Differential Responses of Pediatric and Adult Primary Epithelial Cells to Human Metapneumovirus and Respiratory Syncytial Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
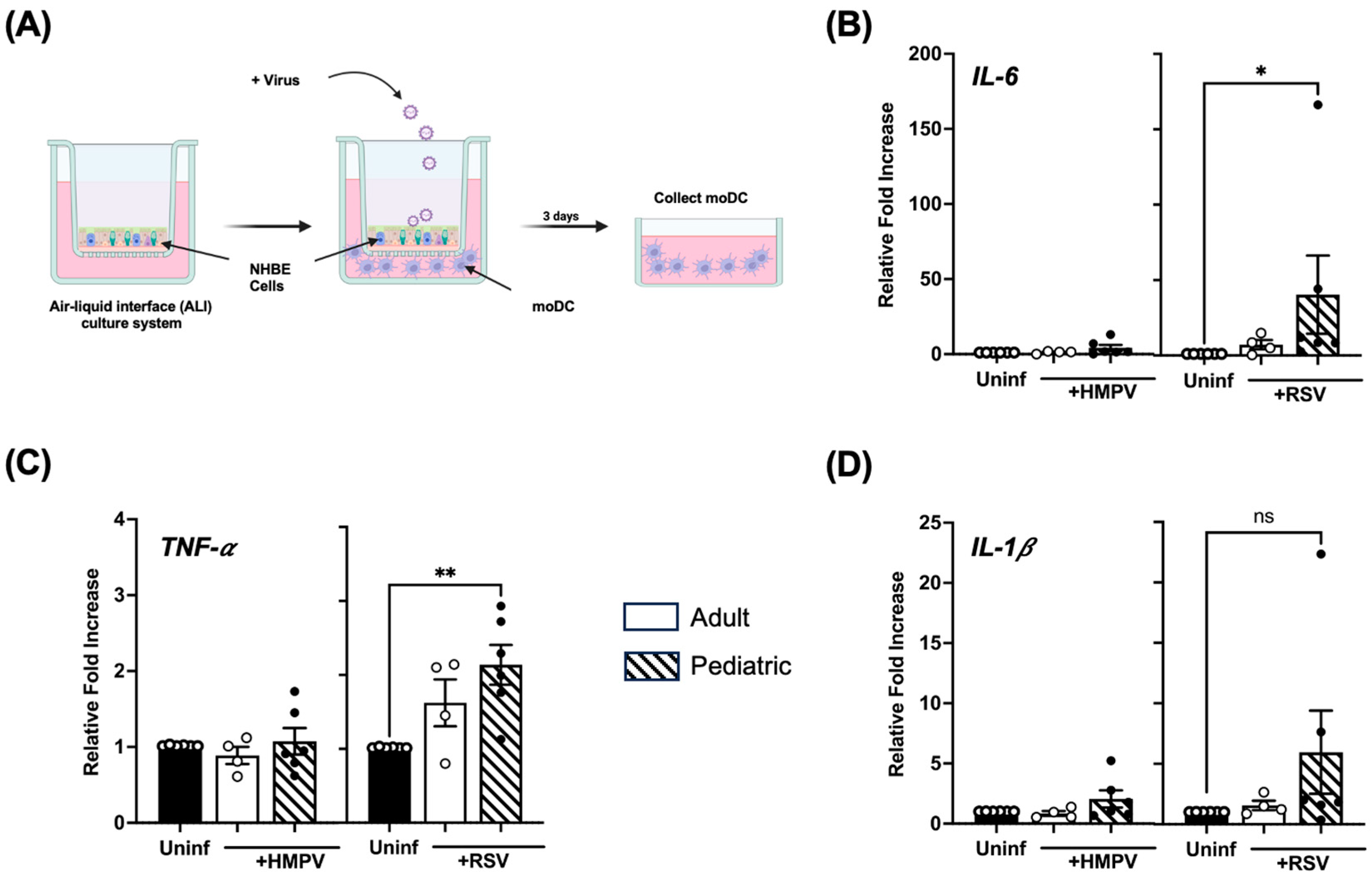
2.2. Establishment of Monocyte-Derived Dendritic Cells
2.3. Virus Stock
2.4. Viral Infection
2.5. RNA Extraction
2.6. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
2.7. Multiplex Cytokine Expression Analysis
2.8. Statistical Analysis
3. Results
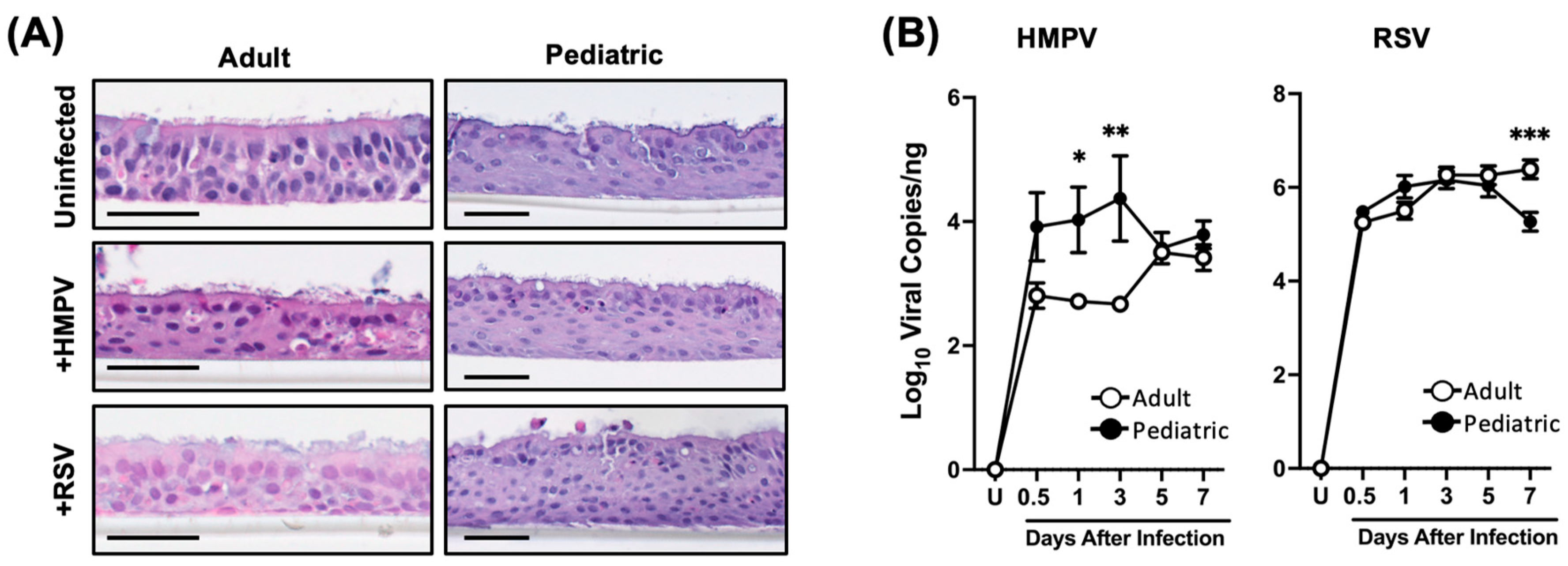
3.1. Differential Susceptibility of Pediatric and Adult NHBE Cells to HMPV and RSV Infection
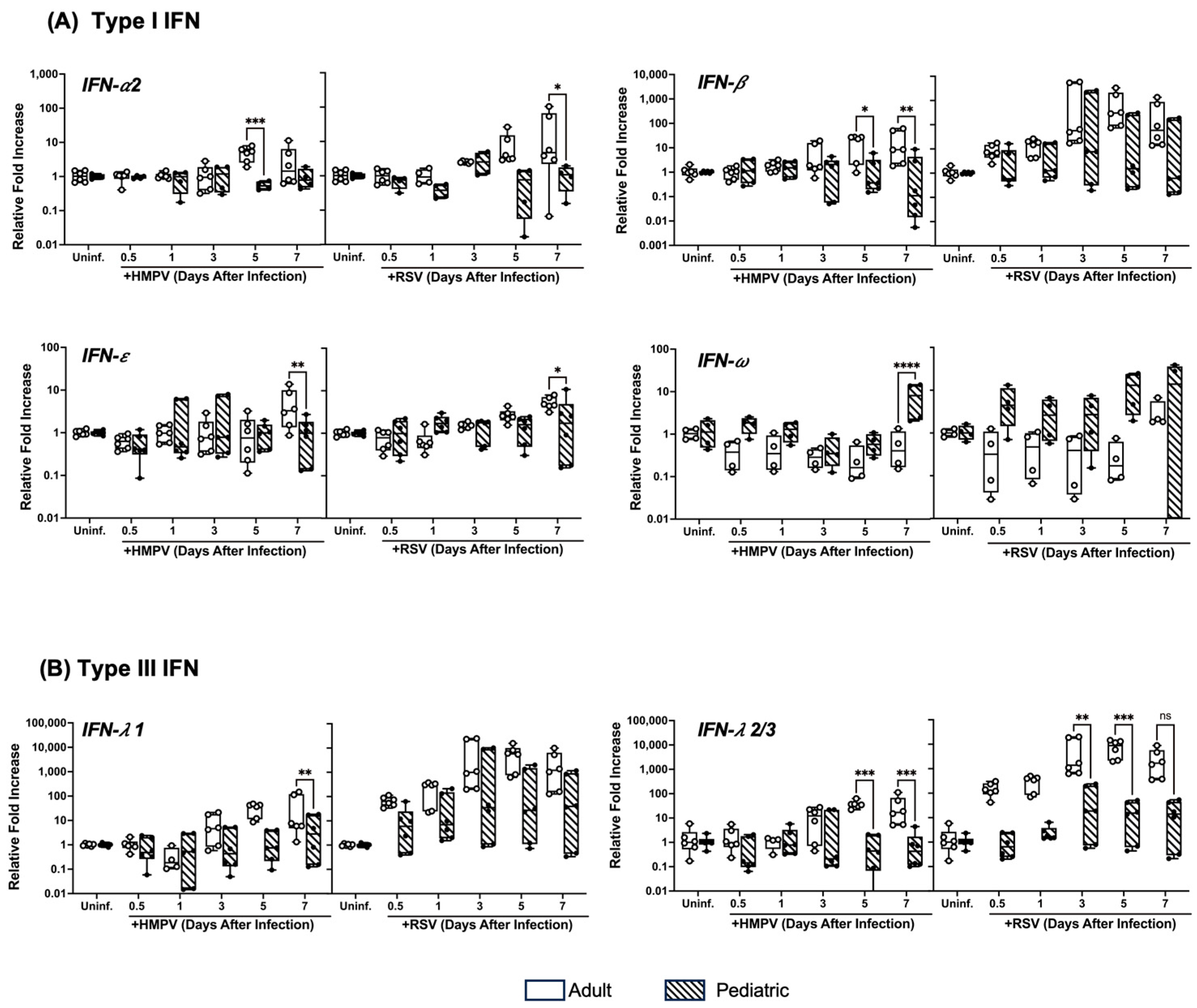
3.2. Pediatric NHBE Cells Express IFN Responses That Are Distinct from Those in Adult Cells upon Infection with RSV or HMPV
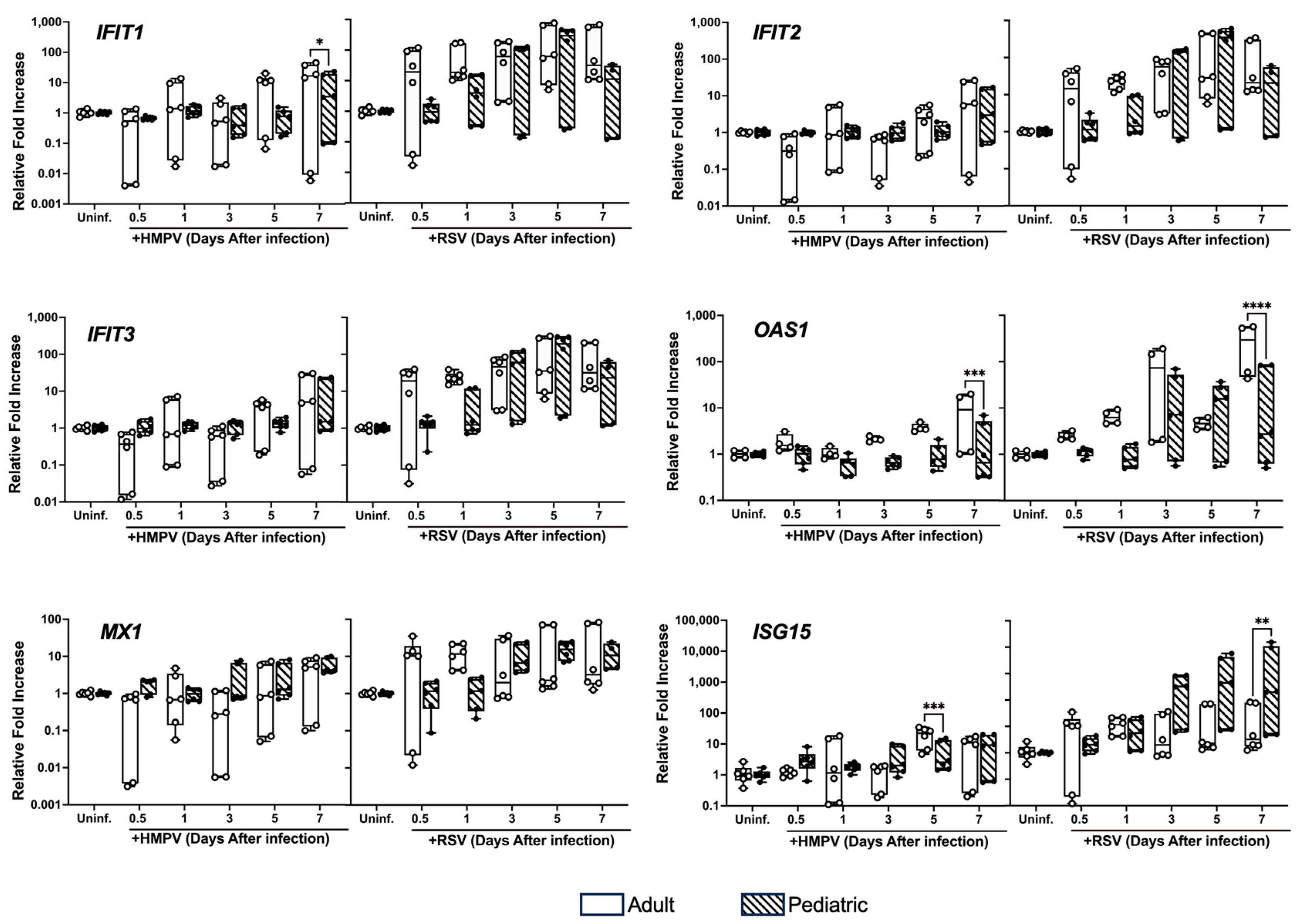
3.3. Differential Expression of ISGs in NHBE Cells from Adults and Children Infected with HMPV and RSV
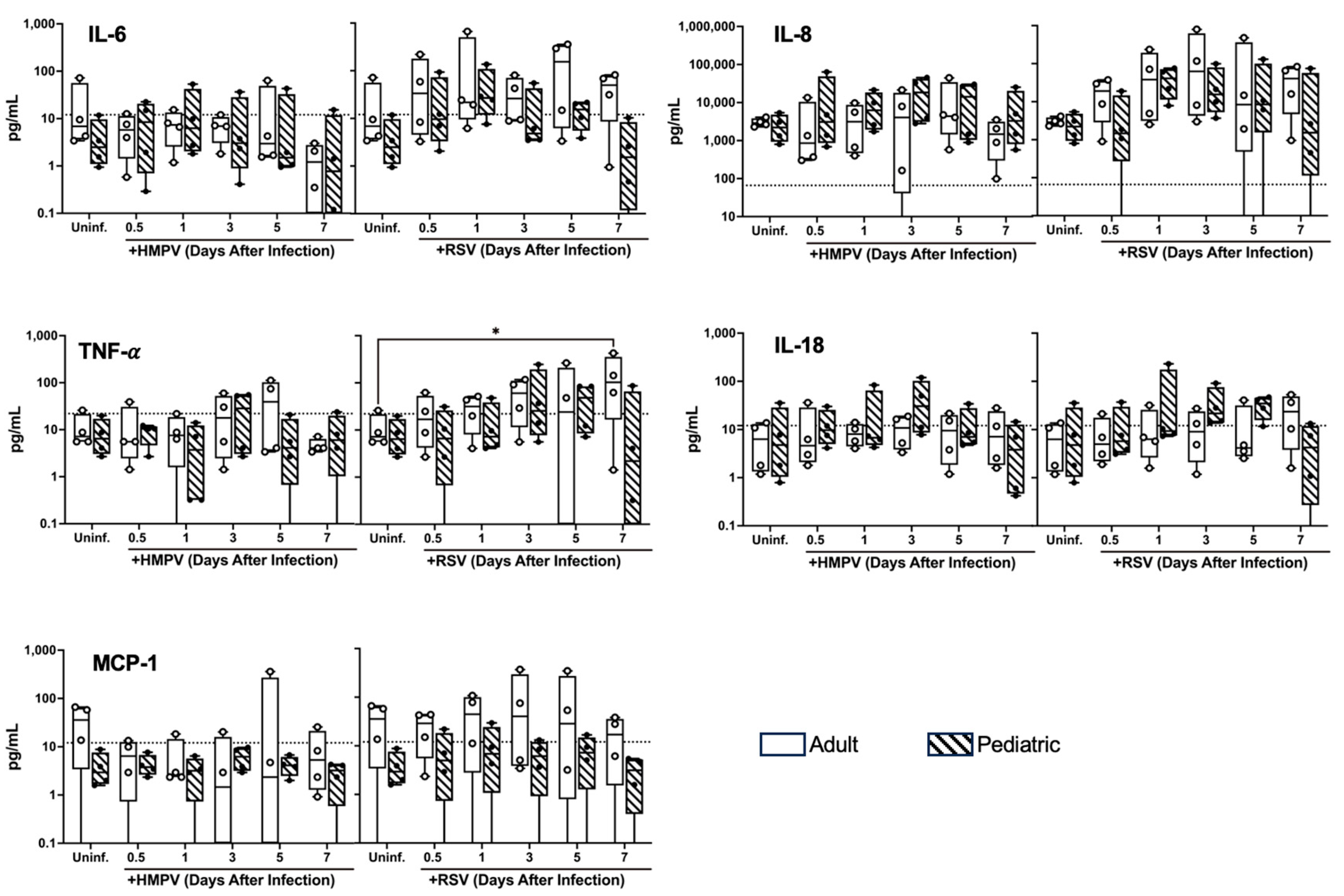
3.4. Differential Cytokine Expression of NHBE Cells from Adults and Children Infected with HMPV and RSV
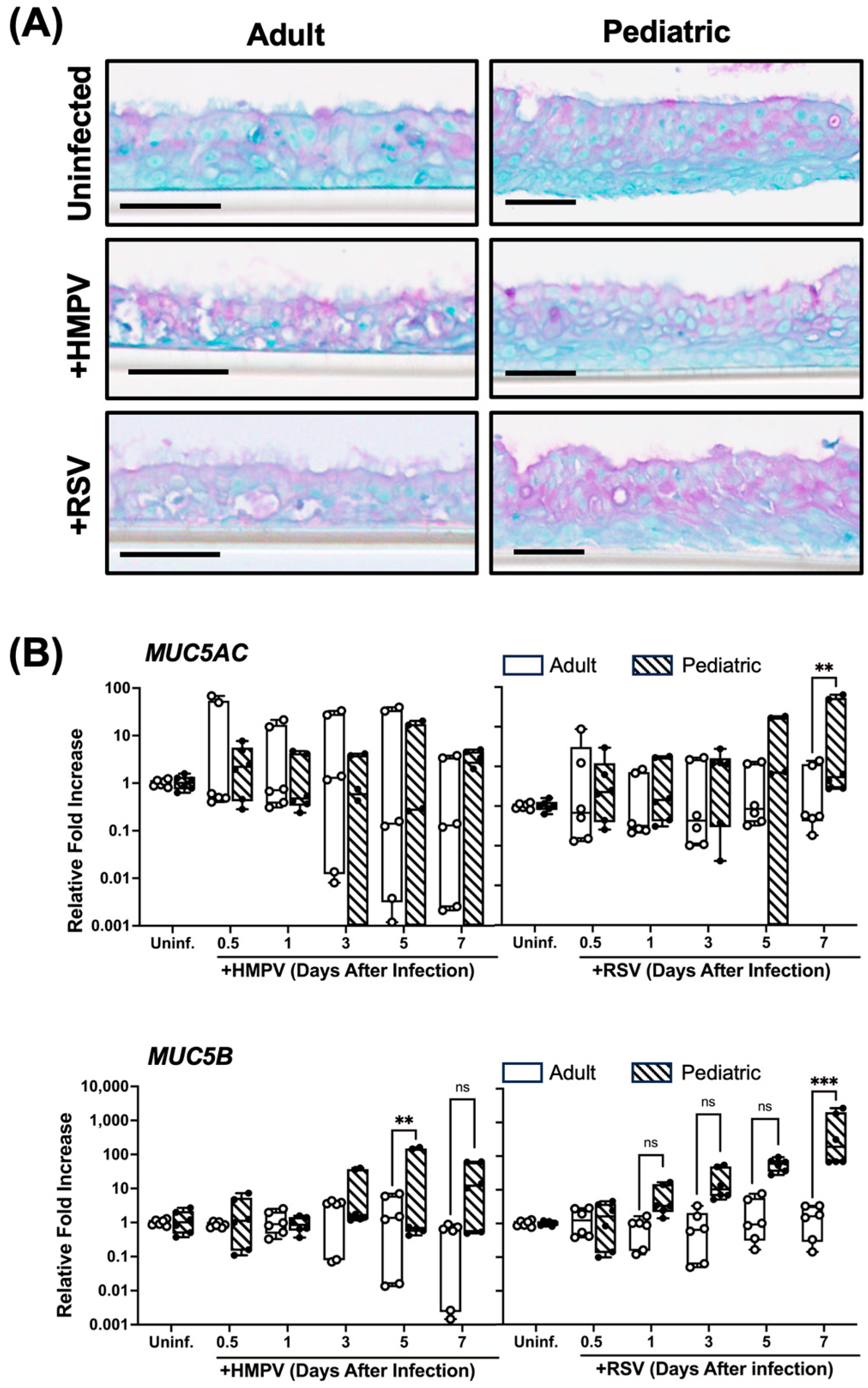
3.5. Higher Mucin Expression in RSV-Infected Pediatric NHBE Cells than in Adult Cells
3.6. Infection of Pediatric NHBE Cells by HMPV or RSV Drives Higher Stimulation of Inflammatory Cytokines in Dendritic Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Soto, J.A.; Stranger, V.; Rivera, T.; Vásquez, A.E.; Kalergis, A.M. Host Components That Modulate the Disease Caused by hMPV. Viruses 2021, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.; et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef]
- Pierangeli, A.; Piralla, A.; Uceda Renteria, S.; Giacomel, G.; Lunghi, G.; Pagani, E.; Giacobazzi, E.; Vian, E.; Biscaro, V.; Piccirilli, G.; et al. Multicenter epidemiological investigation and genetic characterization of respiratory syncytial virus and metapneumovirus infections in the pre-pandemic 2018–2019 season in northern and central Italy. Clin. Exp. Med. 2022, 23, 2725–2737. [Google Scholar] [CrossRef] [PubMed]
- Azar, B.; Hashavya, S.; Ohana Sarna Cahan, L.; Reif, S.; Gross, I. Bronchiolitis Due to RSV and HMPV—Epidemiology, Clinical Course, and Prognosis: Experience of a Single Tertiary Center. Clin. Pediatr. 2023, 62, 1032–1039. [Google Scholar] [CrossRef]
