Assessment of Response and Safety of Bulevirtide Treatment in Patients with Chronic Delta Virus Infection: The ARISTOTLE Pilot Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Endpoints of Study
2.2. Statistical Analysis
3. Results
Adverse Events and Safety Profile
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, L.F.; Ford, E.; Purcell, R.H.; London, W.T.; Phillips, J.; Gerin, J.L. The size of the hepatitis delta agent. J. Med Virol. 1989, 27, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Urban, S.; Neumann-Haefelin, C.; Lampertico, P. Hepatitis D virus in 2021: Virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut 2021, 70, 1782–1794. [Google Scholar] [CrossRef]
- Taylor, J.M. Infection by Hepatitis Delta Virus. Viruses 2020, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Gish, R.; Jacobson, I.M.; Hu, K.Q.; Wedemeyer, H.; Martin, P. Diagnosis and Management of Hepatitis Delta Virus Infection. Dig. Dis. Sci. 2023, 68, 3237–3248. [Google Scholar] [CrossRef]
- Lucifora, J.; Delphin, M. Current knowledge on hepatitis delta virus replication. Antivir. Res. 2020, 179, 104812. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vargas, J.; Amirache, F.; Boson, B.; Mialon, C.; Freitas, N.; Sureau, C.; Fusil, F.; Cosset, F.L. Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo. Nat. Commun. 2019, 10, 2098. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hepatitis D Fact Sheet; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Chen, H.Y.; Shen, D.T.; Ji, D.Z.; Han, P.-C.; Zhang, W.-M.; Ma, J.-F.; Chen, W.-S.; Goyal, H.; Pan, S.; Xu, H.-G. Prevalence and burden of hepatitis D virus infection in the global population: A systematic review and meta-analysis. Gut 2019, 68, 512–521. [Google Scholar] [CrossRef]
- Stockdale, A.J.; Kreuels, B.; Henrion, M.Y.R.; Giorgi, E.; Kyomuhangi, I.; de Martel, C.; Hutin, Y.; Geretti, A.M. The global prevalence of hepatitis D virus infection: Systematic review and metaanalysis. J. Hepatol. 2020, 73, 523–532. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.Y.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Rizzetto, M. Hepatitis D Virus. In Clinical Epidemiology of Chronic Liver Disease; Wong, J.R., Gish, R.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 135–148. [Google Scholar]
- Niro, G.A.; Ciancio, A.; Gaeta, G.B.; Smedile, A.; Marrone, A.; Olivero, A.; Stanzione, M.; David, E.; Brancaccio, G.; Fontana, R.; et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006, 44, 713–720. [Google Scholar] [CrossRef]
- Niro, G.A.; Rosina, F.; Rizzetto, M. Treatment of hepatitis D. J. Viral Hepat. 2005, 12, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Yurdaydin, C.; Hardtke, S.; Caruntu, F.A.; Curescu, M.G.; Yalcin, K.; Akarca, U.S.; Gürel, S.; Zeuzem, S.; Erhardt, A.; et al. Peginterferon Alfa-2a Plus Tenofovir Disoproxil Fumarate for Hepatitis D (HIDIT-II): A Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Infect. Dis. 2019, 19, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Markert, C.; Hohmann, N.; Carls, A.; Mikus, G.; Lehr, T.; Alexandrov, A.; Haag, M.; Schwab, M.; Urban, S.; et al. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J. Hepatol. 2016, 65, 483–489. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/hepcludex (accessed on 14 November 2024).
