1. Introduction
Traditional vaccines remain the fundamental tool in preventing and controlling infectious diseases in animal husbandry. Through presenting antigenic components that resemble the pathogens encountered during natural infection, these vaccines elicit host-specific immune responses to establish protective immunity. The main categories comprise inactivated vaccines, live attenuated vaccines, classical subunit vaccines (produced through conventional purification techniques), and toxoid vaccines (targeting toxin-mediated bacterial diseases). Despite their extensive clinical application, traditional vaccines exhibit inherent limitations that constrain their therapeutic potential [
1]. A critical evaluation of inactivated vaccines reveals the following advantages and disadvantages. Advantages: (1) Enhanced biosafety. The non-replicative nature of inactivated pathogens eliminates infection risks, particularly critical for vulnerable populations including neonatal and pregnant animals. (2) Improved thermostability. Most formulations maintain immunogenicity under ambient or refrigerated conditions (2–8 °C), with oil-adjuvanted variants demonstrating enhanced temperature tolerance. This characteristic reduces reliance on cold-chain infrastructure, lowering operational expenses and particularly benefiting remote small-scale farming operations. (3) Diagnostic differentiation capacity. When implemented alongside serological monitoring systems (e.g., ELISA-based platforms), these vaccines enable discrimination between natural infection antibodies and vaccine-induced immunity, facilitating epidemiological surveillance in eradication programs. Disadvantages: (1) Restricted immunogenicity profile. Inactivated vaccines primarily induce humoral immunity (antibody-mediated responses) while inadequately stimulating cell-mediated immunity, resulting in suboptimal efficacy against intracellular pathogens. (2) Demanding vaccination protocols. The necessity for multiple booster administrations at specified intervals increases husbandry management complexity and labor requirements. (3) Adjuvant-associated complications. Oil-emulsified formulations frequently induce granulomatous inflammation at injection sites, which may lead to reduced feed conversion efficiency and impaired weight gain in production animals.
Egg yolk antibodies (IgY), a distinct immunoglobulin class unique to oviparous vertebrates (amphibians, avians, and reptiles), undergo selective transport from maternal circulation to yolk sacs during oocyte maturation through receptor-mediated mechanisms [
2,
3]. While sharing functional similarities with mammalian IgG in antigen recognition, IgY demonstrates structural divergence including heavier chains and additional constant domains [
4,
5]. This avian immunoglobulin exhibits three key advantages for therapeutic applications: (1) low immunogenicity in mammals, (2) remarkable thermal and pH stability maintaining activity from 4 to 56 °C and pH 4–11, and (3) cost-effective production through natural egg-laying processes [
4,
5]. These characteristics have enabled widespread applications in passive immunization, showing efficacy against mammalian pathogens such as SARS-CoV-2 [
3] and avian viruses including Newcastle disease virus, infectious bursal disease virus (IBDV) [
6], avian influenza [
6], and poultry reovirus [
7]. Mechanistically, IgY exerts dual immunomodulatory functions through antigen-specific Fab regions that neutralize pathogens, coupled with Fc-mediated interactions that enhance phagocytosis and modulate cytokine production [
8].
Fowl Adenovirus Serotype 4 (FAdV-4) and Porcine Epidemic Diarrhea Virus (PEDV) continue to pose significant threats to global livestock production. Current vaccine development against FAdV-4 primarily focuses on inactivated vaccines, subunit vaccines, and genetically engineered candidates [
8]. Nevertheless, the established protective immunity generally requires 14–21 days post-vaccination, resulting in two critical vulnerability periods: a complete lack of protection during the initial 1–14 days phase and potential immune failure after 21 days.
Akin to FAdV-4 vaccines, conventional PEDV vaccines demonstrate suboptimal protective efficacy when compared to ideal vaccine standards, necessitating the development of novel immunization strategies. To date, no effective vaccine has been developed, due to the continual mutation of the PEDV genome and the low efficacy in inducing mucosal immunity [
9,
10]. Obtaining sufficient maternal antibody from the colostrum and milk remains the most promising and effective strategy to protect neonatal suckling piglets against PEDV [
11,
12].