- Ng, D.C.E.; Liew, C.H.; Tan, K.K.; Awang, E.H.B.; Nazri, F.N.B.A.; Maran, A.K.T.; Mohan, V.A.A.L.C.; Ramachandran, D.; Chok, M.; Teh, C.H.; et al. Clinical comparison of HMPV and RSV infections in hospitalised Malaysian children: A propensity score matched study. Clin. Respir. J. 2024, 18, e13747. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.; Nguyen, D.C.; Briggs, B.J.; Nam, H.H. The burden of RSV, hMPV, and PIV amongst hospitalized adults in the United States from 2016 to 2019. J. Hosp. Med. 2024, 19, 581–588. [Google Scholar] [CrossRef]
- Hillyer, P.; Shepard, R.; Uehling, M.; Krenz, M.; Sheikh, F.; Thayer, K.R.; Huang, L.; Yan, L.; Panda, D.; Luongo, C.; et al. Differential Responses by Human Respiratory Epithelial Cell Lines to Respiratory Syncytial Virus Reflect Distinct Patterns of Infection Control. J. Virol. 2018, 92, e02202–e02217. [Google Scholar] [CrossRef]
- Rijsbergen, L.C.; Van Dijk, L.L.A.; Engel, M.F.M.; De Vries, R.D.; De Swart, R.L. In Vitro Modelling of Respiratory Virus Infections in Human Airway Epithelial Cells—A Systematic Review. Front. Immunol. 2021, 12, 683002. [Google Scholar] [CrossRef]
- Persson, B.D.; Jaffe, A.B.; Fearns, R.; Danahay, H. Respiratory Syncytial Virus Can Infect Basal Cells and Alter Human Airway Epithelial Differentiation. PLoS ONE 2014, 9, e102368. [Google Scholar] [CrossRef]
- Bao, X.; Liu, T.; Spetch, L.; Kolli, D.; Garofalo, R.P.; Casola, A. Airway epithelial cell response to human metapneumovirus infection. Virology 2007, 368, 91–101. [Google Scholar] [CrossRef]
- Kinder, J.T.; Moncman, C.L.; Barrett, C.; Jin, H.; Kallewaard, N.; Dutch, R.E. Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies. J. Virol. 2020, 94, e01068-20. [Google Scholar] [CrossRef]
- Chuquimia, O.D.; Petursdottir, D.H.; Periolo, N.; Fernández, C. Alveolar Epithelial Cells Are Critical in Protection of the Respiratory Tract by Secretion of Factors Able To Modulate the Activity of Pulmonary Macrophages and Directly Control Bacterial Growth. Infect. Immun. 2013, 81, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Casola, A.; Suarez, G.; Yu, X.; Spetch, L.; Peeples, M.E.; Garofalo, R.P. Differential Response of Dendritic Cells to Human Metapneumovirus and Respiratory Syncytial Virus. Am. J. Respir. Cell Mol. Biol. 2006, 34, 320–329. [Google Scholar] [CrossRef]
- Biacchesi, S.; Skiadopoulos, M.H.; Tran, K.C.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Recovery of human metapneumovirus from cDNA: Optimization of growth in vitro and expression of additional genes. Virology 2004, 321, 247–259. [Google Scholar] [CrossRef]