- Jachs, M.; Schwarz, C.; Panzer, M.; Binter, T.; Aberle, S.W.; Hartl, L.; Dax, K.; Aigner, E.; Stättermayer, A.F.; Munda, P.; et al. Response-guided long-term treatment of chronic hepatitis D patients with bulevirtide-results of a “real world” study. Aliment. Pharmacol. Ther. 2022, 56, 144–154. [Google Scholar] [CrossRef]
- Wilson, B.E.; Booth, C.M. Real-World Data: Bridging the Gap between Clinical Trials and Practice. EClinicalMedicine 2024, 78, 102915. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Aleman, S.; Brunetto, M.R.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. A Phase 3, Randomized Trial of Bulevirtide in Chronic Hepatitis D. N. Engl. J. Med. 2023, 389, 22–32. [Google Scholar] [CrossRef]
- Lim, J.K.; Flamm, S.L.; Singh, S.; Falck-Ytter, Y.T.; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology 2017, 152, 1536–1543. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis delta virus. J. Hepatol. 2023, 79, 433–460. [Google Scholar] [CrossRef]
- Ghany, M.G.; Buti, M.; Lampertico, P.; Lee, H.M.; 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference Faculty. Guidance on Treatment Endpoints and Study Design for Clinical Trials Aiming to Achieve Cure in Chronic Hepatitis B and D: Report from the 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference. J. Hepatol. 2023, 79, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Wranke, A.; Wedemeyer, H. Antiviral therapy of hepatitis delta virus infection–progress and challenges towards cure. Curr. Opin. Virol. 2016, 20, 112–118. [Google Scholar] [CrossRef]
- Lampertico, P.; Anolli, M.P.; Roulot, D.; Wedemeyer, H. Antiviral Therapy for Chronic Hepatitis Delta: New Insights from Clinical Trials and Real-Life Studies. Gut 2024, online ahead of print. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Aleman, S.; Brunetto, M.; Blank, A.; Andreone, P.; Bogomolov, P.; Chulanov, V.; Mamonova, N.; Geyvandova, N.; Morozov, V.; et al. Bulevirtide monotherapy in patients with chronic HDV: Efficacy and safety results through week 96 from a phase III randomized trial. J. Hepatol. 2024, 81, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Sapuk, A.; Steinhoff, L.; Huenninghaus, K.; Willuweit, K.; Rashidi Alavijeh, J.; Hild, B.; Asar, L.; Schmidt, H.H.; Schramm, C. Long-Term Treatment with Bulevirtide in Patients with Chronic Hepatitis D and Advanced Chronic Liver Disease. Can. J. Gastroenterol. Hepatol. 2024, 2024, 2364031. [Google Scholar] [CrossRef]