This study developed a dual-pathogen composite vaccine combining IgY with inactivated antigens against FAdV-4 and PEDV. The inactivated virus was used as the aqueous phase and mixed with the oil adjuvant in a certain ratio to prepare a water-in-oil emulsion. Then, the emulsion was combined with the antibody at an appropriate ratio and sheared to prepare a stable complex. The innermost layer of the complex was the antigen, the outermost layer was the antibody, and the middle layer was the oil emulsion. The antigen and antibody were isolated by the oil phase and could not undergo specific binding. We systematically evaluated the vaccine’s immunogenic properties and protective efficacy through in vivo models.
2. Materials and Methods
2.1. Viruses and Animals
The PEDV CH/HNXX/201606 strain (GenBank accession no. MK124712), maintained in our laboratory, was titrated in Vero cells (CVCC CL28) using a plaque assay (10
5.7 TCID
50/mL). The FAdV-4 strain (designated WZ; GenBank accession no. MZ508442) was isolated from hepatic tissue samples of a commercial broiler flock in Henan Province, China. The viral titer of FAdV-4 was quantified by TCID
50 assay in LMH cell (ATCC CRL-2117) cultures (10
7.8/0.1 mL). The assay was conducted using standardized endpoint dilution methods [
13,
14].
Specific pathogen-free (SPF) chickens and BALB/c mice were housed in isolators and individual Ventilated Cages. Fertilized SPF eggs from certified parent flocks were incubated in compliance with AAALAC International guidelines. The embryonation process was conducted under controlled incubation conditions (37.8 °C, 55–60% relative humidity). All animal experimental protocols were ethically reviewed and approved by the Institutional Animal Care and Use Committee of Henan Agricultural University (Approval ID: HNND2019061097).
2.2. Preparation of Inactivated Vaccine
The inactivated FAdV-4 and PEDV oil-emulsified vaccines were prepared from their respective parental viral strains. Each viral suspension was inactivated by chemical treatment with 0.1% formalin at 37 °C for 24 h. Residual formaldehyde was subsequently neutralized by adding 1 M glycine. The inactivated virus preparations were emulsified with a commercial oil adjuvant (ISA 71 VG, SEPPIC Chemical Specialities Co., Ltd., Shanghai, China) at an antigen-to-adjuvant ratio of 4:7 (weight-to-weight, w/w). Negative control vaccines were prepared using identical procedures, with phosphate-buffered saline replacing viral antigens.
2.3. Preparation of Egg Yolk Antibodies
To generate anti-FAdV-4 egg yolk antibodies, five specific pathogen-free (SPF) laying hens (aged 160 days) were immunized with an inactivated oil-emulsion antigens. Each hen received three 0.3 mL intramuscular chest injections at 21-day intervals (administered at 160, 181, and 202 days of age), completing the immunization series over a six-week period. The yolk antibodies were subsequently purified through affinity chromatography using a HiTrap™ IgY Purification HP column (Cytiva, Shanghai, China), yielding purified IgY preparations.
