Comparative Clinical Outcomes of Major Respiratory Viruses in Hospitalized Adults During the Post-Pandemic Period: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
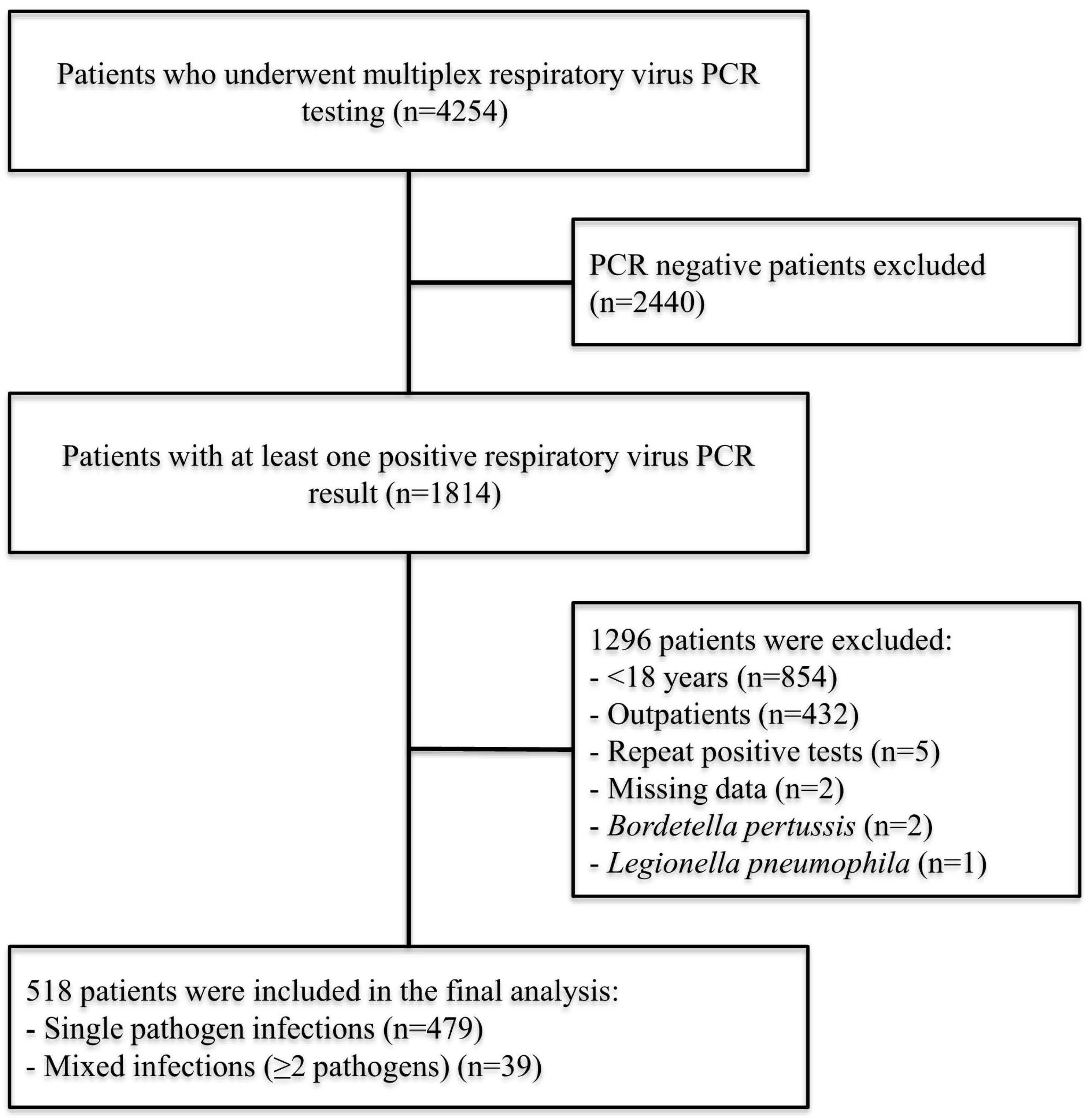
2.2. Patient Population
2.3. Data Collection
2.4. Definitions
2.5. Microbiological Diagnosis
2.6. Outcomes
2.7. Ethics Approval and Consent to Participate
2.8. Statistical Analysis
3. Results
3.1. Description of the Cohort
3.2. Baseline Characteristics and Distribution of Detected Respiratory Viruses
3.3. Clinical and Laboratory Characteristics by Virus
3.4. Comparison Between Influenza and SARS-CoV-2 Infections
3.5. Determining the Factors Affecting Mortality
3.6. Independent Predictors of In-Hospital Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bender, R.G.; Sirota, S.B.; Swetschinski, L.R.; Dominguez, R.-M.V.; Novotney, A.; Wool, E.E.; Ikuta, K.S.; Vongpradith, A.; Rogowski, E.L.B.; Doxey, M.; et al. Global, Regional, and National Incidence and Mortality Burden of Non-COVID-19 Lower Respiratory Infections and Aetiologies, 1990–2021: A Systematic Analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [Google Scholar] [CrossRef]
- Van Doorn, H.R.; Yu, H. Viral Respiratory Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 284–288. ISBN 978-0-323-55512-8. [Google Scholar]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal); WHO: Geneva, Switzerland, 2024; Available online: https://www.Who.Int/News-Room/Fact-Sheets/Detail/Influenza-(Seasonal) (accessed on 9 September 2025).