- Negro, F.; Lok, A.S. Hepatitis D: A Review. JAMA 2023, 330, 2376–2387. [Google Scholar] [CrossRef]
- Degasperi, E.; Anolli, M.P.; Jachs, M.; Reiberger, T.; De Ledinghen, V.; Metivier, S.; D’Offizi, G.; di Maria, F.; Schramm, C.; Schmidt, H.; et al. Real-World Effectiveness and Safety of Bulevirtide Monotherapy for up to 96 Weeks in Patients with HDV-Related Cirrhosis. J. Hepatol. 2025, online ahead of print. [Google Scholar] [CrossRef]
- Killer, A.; Gliga, S.; Lohr, C.; Weigel, C.; Ole Jensen, B.E.; Lübke, N.; Walker, A.; Timm, J.; Bode, J.; Luedde, T.; et al. Dynamics of Virological and Clinical Response Parameters of Bulevirtide Treatment for Hepatitis D: Real-World Data. Gastro Hep Adv. 2024, 3, 353–360. [Google Scholar] [CrossRef]
- Lucifora, J.; Alfaiate, D.; Pons, C.; Michelet, M.; Ramirez, R.; Fusil, F.; Amirache, F.; Rossi, A.; Legrand, A.F.; Charles, E.; et al. Hepatitis D Virus Interferes with Hepatitis B Virus RNA Production via Interferon-Dependent and -Independent Mechanisms. J. Hepatol. 2023, 78, 958–970. [Google Scholar] [CrossRef]
- Gopalakrishna, H.; Mironova, M.; Dahari, H.; Koh, C.; Heller, T. Advances and Challenges in Managing Hepatitis D Virus: Evolving Strategies. Curr. Hepatol. Rep. 2024, 23, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, E.; Anolli, M.P.; D’Offizi, G.; Brunetto, M.R.; Verucchi, G.; Ciancio, A.; Mangia, A.; Santantonio, T.A.; Coppola, N.; Pellicelli, A.; et al. Baseline predictors of virological and biochemical responses in HDV compensated cirrhotic patients treated with Bulevirtide monotherapy (HEP4Di study). Dig. Liver Dis. 2024, 56 (Suppl. S1), S32–S33. [Google Scholar] [CrossRef]
- Siddiqui, M.; Manansala, J.S.; Abdulrahman, H.A.; Nasrallah, G.K.; Smatti, M.K.; Younes, N.; Althani, A.A.; Yassine, H.M. Immune Modulatory Effects of Vitamin D on Viral Infections. Nutrients 2020, 12, 2879. [Google Scholar] [CrossRef] [PubMed]
- LBishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Sandmann, L.; Wedemeyer, H. Interferon-based treatment of chronic hepatitis D. Liver Int. 2023, 43 (Suppl. S1), 69–79. [Google Scholar] [CrossRef]
- Abdrakhman, A.; Ashimkhanova, A.; Almawi, W.Y. Effectiveness of pegylated interferon monotherapy in the treatment of chronic hepatitis D virus infection: A meta-analysis. Antivir. Res. 2021, 185, 104995. [Google Scholar] [CrossRef]
