A Case of Severe Respiratory Failure Caused by Metapneumovirus and Influenza Virus in a Patient with HIV Infection
Abstract
1. Introduction
2. Case Presentation
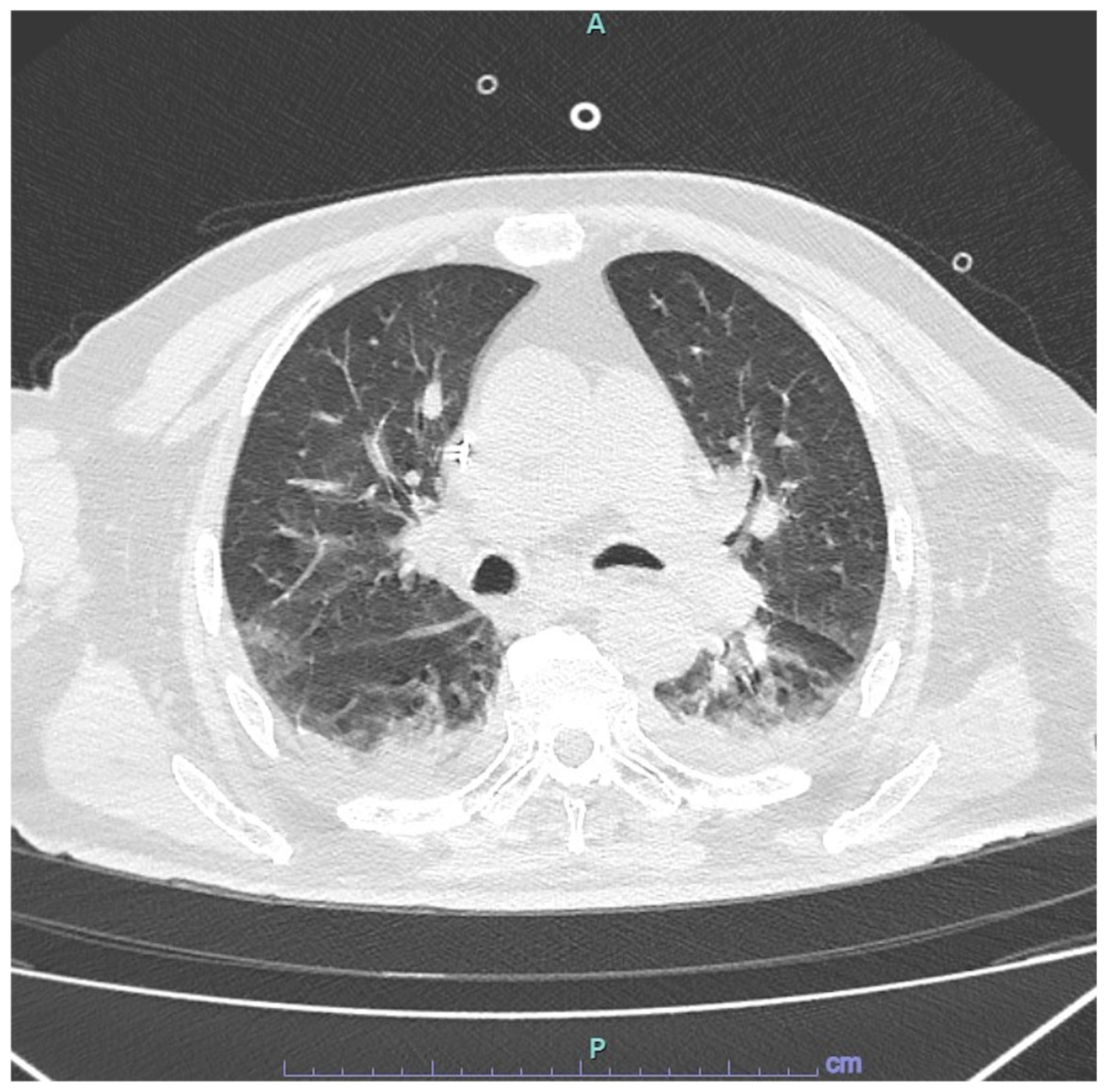
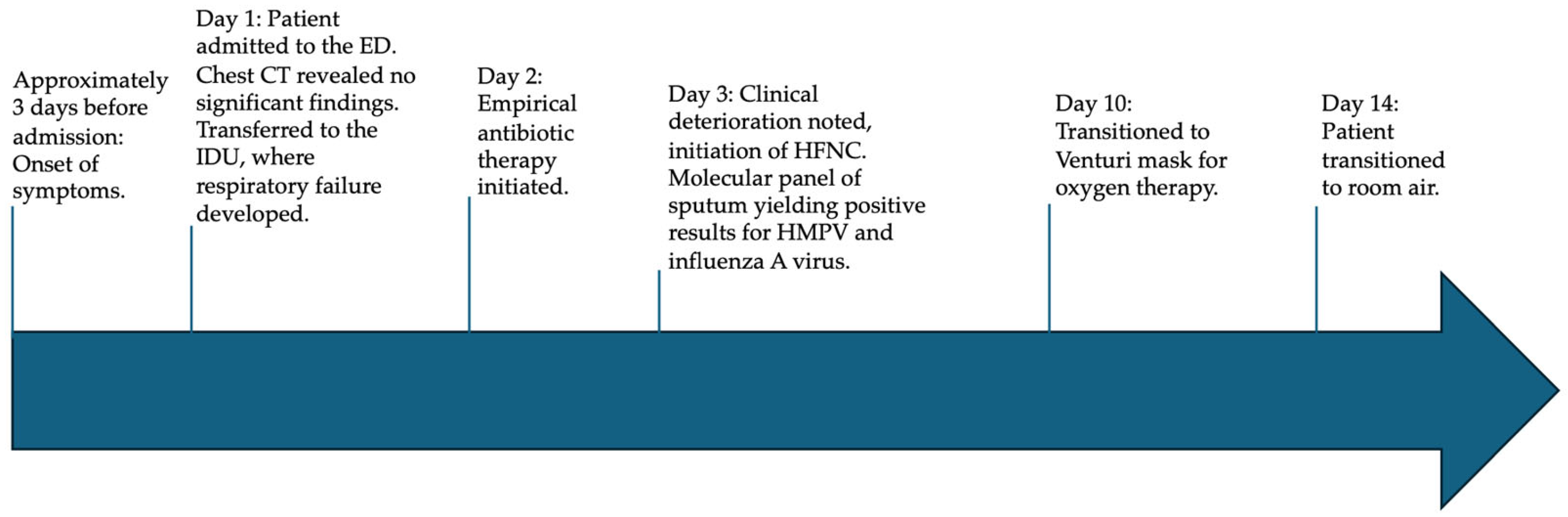
2.1. Presentation to Emergency Department
2.2. Admission to Infectious Disease Unit
2.3. Microbiological Results on Respiratory Samples
3. Discussion
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABG | Arterial blood gas |
| CRP | C-reactive protein |
| CT | Computed tomography |
| HMPV | Human metapneumovirus |
| HFNC | high-flow nasal cannula |
| PLWH | people living with HIV |
References
- Iyer, V.G.; Deb, N.; Javed, M.; Jaiswal, V.; Sah, R. Human Metapneumovirus-Understanding a growing respiratory threat. QJM 2025, hcaf027. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Nanjappa, S.; Cooper, C.D.; Greene, J.N. Human Metapneumovirus Infection in Immunocompromised Patients. Cancer Control 2016, 23, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Thomas, M. Human Metapneumovirus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kapandji, N.; Darmon, M.; Valade, S.; Salmona, M.; Legoff, J.; Zafrani, L.; Azoulay, E.; Lemiale, V. Clinical significance of human metapneumovirus detection in critically ill adults with lower respiratory tract infections. Ann. Intensive Care 2023, 13, 21. [Google Scholar] [CrossRef]
- Basta, M.N. Severe Acute Respiratory Distress Syndrome in an Adult Patient with Human Metapneumovirus Infection Successfully Managed with Veno-Venous Extracorporeal Membrane Oxygenation. Semin. Cardiothorac. Vasc. Anesth. 2024. [Google Scholar] [CrossRef]
- Lefebvre, A.; Manoha, C.; Bour, J.B.; Abbas, R.; Fournel, I.; Tiv, M.; Pothier, P.; Astruc, K.; Aho-Glélé, L.S. Human metapneumovirus in patients hospitalized with acute respiratory infections: A meta-analysis. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2016, 81, 68–77. [Google Scholar] [CrossRef]
- Haas, L.E.; Thijsen, S.F.; van Elden, L.; Heemstra, K.A. Human metapneumovirus in adults. Viruses 2013, 5, 87–110. [Google Scholar] [CrossRef]