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://Data.Who.Int/Dashboards/Covid19/Cases (accessed on 26 September 2025).
- Clinical Management of COVID-19: Living Guideline, June 2025. Available online: https://www.who.int/publications/i/item/B09467 (accessed on 26 September 2025).
- Ambrosch, A.; Luber, D.; Klawonn, F.; Kabesch, M. Focusing on Severe Infections with the Respiratory Syncytial Virus (RSV) in Adults: Risk Factors, Symptomatology and Clinical Course Compared to Influenza A/B and the Original SARS-CoV-2 Strain. J. Clin. Virol. 2023, 161, 105399. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.U.; Park, D.; Kim, I.-H.; Kim, S.; Yoo, H.M. Development of Human Rhinovirus RNA Reference Material Using Digital PCR. Genes 2023, 14, 2210. [Google Scholar] [CrossRef] [PubMed]
- Surie, D. Disease Severity of Respiratory Syncytial Virus Compared with COVID-19 and Influenza Among Hospitalized Adults Aged ≥60 Years—IVY Network, 20 U.S. States, February 2022–May 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Morelli, T.; Freeman, A.; Staples, K.J.; Wilkinson, T.M.A. Hidden in Plain Sight: The Impact of Human Rhinovirus Infection in Adults. Respir. Res. 2025, 26, 120. [Google Scholar] [CrossRef]
- Pozzetto, B.; Grattard, F.; Pillet, S. Multiplex PCR Theranostics of Severe Respiratory Infections. Expert Rev. Anti Infect. Ther. 2010, 8, 251–253. [Google Scholar] [CrossRef][Green Version]
- Mahony, J.B.; Petrich, A.; Smieja, M. Molecular Diagnosis of Respiratory Virus Infections. Crit. Rev. Clin. Lab. Sci. 2011, 48, 217–249. [Google Scholar] [CrossRef]
- Calderaro, A.; Buttrini, M.; Farina, B.; Montecchini, S.; De Conto, F.; Chezzi, C. Respiratory Tract Infections and Laboratory Diagnostic Methods: A Review with A Focus on Syndromic Panel-Based Assays. Microorganisms 2022, 10, 1856. [Google Scholar] [CrossRef]
- Peñarrubia, L.; Reister, S.; Jiménez-Guzmán, S.; Porco, R.; Congost-Teixidor, C.; Pueyo, G.; Camprubí-Font, C.; Vara, K.; Cardenosa, M.d.l.C.; Contreras, M.; et al. Molecular Diagnostics Using the QIAstat-Dx Syndromic Device for Covering Avian Influenza Pandemic Preparedness. Heliyon 2024, 10, e40645. [Google Scholar] [CrossRef]
- Ishikane, M.; Unoki-Kubota, H.; Moriya, A.; Kutsuna, S.; Ando, H.; Kaburagi, Y.; Suzuki, T.; Iwamoto, N.; Kimura, M.; Ohmagari, N. Evaluation of the QIAstat-Dx Respiratory SARS-CoV-2 Panel, a Rapid Multiplex PCR Method for the Diagnosis of COVID-19. J. Infect. Chemother. 2022, 28, 729–734. [Google Scholar] [CrossRef]
- Manno, M.; Pavia, G.; Gigliotti, S.; Pantanella, M.; Barreca, G.S.; Peronace, C.; Gallo, L.; Trimboli, F.; Colosimo, E.; Lamberti, A.G.; et al. Respiratory Virus Prevalence Across Pre-, During-, and Post-SARS-CoV-2 Pandemic Periods. Viruses 2025, 17, 1040. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, M.; Bonura, F.; Cacioppo, F.; Palazzotto, E.; Filizzolo, C.; Russo, S.; Pistoia, D.; Capra, G.; Ferraro, D.; Giammanco, G.M.; et al. Post-COVID-19 Epidemiology of Viral Infections in Adults Hospitalized with Acute Respiratory Syndromes in Palermo, South of Italy. Pathogens 2025, 14, 997. [Google Scholar] [CrossRef]
