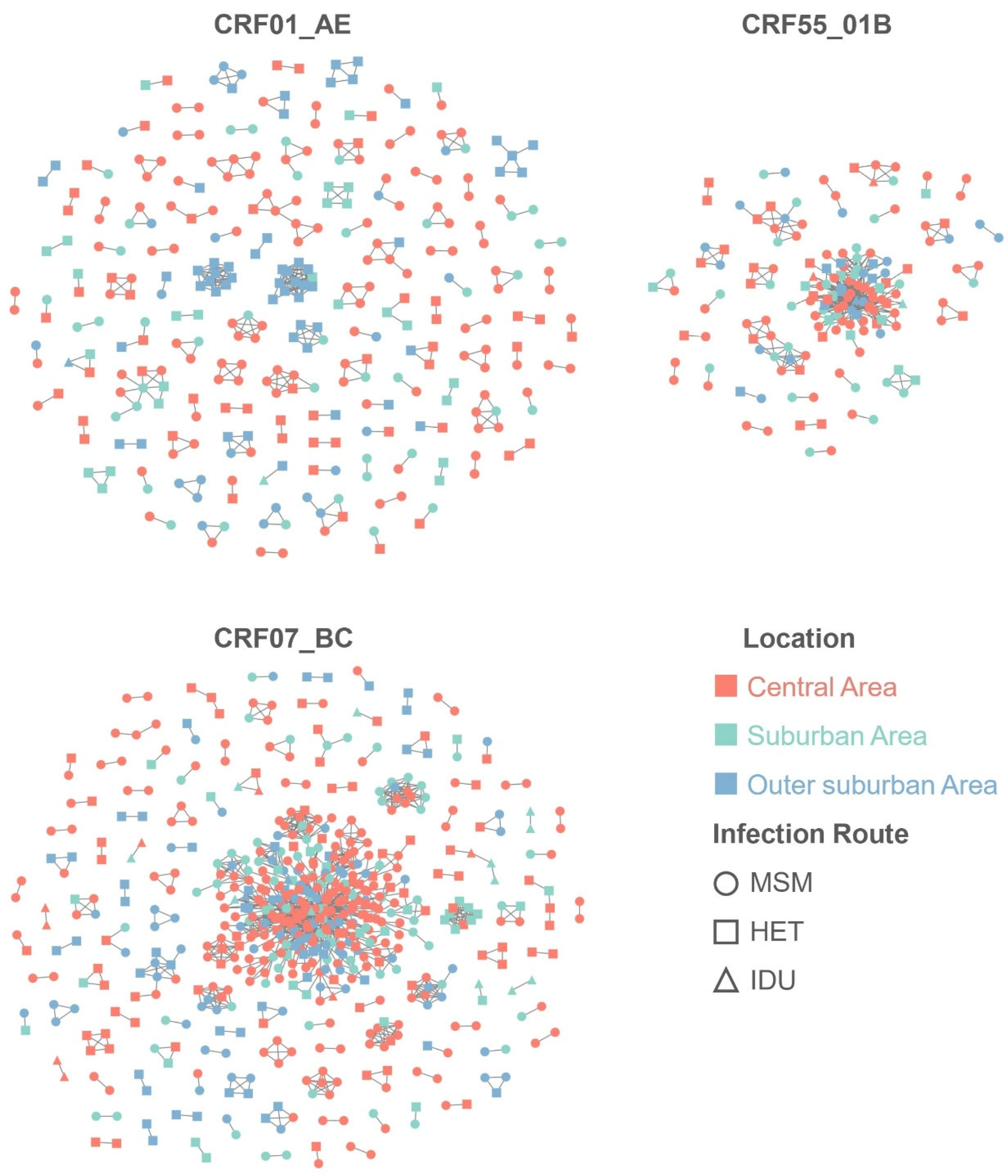

| Area | Overall | CRF01_AE | CRF07_BC | CRF55_01B | ||||
|---|---|---|---|---|---|---|---|---|
| Assortativity | p | Assortativity | p | Assortativity | p | Assortativity | p | |
| Guangzhou | 0.227 | <0.001 | 0.512 | <0.001 | 0.160 | <0.001 | 0.060 | <0.001 |
| Central area | 0.215 | <0.001 | 0.501 | <0.001 | 0.158 | <0.001 | 0.067 | 0.038 |
| Suburban area | 0.120 | <0.001 | 0.235 | <0.001 | 0.086 | 0.002 | 0.092 | 0.017 |
| Outer suburban area | 0.344 | <0.001 | 0.693 | <0.001 | 0.253 | <0.001 | 0.026 | 0.174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Lu, Y.; Li, S.; Wu, H.; Hu, J.; Luo, Y.; Li, Q.; Lai, L.; Huang, W.; Gu, J.; et al. A Spatiotemporal Analysis of a High-Resolution Molecular Network Reveals Shifts of HIV-1 Transmission Hotspots in Guangzhou, China. Viruses 2025, 17, 384. https://doi.org/10.3390/v17030384
Yan H, Lu Y, Li S, Wu H, Hu J, Luo Y, Li Q, Lai L, Huang W, Gu J, et al. A Spatiotemporal Analysis of a High-Resolution Molecular Network Reveals Shifts of HIV-1 Transmission Hotspots in Guangzhou, China. Viruses. 2025; 17(3):384. https://doi.org/10.3390/v17030384
Chicago/Turabian StyleYan, Huanchang, Yifan Lu, Shunming Li, Hao Wu, Jingyang Hu, Yefei Luo, Qingmei Li, Lingxuan Lai, Weiping Huang, Jing Gu, and et al. 2025. "A Spatiotemporal Analysis of a High-Resolution Molecular Network Reveals Shifts of HIV-1 Transmission Hotspots in Guangzhou, China" Viruses 17, no. 3: 384. https://doi.org/10.3390/v17030384
APA StyleYan, H., Lu, Y., Li, S., Wu, H., Hu, J., Luo, Y., Li, Q., Lai, L., Huang, W., Gu, J., Ma, L., Hao, Y., Han, Z., Chen, X.-l., & Liu, Y. (2025). A Spatiotemporal Analysis of a High-Resolution Molecular Network Reveals Shifts of HIV-1 Transmission Hotspots in Guangzhou, China. Viruses, 17(3), 384. https://doi.org/10.3390/v17030384