- Cunha, B.A.; Irshad, N.; Connolly, J.J. Adult human metapneumonovirus (hMPV) pneumonia mimicking Legionnaire’s disease. Heart Lung 2016, 45, 270–272. [Google Scholar] [CrossRef]
- Makhlouf, A.; Peipoch, L.; Duport, P.; Darrieux, E.; Reguerre, Y.; Ramful, D.; Alessandri, J.L.; Levy, Y. First Case of Acute Myocarditis Caused by Metapneumovirus in an Immunocompromised 14-year-old Girl. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2022, 26, 745–747. [Google Scholar] [CrossRef]
- Lademann, H.; Bertsche, A.; Petzold, A.; Zack, F.; Büttner, A.; Däbritz, J.; Hauenstein, C.; Bahn, E.; Spang, C.; Reuter, D.; et al. Acute Disseminated Encephalomyelitis with Seizures and Myocarditis: A Fatal Triad. Medicina 2020, 56, 277. [Google Scholar] [CrossRef]
- Klein, M.B.; Yang, H.; DelBalso, L.; Carbonneau, J.; Frost, E.; Boivin, G. Viral pathogens including human metapneumovirus are the primary cause of febrile respiratory illness in HIV-infected adults receiving antiretroviral therapy. J. Infect. Dis. 2010, 201, 297–301. [Google Scholar] [CrossRef]
- Tran, D.H.; Sameed, M.; Marciniak, E.T.; Verceles, A.C. Human Metapneumovirus Pneumonia Precipitating Acute Respiratory Distress Syndrome in an Adult Patient. Cureus 2021, 13, e16434. [Google Scholar] [CrossRef]
- Kenmoe, S.; Bigna, J.J.; Fatawou Modiyingi, A.; Ndangang, M.S.; Ngoupo, P.A.; Simo, F.B.N.; Tchatchouang, S.; Temfack, E.; Njouom, R. Case fatality rate and viral aetiologies of acute respiratory tract infections in HIV positive and negative people in Africa: The VARIAFRICA-HIV systematic review and meta-analysis. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019, 117, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Groome, M.J.; Moyes, J.; Cohen, C.; Walaza, S.; Tempia, S.; Pretorius, M.; Hellferscee, O.; Chhagan, M.; Haffejee, S.; Dawood, H.; et al. Human metapneumovirus-associated severe acute respiratory illness hospitalisation in HIV-infected and HIV-uninfected South African children and adults. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2015, 69, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.biomerieux.com/us/en/our-offer/clinical-products/biofire-pneumonia-panel.html (accessed on 30 January 2025).