Subsequently, we evaluated the neutralizing capacity of these antibodies using a standardized virus neutralization assay [
7]. The experimental procedure was conducted as follows: Two 96-well cell culture plates were prepared for parallel processing. One plate received 4 × 10
4.0 cells per well and was incubated for 24 h to achieve 80% confluency prior to infection. The second plate was preloaded with 100 µL of DME/F-12 medium per well for antibody serial dilution. Antibody titration began with 100 µL of IgY antibody placed in quadruplicate wells (A1, B1, C1, D1), followed by serial two-fold dilutions prepared across 21–22 successive wells. Viral stock of FAdV-4 WZ strain (third passage in F3 cells) was adjusted to 200 TCID
50/0.1 mL based on the TCID
50 value established in Experiment 1. Equal volumes (100 µL) of diluted viral solution were combined with each antibody dilution, thoroughly mixed by pipetting, and incubated for neutralization (1 h at 37 °C, 5% CO
2). The cell monolayer plate was prepared by replacing the existing medium with 100 µL fresh DME/F-12 containing 2% FBS. The antibody-virus complexes were then transferred to corresponding wells of the pre-incubated cell plate. Three essential control groups were established: cell controls (medium only), negative serum controls (non-immune IgY), and virus controls (virus inoculum without antibody). Plates were maintained in a 5% CO
2 incubator at 37 °C with daily microscopic observation for cytopathic effects (CPE) over 7 days. Neutralization titers were determined by the Reed-Muench method, expressed as the highest antibody dilution achieving 50% protection against CPE development (NT
50).
2.4. Optimization and Preparation of Composite Vaccines
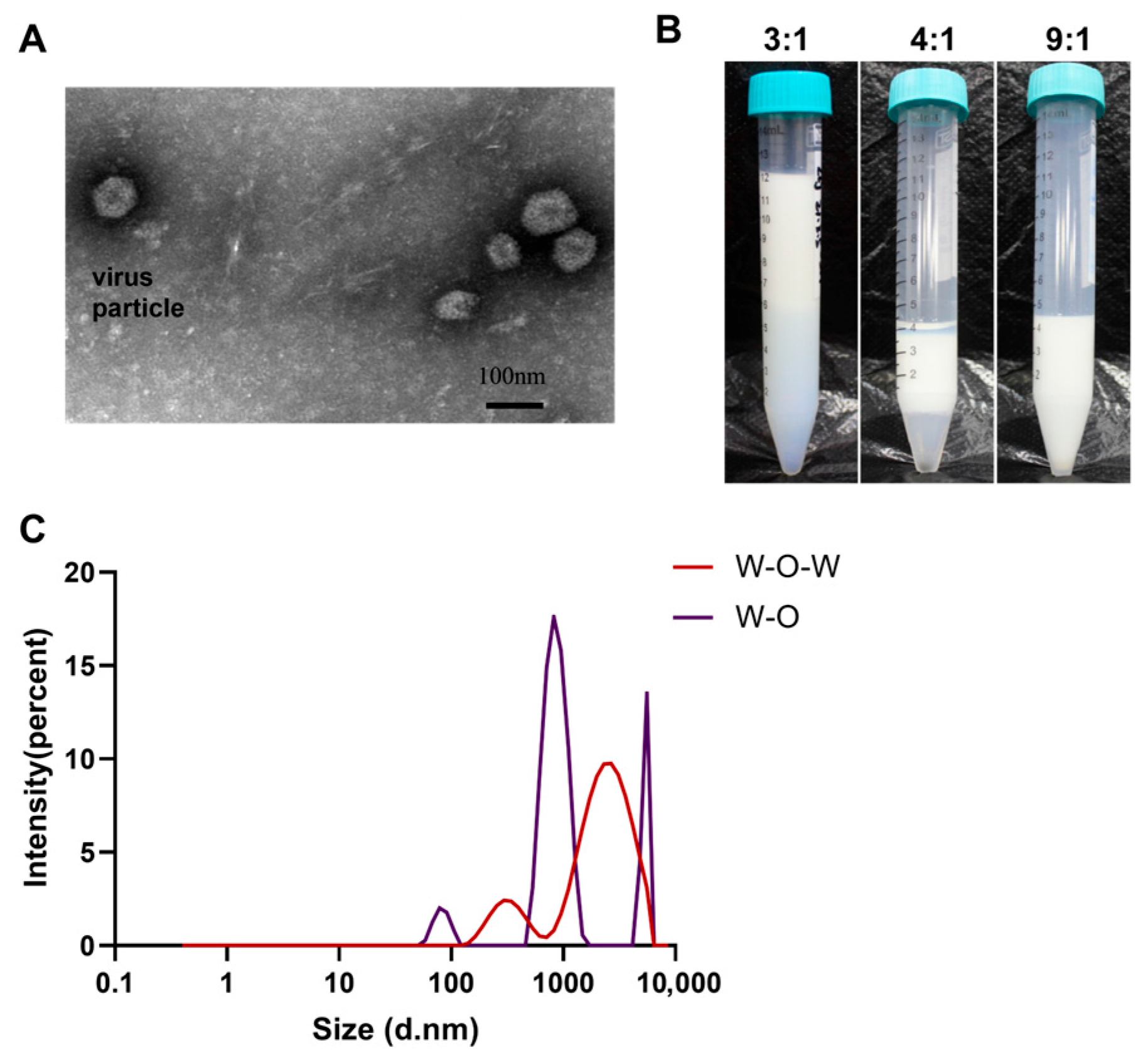
The water-in-oil-in-water (W/O/W) composite vaccine was prepared using a two-step emulsification protocol. Initially, the inactivated viral antigen was homogenized with the oil emulsion adjuvant at a 4:7 (w/w) ratio following established methodology. Purified IgY antibodies were diluted 20-fold in phosphate-buffered saline (PBS) to prepare the aqueous antibody solution. For formulation optimization, the primary water-in-oil (W/O) vaccine emulsion was then mixed with the specific yolk-derived IgY antibody solution in three distinct volume ratios (W/O vaccine: antibody solution = 3:1, 4:1, 9:1, v/v). This secondary emulsification was performed by gradually introducing the oil phase into the aqueous antibody solution under controlled low-speed shearing conditions (1100 rpm for 5 min) to ensure proper droplet formation and interface stabilization.