- Liu, H.; Zakrzewicz, D.; Nosol, K.; Irobalieva, R.N.; Mukherjee, S.; Bang-Sørensen, R.; Goldmann, N.; Kunz, S.; Rossi, L.; Kossiakoff, A.A.; et al. Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP. Nat. Commun. 2024, 15, 2476. [Google Scholar] [CrossRef]
- Asselah, T.; Chulanov, V.; Lampertico, P.; Wedemeyer, H.; Streinu-Cercel, A.; Pântea, V.; Lazar, S.; Placinta, G.; Gherlan, G.S.; Bogomolov, P.; et al. Bulevirtide Combined with Pegylated Interferon for Chronic Hepatitis D. N. Engl. J. Med. 2024, 391, 133–143. [Google Scholar] [CrossRef]
- Scudiero, O.; Nigro, E.; Elce, A.; Colavita, I.; Esposito, A.; Riccio, M.P.; D’Argenio, V.; Monda, V.; Monda, M.; Messina, A.; et al. PPARγ and ADRB3 Polymorphisms Analysis and Irisin Expression in Professional Water Polo Players. Sport Sci. Health 2017, 13, 395–401. [Google Scholar] [CrossRef][Green Version]
- Nigro, E.; Sangiorgio, D.; Scudiero, O.; Monaco, M.L.; Polito, R.; Villone, G.; Daniele, A. Gene Molecular Analysis and Adiponectin Expression in Professional Water Polo Players. Cytokine 2016, 81, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Buti, M.; Wedemeyer, H.; Aleman, S.; Chulanov, V.; Morozov, V.; Sagalova, O.; Stepanova, T.; Gish, R.G.; Lloyd, A.; Kaushik, A.M.; et al. Patient-Reported Outcomes in Chronic Hepatitis Delta: An Exploratory Analysis of the Phase III MYR301 Trial of Bulevirtide. J. Hepatol. 2025, 82, 28–36. [Google Scholar] [CrossRef] [PubMed]
| Variable | Baseline (n = 108) |
|---|---|
| Age, y, mean (SD) | 53.5 (12.2) |
| Sex, n (%) | |
| M | 68 (63.0) |
| F | 40 (37.0) |
| Nationality, n (%) | |
| Italian | 67 (62.0) |
| Romanian | 22 (20.4) |
| Other | 19 (17.6) |
| BMI, median [IQR] | 24.3 [22.7–26.6] |
| Cirrhosis, n (%) | 85 (89.5) |
| Stiffness (kPa), median [IQR] | 12.8 [9.8–17.6] |
| Fibrosis stage, n (%) | |
| Absence | 7 (6.5) |
| Mild | 22 (20.4) |
| Advanced | 63 (58.3) |
| Not performed | 16 (14.8) |
| CAP (db/m), mean (SD) | 214.7 (57.6) |
| Steatosis stage, n (%) | |
| S0 | 49 (45.4) |
| S1 | 7 (6.5) |
| S2 | 7 (6.5) |
| S3 | 5 (4.6) |
| Not performed/available | 40 (37.0) |
| History of HCC, n (%) | 9 (8.3) |
| Active | 4 (3.7) |
| Previous interferon, n (%) | 38 (35.2) |
| HDV RNA (UI/mL), median [IQR] | 42,660 [3205–367,420] |
| HBV DNA not detected, n (%) | 89 (82.4) |
| HBV treatment, n (%) | |
| Entecavir | 52 (48.1) |
| Tenofovir | 56 (51.9) |
| HCV treated, n (%) | 9 (8.3) |
| HIV positive, n (%) | 5 (4.6) |
| Progression Time | ||||||
|---|---|---|---|---|---|---|