- Hong, S.; Zhu, M.; Huang, Y.; Chen, L.; Han, D.; Qiu, W.; Li, S.; Ding, L.; Zheng, H.; Guo, F.; et al. Post-Pandemic Resurgence of Respiratory Virus Infections: Age and Department-Specific Patterns in a Chinese Tertiary Hospital (2020–2024). BMC Infect. Dis. 2025, 25, 1431. [Google Scholar] [CrossRef] [PubMed]
- Naouri, D.; Pham, T.; Dres, M.; Vuagnat, A.; Beduneau, G.; Mercat, A.; Combes, A.; Kimmoun, A.; Schmidt, M.; Demoule, A.; et al. Differences in Clinical Characteristics and Outcomes between COVID-19 and Influenza in Critically Ill Adult Patients: A National Database Study. J. Infect. 2023, 87, 120–127. [Google Scholar] [CrossRef]
- López Montesinos, I.; Arrieta-Aldea, I.; Dicastillo, A.; Zuccarino, F.; Sorli, L.; Guerri-Fernández, R.; Arnau-Barrés, I.; Montero, M.M.; Siverio-Parès, A.; Durán, X.; et al. Comparison of Hospitalized Coronavirus Disease 2019 and Influenza Patients Requiring Supplemental Oxygen in a Cohort Study: Clinical Impact and Resource Consumption. Clin. Infect. Dis. 2022, 75, 2225–2238. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Mortality in Patients Hospitalized for COVID-19 vs Influenza in Fall-Winter 2023–2024. JAMA 2024, 331, 1963–1965. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Svalgaard, I.B.; Lomholt, F.K.; Emborg, H.-D.; Christiansen, L.E.; Soborg, B.; Hviid, A.; Vestergaard, L.S. The Hospital and Mortality Burden of COVID-19 Compared with Influenza in Denmark: A National Observational Cohort Study, 2022–2024. Lancet Infect. Dis. 2025, 25, 616–624. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Liu, Y.; Calzavara, A.; Sundaram, M.E.; Djebli, M.; Darvin, D.; Baral, S.; Kustra, R.; Kwong, J.C.; Mishra, S. Predictors of All-cause Mortality among Patients Hospitalized with Influenza, Respiratory Syncytial Virus, or SARS-CoV-2. Influenza Other Respir. Viruses 2022, 16, 1072–1081. [Google Scholar] [CrossRef]
- Kassaw, G.; Mohammed, R.; Tessema, G.M.; Yesuf, T.; Lakew, A.M.; Tarekegn, G.E. Outcomes and Predictors of Severe Community-Acquired Pneumonia Among Adults Admitted to the University of Gondar Comprehensive Specialized Hospital: A Prospective Follow-up Study. Infect. Drug Resist. 2023, 16, 619–635. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y.; Liu, Y.; Liu, X.; Gu, L.; Zhang, X.; Pu, Z.; Yang, G.; Liu, B.; Nie, Q.; et al. Disease Severity and Clinical Outcomes of Community-Acquired Pneumonia Caused by Non-Influenza Respiratory Viruses in Adults: A Multicentre Prospective Registry Study from the CAP-China Network. Eur. Respir. J. 2019, 54, 1802406. [Google Scholar] [CrossRef] [PubMed]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Shlomai, A.; Goldberg, E.; Sklan, E.H. Secondary Bacterial Infection in COVID-19 Patients Is a Stronger Predictor for Death Compared to Influenza Patients. Sci. Rep. 2021, 11, 12703. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, P.; Karlsson Valik, J.; Abdel-Halim, L.; Alfvén, T.; Nauclér, P. Outcomes of SARS-CoV-2 Omicron Variant Infections Compared With Seasonal Influenza and Respiratory Syncytial Virus Infections in Adults Attending the Emergency Department: A Multicenter Cohort Study. Clin. Infect. Dis. 2024, 78, 900–907. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 518) | |