- Guerrero-Plata, A.; Baron, S.; Poast, J.S.; Adegboyega, P.A.; Casola, A.; Garofalo, R.P. Activity and Regulation of Alpha Interferon in Respiratory Syncytial Virus and Human Metapneumovirus Experimental Infections. J. Virol. 2005, 79, 10190–10199. [Google Scholar] [CrossRef] [PubMed]
- Ueba, O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med. Okayama 1978, 32, 265–272. [Google Scholar]
- Kisch, A.L.; Johnson, K.M. A plaque assay for respiratory syncytial virus. Proc. Soc. Exp. Biol. Med. 1963, 112, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peeples, M.E.; Boucher, R.C.; Collins, P.L.; Pickles, R.J. Respiratory Syncytial Virus Infection of Human Airway Epithelial Cells Is Polarized, Specific to Ciliated Cells, and without Obvious Cytopathology. J. Virol. 2002, 76, 5654–5666. [Google Scholar] [CrossRef]
- Talukdar, S.N.; Osan, J.; Ryan, K.; Grove, B.; Perley, D.; Kumar, B.D.; Yang, S.; Dallman, S.; Hollingsworth, L.; Bailey, K.L.; et al. RSV-induced expanded ciliated cells contribute to bronchial wall thickening. Virus Res. 2023, 327, 199060. [Google Scholar] [CrossRef]
- Kuiken, T.; Van Den Hoogen, B.G.; Van Riel, D.A.J.; Laman, J.D.; Van Amerongen, G.; Sprong, L.; Fouchier, R.A.M.; Osterhaus, A.D.M.E. Experimental Human Metapneumovirus Infection of Cynomolgus Macaques (Macaca fascicularis) Results in Virus Replication in Ciliated Epithelial Cells and Pneumocytes with Associated Lesions throughout the Respiratory Tract. Am. J. Pathol. 2004, 164, 1893–1900. [Google Scholar] [CrossRef]
- Guo, T.J.F.; Singhera, G.K.; Leung, J.M.; Dorscheid, D.R. Airway Epithelial-Derived Immune Mediators in COVID-19. Viruses 2023, 15, 1655. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, V.G.; Lukassen, S.; Drechsler, M.; Loske, J.; Burkart, S.S.; Wüst, S.; Jacobsen, E.-M.; Röhmel, J.; Mall, M.A.; Debatin, K.-M.; et al. Enhanced Airway Epithelial Response to SARS-CoV-2 Infection in Children is Critically Tuned by the Cross-Talk Between Immune and Epithelial Cells. BioRxiv 2023, 27, e57912. [Google Scholar] [CrossRef]
- Glaser, L.; Coulter, P.J.; Shields, M.; Touzelet, O.; Power, U.F.; Broadbent, L. Airway Epithelial Derived Cytokines and Chemokines and Their Role in the Immune Response to Respiratory Syncytial Virus Infection. Pathogens 2019, 8, 106. [Google Scholar] [CrossRef]