Vaccine stability was evaluated through centrifugation testing (3000 rpm × 15 min) with formulations demonstrating ≤5% (v/v) aqueous phase precipitation considered acceptable for physical stability. For comprehensive characterization, vaccine formulations underwent dynamic light scattering (DLS) analysis (25 °C, 0.1 mg/mL in PBS) to determine particle size distribution and zeta potential parameters critical for assessing colloidal stability and surface charge properties.
The composite vaccine containing inactivated virus and specific yolk immunoglobulin (IgY) was prepared using a two-step emulsification method. Each 0.5 mL dose consisted of 106 TCID50 inactivated virus mixed with IgY at a 9:1 (v/v) ratio (virus suspension/IgY solution). During formulation, antigen–antibody complexes were formed via specific binding between viral surface proteins and therapeutic antibodies in IgY. The emulsion was vortex-mixed at moderate speed (500 rpm) for 5 min to ensure component homogeneity while maintaining complex stability. The final vaccine formulation was stored at 4–8 °C prior to immunization.
2.5. FAdV-4 Vaccination and Challenge
Specific pathogen-free (SPF) chickens were housed in isolators with ad libitum access to water and standard poultry feed. The study cohorts (n = 65/group) comprised three randomly assigned treatment groups: Group A received 0.5 mL of FAdV-4 immune complex formulation (containing FAdV-4 antigen–antibody complexes) via pectoral muscle injection at 14 days post-hatch; Group B was administered 0.5 mL/bird of inactivated FAdV-4 vaccine formulated in oil emulsion adjuvant through the same intramuscular route; Group C served as the emulsion control with equivalent-volume injections of PBS-formulated oil emulsion.
To assess vaccination safety, longitudinal growth monitoring was implemented with weekly recording of body weight (g). Blood collection protocols occurred weekly from vaccination week 1 through trial termination. Serum samples were centrifuged (3000× g, 10 min) and stored at −80 °C pending FAdV-4 neutralizing antibody quantification using virus neutralization assays and ELISA tests.