|---|---|
| Age, mean ± SD [min–max] | 62.8 ± 17.6 [18–94] |
| Sex Male, n (%) | 282 (54.4) |
| Presence of comorbidity, n (%) † Diabetes mellitus Cardiovascular disease Chronic lung disease (COPD, asthma) Hematological malignancy Solid tumor | 476 (91.9) 145 (28.0) 143 (27.6) 104 (20.1) 102 (19.7) 98 (18.9) |
| Charlson Comorbidity Index, median (IQR) [min–max] | 5 (2–7) [0–15] |
| Immunosuppression, n (%) | 223 (43.1) |
| Vaccination status, n (%) ‡ Influenza vaccination COVID-19 vaccination Pneumococcal Vaccination | 37 (7.1) 469 (90.5) 151 (29.2) |
| Clinical findings at the time of sample collection, n (%) § Fever Cough Dyspnea | 161 (31.1) 279 (53.9) 161 (31.1) |
| Oxygen requirement at sample collection, n (%) | 262 (50.5) |
| Laboratory findings at the time of sample collection Leukocyte count (cells/µL), median (IQR) Neutrophil count (cells/µL), median (IQR) Lymphocyte count (cells/µL), median (IQR) CRP (mg/L), median (IQR) | 7500 (4600–11,350) 5540 (2937–8742) 870 (520–1400) 74.0 (33.0–166.0) |
| Radiological evidence of pneumonia, n (%) | 109 (21.0) |
| Secondary bacterial infection, n (%) | 53 (10.2) |
| Location at the time of sample collection, n (%) Ward ICU | 361 (69.7) 157 (30.3) |
| ICU admission after sample collection, (n = 361), n (%) ¶ | 96/361 (26.6) |
| Length of stay in ward (days) median (IQR) [min–max] | 10 (4–19) [0–143] |
| Length of stay in ICU (days), median (IQR) [min–max] | 0 (0–10) [0–176] |
| Total length of hospital stay (days), median (IQR) [min–max] | 16 (9–29) [0–176] |
| Status on day 30, n (%) Discharged Follow-up ongoing In intensive care unit Deceased | 299 (57.7) 52 (10.0) 44 (8.5) 123 (23.7) |
| Overall in-hospital mortality, n (%) | 163 (31.5) |
| Time from sample collection to death, median (IQR) [min–max] (n = 163) | 14 (6–25) [0–173] |
| Time from hospital admission to death, median (IQR) [min–max] (n = 163) | 23 (13–37) [2–176] |
| Detected Pathogen | n | % |
|---|---|---|
| SARS-CoV-2 | 169 | 32.6 |
| Influenza A | 167 | 32.2 |
| Rhinovirus | 72 | 13.9 |
| Respiratory syncytial virus (RSV) A + B | 52 | 10.0 |
| Influenza B | 24 | 4.6 |
| Parainfluenza virus 3 | 19 | 3.7 |
| Human metapneumovirus | 15 | 3.0 |
| Human coronavirus OC43 | 11 | 2.1 |
| Adenovirus | 10 | 1.9 |
| Human coronavirus 229E | 6 | 1.2 |
| Human coronavirus HKU1 | 6 | 1.2 |
| Human coronavirus NL63 | 6 | 1.2 |
| Enterovirus | 3 | 0.6 |
| Parainfluenza virus 2 | 2 | 0.4 |
| Parainfluenza virus 1 | 1 | 0.2 |
| Parainfluenza virus 4 | 1 | 0.2 |