- Duan, W.; Cen, Y.; Lin, C.; Ouyang, H.; Du, K.; Kumar, A.; Wang, B.; Avolio, J.; Grasemann, H.; Moraes, T.J. Inflammatory epithelial cytokines after in vitro respiratory syncytial viral infection are associated with reduced lung function. ERJ Open Res. 2021, 7, 00365–02021. [Google Scholar] [CrossRef] [PubMed]
- Belikova, M.; Säfholm, J.; Al-Ameri, M.; Orre, A.C.; Dahlén, S.E.; Adner, M. Combined exposure to the alarmins TSLP, IL-33 and IL-25 enhances mast cell-dependent contractions of human bronchi. Clin. Exp. Allergy 2023, 53, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Claudio, E.; Wang, H.; Kamenyeva, O.; Tang, W.; Ha, H.-L.; Siebenlist, U. IL-25 Orchestrates Activation of Th Cells via Conventional Dendritic Cells in Tissue to Exacerbate Chronic House Dust Mite–Induced Asthma Pathology. J. Immunol. 2019, 203, 2319–2327. [Google Scholar] [CrossRef]
- Hong, H.; Liao, S.; Chen, F.; Yang, Q.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804. [Google Scholar] [CrossRef]
- Becker, M.E.; Martin-Sancho, L.; Simons, L.M.; McRaven, M.D.; Chanda, S.K.; Hultquist, J.F.; Hope, T.J. Live imaging of airway epithelium reveals that mucociliary clearance modulates SARS-CoV-2 spread. Nat. Commun. 2024, 15, 9480. [Google Scholar] [CrossRef]
- Kaler, L.; Engle, E.M.; Corkran, M.; Iverson, E.; Boboltz, A.; Ignacio, M.A.; Yeruva, T.; Scull, M.A.; Duncan, G.A. Mucus Physically Restricts Influenza A Viral Particle Access to the Epithelium. Adv. Biol. 2025, 12, e2400329. [Google Scholar] [CrossRef]
- Rajan, D.; O’Keefe, E.L.; Travers, C.; McCracken, C.; Geoghegan, S.; Caballero, M.T.; Acosta, P.L.; Polack, F.; Anderson, L.J. MUC5AC Levels Associated With Respiratory Syncytial Virus Disease Severity. Clin. Infect. Dis. 2018, 67, 1441–1444. [Google Scholar] [CrossRef]
- Vargas, S.O.; Kozakewich, H.P.; Perez-Atayde, A.R.; McAdam, A.J. Pathology of human metapneumovirus infection: Insights into the pathogenesis of a newly identified respiratory virus. Pediatr. Dev. Pathol. 2004, 7, 478–486; discussion 421. [Google Scholar] [CrossRef]
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial cell–derived cytokines: More than just signaling the alarm. J. Clin. Investig. 2019, 129, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Malinczak, C.-A.; Parolia, A.; Fonseca, W.; Morris, S.; Rasky, A.J.; Bawa, P.; Zhang, Y.; Mire, M.M.; Ziegler, S.F.; Ptaschinski, C.; et al. TSLP-Driven Chromatin Remodeling and Trained Systemic Immunity after Neonatal Respiratory Viral Infection. J. Immunol. 2021, 206, 1315–1328. [Google Scholar] [CrossRef]