| Time 0 (Baseline) | Time 1 (=6 Months) | |||||
| Variable | Responder (n = 59) | Non-Responder (n = 49) | p | Responder (n = 59) | Non-Responder (n = 49) | p |
| PLT × 103 (U/mL), median [IQR] | 103.0 [78.0–153.0] | 121.0 [80.0–183.0] | 0.411 | 121.0 [80.0–183.0] | 125.0 [95.0–170.0] | 0.784 |
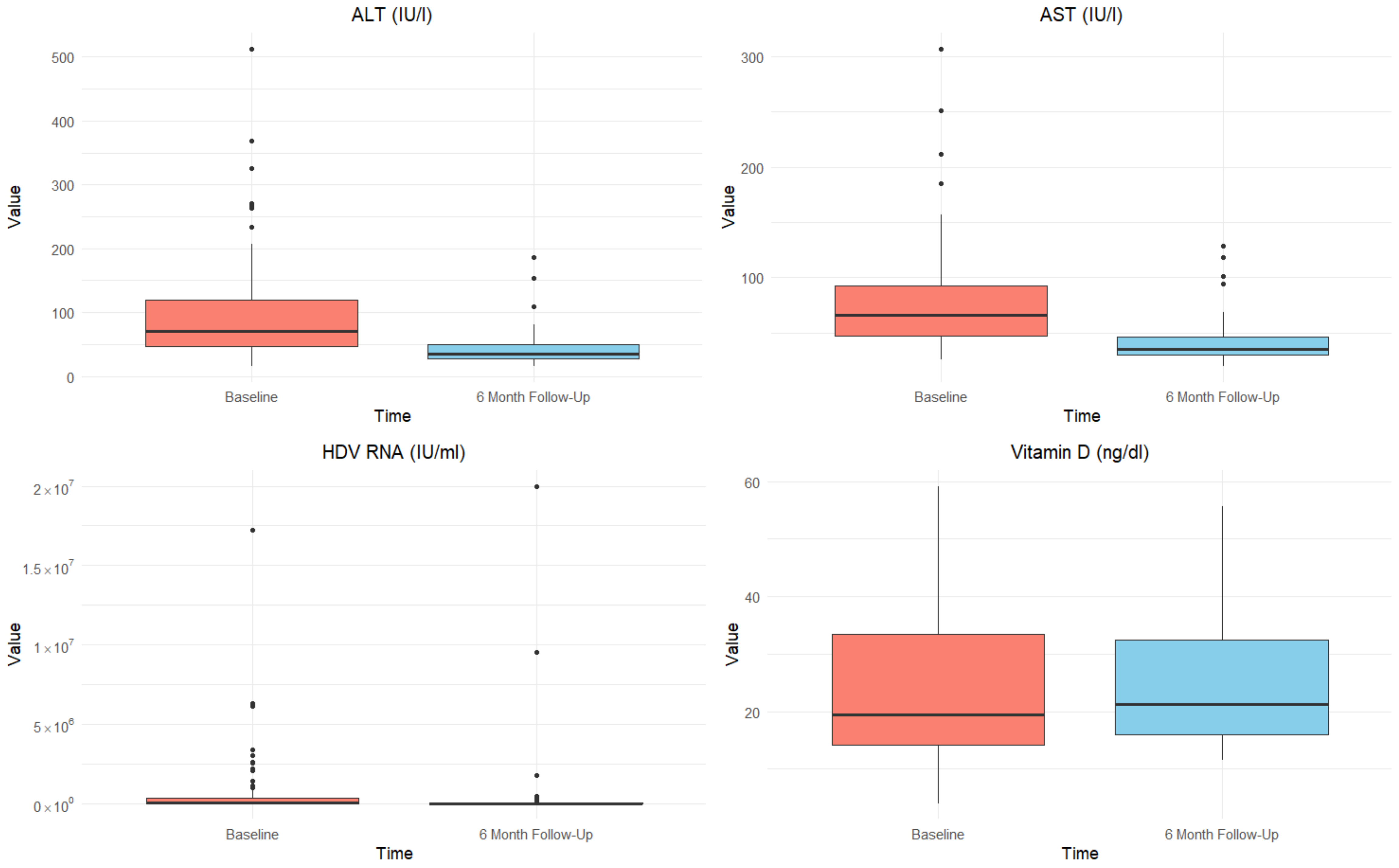
| ALT (U/mL), median [IQR] | 67.0 [44.0–116.3] | 75.0 [47.8–125.3] | 0.274 | 31.5 [24.0–36.5] | 45.5 [34.0–64.0] | 0.001 |
| AST (U/mL), median [IQR] | 66.0 [46.5–91.0] | 66.0 [48.8–99.0] | 0.772 | 32.5 [28.0–38.0] | 43.0 [33.0–50.0] | 0.021 |
| ALP (U/mL), median [IQR] | 88.0 [68.3–114.3] | 91.0 [72.0–119.0] | 0.858 | 82.0 [72.3–97.8] | 87.0 [65.8–110.5] | 0.996 |
| GGT (U/mL), median [IQR] | 59.0 [34.3–84.0] | 63.0 [29.8–91.3] | 0.939 | 37.0 [23.3–52.3] | 40.5 [29.5–70.5] | 0.226 |
| Albumin (g/dL), median [IQR] | 3.9 [3.6–4.2] | 4.1 [3.8–4.4] | 0.020 | 4.1 [3.9–4.4] | 4.1 [3.8–4.4] | 0.598 |
| γ globulin (g/dL), median [IQR] | 1.9 [1.5–2.2] | 1.9 [1.4–2.4] | 0.659 | 1.5 [1.2–1.8] | 1.7 [1.2–1.9] | 0.880 |
| INR, median [IQR] | 1.2 [1.1–1.4] | 1.1 [1.0–1.2] | 0.002 | 1.1 [1.1–1.2] | 1.1 [1.0–1.2] | 0.648 |
| eGFR (mL/min/1.73 m2), median [IQR] | 86.7 [69.3–98.5] | 88.2 [78.0–98.6] | 0.392 | 90.2 [74.3–100.7] | 88.1 [75.0–100.6] | 0.776 |
| Vitamin D (ng/dL), median [IQR] | 14.3 [11.0–21.6] | 32.6 [19.1–39.7] | 0.001 | 20.0 [14.9–29.2] | 38.5 [16.4–47.0] | 0.248 |
| HBsAg (U/mL), median [IQR] | 5722.0 [720.0–10,466.7] | 4968.6 [887.0–9608.0] | 0.813 | 8000.0 [1258.0–10,887.5] | 9427.7 [4431.8–15,568.0] | 0.188 |
| APRI Score, median [IQR] | 1.5 [1.0–2.4] | 1.5 [0.8-2.5] | 0.731 | 1.0 [0.5–2.4] | 1.3 [0.8–1.3] | 0.144 |
| FIB-4, median [IQR] | 3.7 [2.3–5.5] | 2.9 [1.8–5.8] | 0.205 | 2.7 [1.6–4.1] | 2.7 [1.5–4.6] | 0.895 |
| HDV RNA (UI/mL), median [IQR] | 29,800.0 [3100.0–375,000.0] | 45,160.0 [3683.0–363,815.5] | 0.615 | 0.0 * [0.0–291.0] | 14,090.0 [1750.5–69,483.8] | <0.001 |
| Univariable Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | OR | 95% CI | p | OR | 95% CI | p | ||
| Age | 1.03 | 0.99 | 1.07 | 0.051 | 1.02 | 0.95 | 1.10 | 0.509 |
| Sex | 1.41 | 0.64 | 3.12 | 0.391 | ||||
| M | ||||||||
| F | ||||||||
| Nationality | ||||||||
| Italian | 1 | |||||||
| Romanian/Other | 0.30 | 0.13 | 0.68 | 0.004 | 0.32 | 0.03 | 3.54 | 0.355 |
| BMI | 1.04 | 0.94 | 1.15 | 0.451 | ||||
| Cirrhosis | 0.79 | 0.32 | 1.95 | 0.606 | ||||
| Stiffness | 1.04 | 0.99 | 1.09 | 0.100 | ||||