- Beck, J.M.; Rosen, M.J.; Peavy, H.H. Pulmonary complications of HIV infection. Report of the Fourth NHLBI Workshop. Am. J. Respir. Crit. Care Med. 2001, 164, 2120–2126. [Google Scholar] [CrossRef]
- Sepúlveda-Alfaro, J.; Catalán, E.A.; Vallejos, O.P.; Ramos-Tapia, I.; Madrid-Muñoz, C.; Mendoza-León, M.J.; Suazo, I.D.; Rivera-Asin, E.; Silva, P.H.; Alvarez-Mardones, O.; et al. Human metapneumovirus respiratory infection affects both innate and adaptive intestinal immunity. Front. Immunol. 2024, 15, 1330209. [Google Scholar] [CrossRef]
- González, A.E.; Lay, M.K.; Jara, E.L.; Espinoza, J.A.; Gómez, R.S.; Soto, J.; Rivera, C.A.; Abarca, K.; Bueno, S.M.; Riedel, C.A.; et al. Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection. Virulence 2017, 8, 685–704. [Google Scholar] [CrossRef]
- Céspedes, P.F.; Palavecino, C.E.; Kalergis, A.M.; Bueno, S.M. Modulation of Host Immunity by the Human Metapneumovirus. Clin. Microbiol. Rev. 2016, 29, 795–818. [Google Scholar] [CrossRef]
- Sheth, A.N.; Patel, P.; Peters, P.J. Influenza and HIV: Lessons from the 2009 H1N1 influenza pandemic. Curr. HIV/AIDS Rep. 2011, 8, 181–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Sakthivel, S.K.; Bramley, A.; Jain, S.; Haynes, A.; Chappell, J.D.; Hymas, W.; Lenny, N.; Patel, A.; Qi, C.; et al. Serology Enhances Molecular Diagnosis of Respiratory Virus Infections Other than Influenza in Children and Adults Hospitalized with Community-Acquired Pneumonia. J. Clin. Microbiol. 2016, 55, 79–89. [Google Scholar] [CrossRef]
- Rhedin, S.; Lindstrand, A.; Hjelmgren, A.; Ryd-Rinder, M.; Öhrmalm, L.; Tolfvenstam, T.; Örtqvist, Å.; Rotzén-Östlund, M.; Zweygberg-Wirgart, B.; Henriques-Normark, B.; et al. Respiratory viruses associated with community-acquired pneumonia in children: Matched case-control study. Thorax 2015, 70, 847–853. [Google Scholar] [CrossRef]
- Singleton, R.J.; Bulkow, L.R.; Miernyk, K.; DeByle, C.; Pruitt, L.; Hummel, K.B.; Bruden, D.; Englund, J.A.; Anderson, L.J.; Lucher, L.; et al. Viral respiratory infections in hospitalized and community control children in Alaska. J. Med. Virol. 