To assess vaccine-induced protection, five specific pathogen-free (SPF) chickens per group were challenged intramuscularly with the FAdV-4 WZ strain (2 × 106 TCID50/bird) at designated timepoints after immunization. Challenge timepoints were strategically selected to evaluate both immediate and prolonged immunity: immediately post-vaccination (0 h), 12 h, 24 h, and then at weekly intervals spanning weeks 1 through 20 (1, 2, 3, 4, 5, 8, 12, 16, and 20 weeks). Following challenge, all subjects were observed twice daily for clinical manifestations (including lethargy, anorexia, and neurological signs) and mortality outcomes. To ensure diagnostic accuracy, mortalities underwent immediate postmortem examination (<1 h after death) for macroscopic lesion evaluation. Surviving chickens were maintained under observation for a 14-day period to monitor potential disease progression before being humanely euthanized for comprehensive histopathological analysis.
For parallel reproductive assessment, fertilized eggs were collected daily from immunized breeders (hen/rooster ratio = 4:1) starting at 19 weeks on a daily basis. The collected eggs were incubated to produce chicks for vertical transmission studies. At 2 weeks of age, the offspring were challenged via intramuscular administration of the FAdV-4 WZ strain (2 × 106 TCID50/bird). Observation and necropsy protocols were the same as those described for the parent stock.
2.6. PEDV Vaccination and Evaluation
A murine model was utilized to evaluate PEDV vaccine efficacy. Thirty 6-week-old BALB/c mice were randomly allocated into three experimental groups (n = 10/group): the water-in-oil (W/O) emulsion vaccine group, water-in-oil-in-water (W/O/W) emulsion vaccine group, and PBS-formulated oil emulsion group. Subcutaneous immunization was performed in the dorsal flank region with 0.3 mL of respective vaccine formulations. The immunization protocol consisted of prime vaccination on day 0 followed by two booster doses at 14-day intervals (week 2 and week 4 post-prime), with identical injection parameters maintained throughout. Serial blood samples (≈200 μL) were collected via tail vein puncture at seven timepoints: pre-immunization (day 0) and weekly intervals from weeks 1 to 6 post-immunization. Serum fractions were obtained through centrifugation at 3000× g for 15 min, aliquoted into sterile cryovials, and preserved at −80 °C pending serological analysis.
2.7. ELISA Test
Serum samples collected from chickens were initially evaluated for anti-FAdV-4 and anti-PEDV antibody levels using enzyme-linked immunosorbent assay (ELISA) according to standardized protocols. The procedure was conducted as follows: Purified FAdV-4 or PEDV antigen (1.75 μg/well) was immobilized onto 96-well plates through overnight incubation at 4 °C. After removing unbound antigens, the plates were blocked with 5% skimmed milk powder in PBST (phosphate-buffered saline with 0.05% Tween 20) for 2 h at 37 °C. Subsequent to blocking solution removal, three sequential washes with PBST were performed using an automated plate washer. Serum samples from experimental groups were diluted 1:1000 in sample dilution buffer and incubated in antigen-coated wells for 1 h at 37 °C. Following primary antibody incubation and three additional washing cycles, horseradish peroxidase (HRP)-conjugated rabbit anti-chicken or goat anti-mouse IgG (Proteintech, Wuhan, China) was added at a 1:7000 dilution in blocking buffer. The secondary antibody incubation proceeded for 1 h at 37 °C with constant agitation. After five final PBST washes, 100 μL of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Solarbio, Beijing, China) was dispensed into each well, and the colorimetric reaction was allowed to develop for 10 min in darkness. Reaction termination was achieved by adding 50 μL of 2 M sulfuric acid per well. Optical density measurements were obtained at 450 nm using a SpectraMax M5 microplate reader (Thermo Fisher, Shanghai, China). To maintain experimental validity, inactivated FAdV-4/PEDV-positive serum served as positive controls while healthy serum (never exposed to either virus) functioned as negative controls. All samples and controls were analyzed in triplicate wells across three independent assay plates.