| History of HCC | 0.78 | 0.20 | 3.11 | 0.726 | ||||
| Previous interferon | 0.60 | 0.15 | 2.47 | 0.479 | ||||
| HBV DNA | 1.00 | 0.99 | 1.01 | 0.638 | ||||
| PLT | 0.99 | 0.98 | 1.01 | 0.823 | ||||
| ALT | 0.99 | 0.99 | 1.01 | 0.312 | ||||
| AST | 1.00 | 0.99 | 1.01 | 0.846 | ||||
| ALP | 1.00 | 0.99 | 1.01 | 0.909 | ||||
| GGT | 0.99 | 0.98 | 1.01 | 0.606 | ||||
| Albumin | 0.32 | 0.12 | 0.84 | 0.020 | 1.36 | 0.15 | 12.28 | 0.935 |
| γ globulin | 0.80 | 0.31 | 2.03 | 0.634 | ||||
| INR | 1.57 | 0.86 | 2.68 | 0.245 | ||||
| eGFR | 0.99 | 0.97 | 1.02 | 0.357 | ||||
| HBsAg | 1.01 | 0.99 | 1.01 | 0.479 | ||||
| APRI score | 0.99 | 0.76 | 1.28 | 0.929 | ||||
| FIB-4 | 1.02 | 0.90 | 1.17 | 0.717 | ||||
| Vitamin D | 0.92 | 0.87 | 0.98 | 0.013 | 0.93 | 0.88 | 1.02 | 0.182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, L.; Viganò, M.; Ciancio, A.; Caturano, A.; Messina, V.; Niro, G.A.; Capoluongo, N.; Loglio, A.; Marinaro, L.; Marrone, A.; et al. Assessment of Response and Safety of Bulevirtide Treatment in Patients with Chronic Delta Virus Infection: The ARISTOTLE Pilot Observational Study. Viruses 2025, 17, 251. https://doi.org/10.3390/v17020251
Rinaldi L, Viganò M, Ciancio A, Caturano A, Messina V, Niro GA, Capoluongo N, Loglio A, Marinaro L, Marrone A, et al. Assessment of Response and Safety of Bulevirtide Treatment in Patients with Chronic Delta Virus Infection: The ARISTOTLE Pilot Observational Study. Viruses. 2025; 17(2):251. https://doi.org/10.3390/v17020251
Chicago/Turabian StyleRinaldi, Luca, Mauro Viganò, Alessia Ciancio, Alfredo Caturano, Vincenzo Messina, Grazia Anna Niro, Nicolina Capoluongo, Alessandro Loglio, Letizia Marinaro, Aldo Marrone, and et al. 2025. "Assessment of Response and Safety of Bulevirtide Treatment in Patients with Chronic Delta Virus Infection: The ARISTOTLE Pilot Observational Study" Viruses 17, no. 2: 251. https://doi.org/10.3390/v17020251
APA StyleRinaldi, L., Viganò, M., Ciancio, A., Caturano, A., Messina, V., Niro, G. A., Capoluongo, N., Loglio, A., Marinaro, L., Marrone, A., Claar, E., Russello, M., Ciracì, E., Gentilucci, U. V., Pace Palitti, V., Acierno, C., Cosentino, C., Mormone, A., Cotugno, R., ... Izzi, A. (2025). Assessment of Response and Safety of Bulevirtide Treatment in Patients with Chronic Delta Virus Infection: The ARISTOTLE Pilot Observational Study. Viruses, 17(2), 251. https://doi.org/10.3390/v17020251