- El-Qutob, D.; Letran, A. TSLP and asthma: Fellow travelers. Explor. Asthma Allergy 2023, 1, 4–10. [Google Scholar] [CrossRef]
- Nadiger, M.; Sendi, P.; Martinez, P.A.; Totapally, B.R. Epidemiology and Clinical Features of Human Metapneumovirus and Respiratory Syncytial Viral Infections in Children. Pediatr. Infect. Dis. J. 2023, 42, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Rateeb, A.; Abdelrazic, M.; Eid, W.; Abdelrazik, E. COVID-19 IN Children. Minia J. Med. Res. 2022, 33, 197–201. [Google Scholar] [CrossRef]
- Magklara, K.; Kyriakopoulos, M. The impact of the COVID-19 pandemic on children and young people. Psychiatriki 2023, 34, 265–268. [Google Scholar] [CrossRef]
- Rahmat Budaya, A.G.; Esa Darussalam, A.H.; Takahasi, T.F. COVID-19 Comorbidity In Children. OPSearch Am. J. Open Res. 2023, 2, 575–580. [Google Scholar] [CrossRef]
- Andrade, C.A.; Pacheco, G.A.; Gálvez, N.M.S.; Soto, J.A.; Bueno, S.M.; Kalergis, A.M. Innate Immune Components That Regulate the Pathogenesis and Resolution of hRSV and hMPV Infections. Viruses 2020, 12, 637. [Google Scholar] [CrossRef]
- Roussy, J.-F.; Carbonneau, J.; Ouakki, M.; Papenburg, J.; Hamelin, M.-È.; De Serres, G.; Boivin, G. Human metapneumovirus viral load is an important risk factor for disease severity in young children. J. Clin. Virol. 2014, 60, 133–140. [Google Scholar] [CrossRef]
- Bosis, S.; Esposito, S.; Osterhaus, A.D.M.E.; Tremolati, E.; Begliatti, E.; Tagliabue, C.; Corti, F.; Principi, N.; Niesters, H.G.M. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J. Clin. Virol. 2008, 42, 286–290. [Google Scholar] [CrossRef]
- Peng, D.; Zhao, X.; Liu, E.; Huang, Y.; Yang, X.; Zhao, Y.; Chen, X.; Zhang, Z. Analysis of viral load in children infected with human metapneumovirus. Iran. J. Pediatr. 2010, 20, 393–400. [Google Scholar] [PubMed]
- Cormier, S.A.; Shrestha, B.; Saravia, J.; Lee, G.I.; Shen, L.; Devincenzo, J.P.; Kim, Y.-I.; You, D. Limited Type I Interferons and Plasmacytoid Dendritic Cells during Neonatal Respiratory Syncytial Virus Infection Permit Immunopathogenesis upon Reinfection. J. Virol. 2014, 88, 9350–9360. [Google Scholar] [CrossRef]
- Cheemarla, N.R.; Uche, I.K.; McBride, K.; Naidu, S.; Guerrero-Plata, A. In utero tobacco smoke exposure alters lung inflammation, viral clearance, and CD8+ T-cell responses in neonatal mice infected with respiratory syncytial virus. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 317, L212–L221. [Google Scholar] [CrossRef]