2010, 82, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Philippot, Q.; Rammaert, B.; Dauriat, G.; Daubin, C.; Schlemmer, F.; Costantini, A.; Tandjaoui-Lambiotte, Y.; Neuville, M.; Desrochettes, E.; Ferré, A.; et al. Human metapneumovirus infection is associated with a substantial morbidity and mortality burden in adult inpatients. Heliyon 2024, 10, e33231. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.E.; Williams, J.V. Human Metapneumovirus. Microbiol. Spectr. 2015, 14, 237–247. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Analysis | Patient’s Result | Reference Range |
|---|---|---|
| WBC (cells/μL) | 8800 | 4000–11,000 |
| Neutrophils (cells/μL) | 7300 | 4000–7400 |
| Lymphocytes (cells/μL) | 950 | 2000–4800 |
| Monocytes (cells/μL) | 690 | 160–1000 |
| PLT (n/μL) | 163,000 | 150,000–450,000 |
| Hb (g/dL) | 15.1 | 12–16 |
| ALP (U/L) | 98 | 35–104 |
| ALT (U/L) | 20 | 0–35 |
| AST (U/L) | 13 | 0–35 |
| GGT (U/L) | 19 | 5–36 |
| Sodium (mmol/L) | 136 | 132–146 |
| Potassium (mmol/L) | 4.8 | 3.7–5.4 |
| Creatinine (mg/dL) | 1.86 | 0.51–0.95 |
| CRP (mg/L) | 191 | <5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipitò, L.; Mazzola, C.V.; Bono, E.; Gioè, C.; Giammanco, G.M.; Bonura, C.; Cascio, A. A Case of Severe Respiratory Failure Caused by Metapneumovirus and Influenza Virus in a Patient with HIV Infection. Viruses 2025, 17, 289. https://doi.org/10.3390/v17030289
Pipitò L, Mazzola CV, Bono E, Gioè C, Giammanco GM, Bonura C, Cascio A. A Case of Severe Respiratory Failure Caused by Metapneumovirus and Influenza Virus in a Patient with HIV Infection. Viruses. 2025; 17(3):289. https://doi.org/10.3390/v17030289
Chicago/Turabian StylePipitò, Luca, Chiara Vincenza Mazzola, Eleonora Bono, Claudia Gioè, Giovanni M. Giammanco, Celestino Bonura, and Antonio Cascio. 2025. "A Case of Severe Respiratory Failure Caused by Metapneumovirus and Influenza Virus in a Patient with HIV Infection" Viruses 17, no. 3: 289. https://doi.org/10.3390/v17030289
APA StylePipitò, L., Mazzola, C. V., Bono, E., Gioè, C., Giammanco, G. M., Bonura, C., & Cascio, A. (2025). A Case of Severe Respiratory Failure Caused by Metapneumovirus and Influenza Virus in a Patient with HIV Infection. Viruses, 17(3), 289. https://doi.org/10.3390/v17030289