2.8. Neutralization Assay
Serum samples from each group were heat-inactivated at 56 °C for 30 min and subjected to two-fold serial dilutions, starting with an initial dilution of 1:4 (v/v). These diluted serum samples were mixed with 100 TCID50 per well of virus in equal volumes (1:1 ratio, v/v) and incubated at 37 °C under 5% CO2 for 1 h. The resulting virus-serum mixtures were then inoculated onto confluent cell monolayers in 96-well plates. Following a 7-day incubation under standard cell culture conditions (37 °C, 5% CO2, 95% humidity), cells were fixed with crystal violet and microscopically evaluated for cytopathic effects (CPE).
Neutralization activity was determined by comparison with parallel controls: virus-only controls (to verify viral infectivity) and cell-only controls (to assess background cell viability). A serum sample was considered neutralizing if it demonstrated ≥50% reduction in CPE relative to virus-only controls.
2.9. Analysis of Viral Shedding
Following experimental challenge with FAdV-4, cloacal swab samples were collected from both immunized and control chickens. The swab samples were suspended in PBS and stored at 4 °C for 1 h. Subsequent centrifugation at 8000×
g for 10 min separated the liquid components, after which the supernatant was carefully collected. Nucleic acid extraction was then performed using a commercial DNA extraction kit, strictly adhering to the manufacturer’s instructions. To assess FAdV-4 infection dynamics, we implemented quantitative PCR (qPCR) for precise copy number determination [
15]. Viral load quantification employed the 2−ΔΔCt method with β-actin as the endogenous reference control.
2.10. Histopathological Studies
Under experimental challenge conditions, heart, liver, and kidney samples were collected from chickens for histopathological evaluation. Fresh tissue specimens underwent immediate fixation in 10% neutral buffered formalin maintained at 25 ± 2 °C for 16–18 h. This fixation protocol ensured complete tissue preservation for microscopic analysis. The fixed specimens were then processed through a conventional alcohol dehydration series (70–100% ethanol) followed by paraffin embedding using standard histological protocols. Tissue sections of 5 μm thickness were prepared using a rotary microtome. All sections were stained with hematoxylin and eosin following established histological protocols, with blinded evaluation performed by two independent pathologists using an Olympus BX53 light microscope equipped with a DP27 digital camera system(Olympus, Nagano Prefecture, Japan). Microscopic observations were recorded at 200× and 400× magnifications for detailed cellular assessment.
2.11. Statistical Analysis
All data were statistically analyzed using GraphPad Prism 9 and expressed as “mean ± standard deviation”. For survival curves, significance was determined using the log-rank (Mantel–Cox) test. The data were considered significantly different when p < 0.05 between different groups in EXCEL version 14.0 software.
4. Discussion
IgY antibodies have proven to be safe and effective in clinical applications as pathogen-specific biotherapeutics, functioning through high-affinity target binding for microbial neutralization [
3,
4,
5,
6]. After oral administration, these antibodies are efficiently absorbed into the gastrointestinal tract and transported to the bloodstream through enteric circulation. Our findings demonstrate that passive immunization with IgY provides temporary but critical immunoprotective coverage during the maturation phase of vaccine-induced active immunity. The established dual-phase protection model involves multiple synergistic mechanisms: target-binding antibodies exert their protective effects through steric hindrance of viral-receptor interaction, pathogen-agglutination precipitation, opsonophagocytic enhancement, and complement-mediated virolysis—working cooperatively to establish robust antiviral defenses [
16,
17,
18].
First identified in China during the 2015 outbreaks, FAdV-4-associated inclusion body hepatitis with pericardial effusion syndrome has prompted the development of immunization-based control strategies [
13]. While vaccination remains the cornerstone for disease prevention, emerging evidence suggests a critical 3-week vulnerability window exists between vaccine administration and establishment of protective immunity in poultry flocks. Developing vaccines that provide immediate immunoprotection while maintaining long-term efficacy has therefore become a priority for sustainable avian adenovirus control.