- Škorvanová, L.; Švančarová, P.; Svetlíková, D.; Betáková, T. Protective efficacy of IFN-ω AND IFN-λs against influenza viruses in induced A549 cells. Acta Virol. 2015, 59, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Le Pen, J.; Rice, C.M. The antiviral state of the cell: Lessons from SARS-CoV-2. Curr. Opin. Immunol. 2024, 87, 102426. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Perng, Y.-C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; He, W.; Chen, J.; Yang, G.; Chen, L.; Chang, H. Free ISG15 inhibits Pseudorabies virus infection by positively regulating type I IFN signaling. PLoS Pathog. 2022, 18, e1010921. [Google Scholar] [CrossRef]
- Zhao, C.; Collins, M.N.; Hsiang, T.-Y.; Krug, R.M. Interferon-induced ISG15 pathway: An ongoing virus–host battle. Trends Microbiol. 2013, 21, 181–186. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Han, J.; Dakhama, A.; Jia, Y.; Wang, M.; Zeng, W.; Takeda, K.; Shiraishi, Y.; Okamoto, M.; Ziegler, S.F.; Gelfand, E.W. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J. Allergy Clin. Immunol. 2012, 130, 1175–1186.e1179. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Saravia, J.; Siefker, D.; Shrestha, B.; Cormier, S.A. Crawling with Virus: Translational Insights from a Neonatal Mouse Model on the Pathogenesis of Respiratory Syncytial Virus in Infants. J. Virol. 2016, 90, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Laham, F.R.; Israele, V.; Casellas, J.M.; Garcia, A.M.; Lac Prugent, C.M.; Hoffman, S.J.; Hauer, D.; Thumar, B.; Name, M.I.; Pascual, A.; et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J. Infect. Dis. 2004, 189, 2047–2056. [Google Scholar] [CrossRef]
- Sebina, I.; Phipps, S. The Contribution of Neutrophils to the Pathogenesis of RSV Bronchiolitis. Viruses 2020, 12, 808. [Google Scholar] [CrossRef]
- Berdat, P.A.; Wehrle, T.J.; Küng, A.; Achermann, F.; Sutter, M.; Carrel, T.P.; Nydegger, U.E. Age-Specific Analysis of Normal Cytokine Levels in Healthy Infants. Clin. Chem. Lab. Med. 2003, 41, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, W.; Lukacs, N.W.; Ptaschinski, C. Factors Affecting the Immunity to Respiratory Syncytial Virus: From Epigenetics to Microbiome. Front. Immunol. 2018, 9, 226. [Google Scholar] [CrossRef]
- Duchesne, M.; Okoye, I.; Lacy, P. Epithelial cell alarmin cytokines: Frontline mediators of the asthma inflammatory response. Front. Immunol. 2022, 13, 975914. [Google Scholar] [CrossRef]
- Itazawa, T.; Morita, E.; Koga, T.; Okada, K.; Shimizu, T.; Ueda, Y. The Value of IL-25, IL-33, and TSLP in Nasal Secretions as a Biomarker of Severity of Allergic Rhinitis in Children. J. Allergy Clin. Immunol. 2023, 151, AB207. [Google Scholar] [CrossRef]
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The Role of Airway Epithelial Cell Alarmins in Asthma. Cells 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.K.; Céspedes, P.F.; Palavecino, C.E.; León, M.A.; Díaz, R.A.; Salazar, F.J.; Méndez, G.P.; Bueno, S.M.; Kalergis, A.M. Human metapneumovirus infection activates the TSLP pathway that drives excessive pulmonary inflammation and viral replication in mice. Eur. J. Immunol. 2015, 45, 1680–1695. [Google Scholar] [CrossRef]
- Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Siefker, D.; Lee, G.I.; Harding, J.N.; Jones, T.L.; Rovnaghi, C.; Bagga, B.; et al. Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33. PLoS Pathog. 2015, 11, e1005217. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Huang, L.Z.X.; Mykytyn, A.Z.; Wang, C.; Lamers, M.M.; Westendorp, B.; Wubbolts, R.W.; Van Putten, J.P.M.; Bosch, B.-J.; Haagmans, B.L.; et al. Glycosylated extracellular mucin domains protect against SARS-CoV-2 infection at the respiratory surface. PLoS Pathog. 2023, 19, e1011571. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Iverson, E.; Kaler, L.; Boboltz, A.; Scull, M.A.; Duncan, G.A. MUC5B mobilizes and MUC5AC spatially aligns mucociliary transport on human airway epithelium. Sci. Adv. 2022, 8, eabq5049. [Google Scholar] [CrossRef]
- Wu, D.; Xiang, Y. Role of mucociliary clearance system in respiratory diseases. J. Cent. South Univ. Med. Sci. 2023, 48, 275–284. [Google Scholar]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef]
- Schraml, B.U.; Reis e Sousa, C. Defining dendritic cells. Curr. Opin. Immunol. 2015, 32, 13–20. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hemmi, H. Dendritic Cells: Translating Innate to Adaptive Immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, R.P.; Kato, A.; Kern, R.; Kuperman, D.; Avila, P.C. Epithelium: At the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 2007, 120, 1279–1284. [Google Scholar] [CrossRef]