This study developed an innovative active-passive immunization strategy combining formalin-inactivated FAdV-4 with hyperimmune IgY against FAdV-4. Experimental validation in specific-pathogen-free (SPF) Leghorn chicks demonstrated that the immune complex vaccine achieved 100% clinical protection with complete viral clearance by post-challenge from 0 h to 20 weeks, as confirmed through qPCR and histopathological analysis.
Compared to standalone inactivated vaccines, our immune complex formulation demonstrates accelerated initiation, augmented intensity, and extended duration of protective immunity. This superior efficacy is consistent with established mechanisms whereby immune composite vaccines alter the rate or pattern of viral replication while preserving antigen immunogenicity [
19]. The water-oil-water (W/O/W) emulsion, engineered through phase fractionation emulsification, achieved optimal physicochemical stability. This delivery system exhibited exceptional formulation homogeneity and enhanced adjuvant properties that synergistically potentiate rather than compromise native immune functions. In the immune complex, the antibody and antigen were physically isolated by the oil phase, and the antigen surface did not be masked by antibody; therefore, they would not undergo specific binding. The antibody and antigen were absorbed or recognized, respectively, at different times, and they play an important role in passive and active immunity. When the immune complex was injected into the animal body, the outer layer of the antibody aqueous phase, which contains a substantial molar excess of IgY antibodies, could be released into the bloodstream quickly, providing immediate passive immunity. However, the IgY antibody has a half-life of approximately 4.1 days [
20], As it was absorbed gradually, the internal water-in-oil emulsion began to stimulate the immune system. The antigen aqueous phase was recognized by the immune cells, generating active immunity, and protective antibodies began to appear within 10–14 days. So, this immune complex can facilitate a rapid immune response, and the antigen surface could not be masked by preformed antibodies on the immunogenicity of the co-administered antigen.
The vertical transmission of maternal immunity from vaccinated breeders to progeny represents a critical advantage in FAdV-4 management [
8,
21]. As demonstrated in previous studies [
22,
23], vaccine efficacy shows strong correlation with transovarian antibody transfer levels, where maternal antibody titers serve as key determinants of broiler protection. Notably, our immune complex formulation achieved sustained high-level protective efficacy in progeny through 30 weeks post-vaccination while maintaining excellent safety profiles in vaccinated breeders, with histopathological analysis confirming absence of tissue damage. Compared to conventional inactivated vaccines, this dual-action formulation (combining immune complex formation with enhanced antigen presentation) demonstrated accelerated induction of protective immunity through antibody-mediated antigen processing [
1,
7]. The enhanced immunogenicity likely results from improved phagocytosis and intracellular processing of high-affinity immune complexes by professional antigen-presenting cells [
24].
To investigate the potential application of egg yolk antibodies in composite vaccine development for non-avian species, we formulated a combined PEDV-IgY vaccine. IgY from immunized chickens has also been shown to be safe and effective against PEDV in newborn piglets [
25]. In previous studies, due to the incomplete development of the digestive system in newborn piglets, IgY taken through the digestive tract can quickly and effectively penetrate the intestinal wall of newborn piglets, maintain long-term activity in the intestine, and have a significant protective effect on piglets. The IgY produced by purified recombinant PEDV S1 protein immunized hens can also protect piglets against PEDV infection [
26]. Throughout, IgY against PEDV may have been an alternative method of supplementing prophylactic measures like colostral antibodies from sows. In this study, we formed a novel composite vaccine by combining inactivated PEDV antigen with IgY, which triggered sustained humoral immunity and represents a novel application of IgY in PEDV prevention and control. These findings suggest that the egg yolk antibody composite vaccine exhibits potential applicability in non-avian species.
Functionally analogous to mammalian IgG, avian IgY demonstrates inherent immunomodulatory properties. From an economic perspective, the utilization of egg yolk antibodies presents a more cost-effective alternative to IgG. Therefore, the IgY immune complex vaccine represents a promising candidate with significant commercial potential.