- Dodge, I.L.; Carr, M.W.; Cernadas, M.; Brenner, M.B. IL-6 Production by Pulmonary Dendritic Cells Impedes Th1 Immune Responses. J. Immunol. 2003, 170, 4457–4464. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chen, S.H.; Wang, J.Y. Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J. Mol. Med. 2016, 94, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, H.W.; Song, W.J.; Jeon, E.Y.; Bang, B.; Shim, E.J.; Moon, H.G.; Kim, Y.K.; Kang, H.R.; Min, K.U.; et al. TNF-α enhance Th2 and Th17 immune responses regulating by IL23 during sensitization in asthma model. Cytokine 2016, 79, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Maltby, S.; Simpson, J.L.; Eyers, F.; Baines, K.J.; Gibson, P.G.; Foster, P.S.; Yang, M. TNF-α and Macrophages Are Critical for Respiratory Syncytial Virus–Induced Exacerbations in a Mouse Model of Allergic Airways Disease. J. Immunol. 2016, 196, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Paplinska-Goryca, M.; Misiukiewicz-Stepien, P.; Proboszcz, M.; Nejman-Gryz, P.; Gorska, K.; Krenke, R. The Expressions of TSLP, IL-33, and IL-17A in Monocyte Derived Dendritic Cells from Asthma and COPD Patients are Related to Epithelial–Macrophage Interactions. Cells 2020, 9, 1944. [Google Scholar] [CrossRef]
- Smolinska, S.; Antolín-Amérigo, D.; Popescu, F.-D.; Jutel, M. Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma. Int. J. Mol. Sci. 2023, 24, 12725. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babawale, P.I.; Guerrero-Plata, A. Differential Responses of Pediatric and Adult Primary Epithelial Cells to Human Metapneumovirus and Respiratory Syncytial Virus Infection. Viruses 2025, 17, 380. https://doi.org/10.3390/v17030380
Babawale PI, Guerrero-Plata A. Differential Responses of Pediatric and Adult Primary Epithelial Cells to Human Metapneumovirus and Respiratory Syncytial Virus Infection. Viruses. 2025; 17(3):380. https://doi.org/10.3390/v17030380
Chicago/Turabian StyleBabawale, Pius I., and Antonieta Guerrero-Plata. 2025. "Differential Responses of Pediatric and Adult Primary Epithelial Cells to Human Metapneumovirus and Respiratory Syncytial Virus Infection" Viruses 17, no. 3: 380. https://doi.org/10.3390/v17030380
APA StyleBabawale, P. I., & Guerrero-Plata, A. (2025). Differential Responses of Pediatric and Adult Primary Epithelial Cells to Human Metapneumovirus and Respiratory Syncytial Virus Infection. Viruses, 17(3), 380. https://doi.org/10.3390/v17030380