| Total detected pathogens | 564 | 1.089 per sample |
| Total positive samples | 518 | 100.0 |
| Mixed infections (≥2 pathogens) | 39 | 7.5 |
| Variable | Influenza (A + B) (n = 174) ‡ | SARS-CoV-2 (n = 152) | Rhinovirus (n = 58) | RSV (n = 34) | p-Value |
|---|---|---|---|---|---|
| Age, mean ± SD | 64.2 ± 17.6 | 64.5 ± 16.4 | 60.9 ± 19.6 | 62.3 ± 13.1 | 0.488 * |
| Male sex, n (%) | 86/174 (49.4) | 79/152 (52.0) | 34/58 (58.6) | 24/34 (70.6) | 0.117 ** |
| Any comorbidity, n (%) | 158/174 (90.8) | 140/152 (92.1) | 51/58 (87.9) | 32/34 (94.1) | 0.726 ** |
| CCI, median [IQR] | 4.5 [2.0–7.0] | 5.0 [2.5–7.0] | 4.0 [2.0–6.0] | 4.5 [3.0–7.0] | 0.455 * |
| Immunosuppression, n (%) | 57/174 (32.8) a | 72/152 (47.4) b | 22/58 (37.9) ab | 22/34 (64.7) b | <0.001 ** |
| Vaccination status, n (%) | |||||
| - Influenza vaccination | 11/174 (6.3) | 12/152 (7.9) | 3/58 (5.2) | 4/34 (11.8) | 0.630 ** |
| - COVID-19 vaccination | 157/174 (90.2) a | 141/152 (92.8) a | 47/58 (81.0) a | 33/34 (97.1) a | 0.035 ** |
| Clinical and laboratory characteristics at the time of sample collection | |||||
| - Fever, n (%) | 60/174 (34.5) a | 45/152 (29.6) a | 7/58 (12.1) b | 6/34 (17.6) ab | 0.005 ** |
| - Cough, n (%) | 97/174 (55.7) | 73/152 (48.0) | 32/58 (55.2) | 20/34 (58.8) | 0.456 ** |
| - Dyspnea, n (%) | 45/174 (25.9) | 58/152 (38.2) | 14/58 (24.1) | 10/34 (29.4) | 0.068 ** |
| - Oxygen requirement, n (%) | 84/174 (48.3) | 86/152 (56.6) | 27/58 (46.6) | 20/34 (58.8) | 0.312 ** |
| - IN ICU, n (%) | 51/174 (29.3) | 60/152 (39.5) | 17/58 (29.3) | 9/34 (26.5) | 0.174 ** |
| - WBC (cells/µL), median [IQR] | 7.4 [4.8–11.4] b | 7.2 [4.3–11.3] b | 9.9 [6.4–14.0]a | 7.7 [0.9–10.5] b | 0.010 * |
| - ALC (cells/µL), median [IQR] | 0.89 [0.55–1.35] | 0.86 [0.52–1.36] | 0.96 [0.55–1.98] | 0.76 [0.31–1.41] | 0.178 * |
| - CRP (mg/L), median [IQR] | 63.0 [35.2–142.2] | 78.0 [33.0–173.0] | 98.0 [28.2–160.8] | 67.0 [52.0–143.0] | 0.588 * |
| - Radiological evidence of pneumonia, n (%) § | 26/174 (14.9) | 23/152 (15.1) | 17/58 (29.3) | 6/34 (17.6) | 0.070 ** |
| Secondary bacterial infection, n (%) | 9 (5.2) | 20 (13.2) | 7 (12.1) | 2 (5.9) | 0.062 ** |
| ICU admission after sample collection, n (%) | 26/174 (14.9) a | 39/152 (25.7) a | 6/58 (10.3) a | 4/34 (11.8) a | 0.016 ** |
| Total length of hospital stay (days), median [IQR] | 14.0 [9.0–26.0] | 17.5 [9.0–28.5] | 14.0 [8.0–23.0] | 18.5 [10.5–28.5] | 0.158 * |
| In-hospital mortality, n (%) | 45/174 (25.9) ac | 62/152 (40.8) b | 8/58 (13.8) a | 13/34 (38.2) bc | <0.001 ** |
| Time from sample collection to death, (days), median [IQR] (n= 128) | 9 [6–30] | 14 [7–23] | 17 [4–23] | 14 [6–20] | 0.959 * |
| Time from admission to death (days), median [IQR] (n = 128) | 20 [11–37] | 22 [13–35] | 26 [16–32] | 20 [16–42] | 0.994 * |
| Variable | Influenza (A + B) (n = 174) | SARS-CoV-2 (n = 152) | p-Value |
|---|---|---|---|
| Key laboratory marker, median [IQR] ‡ | |||
| Absolute lymphocyte count (cells/µL)—Day 7 | 985 (730–1645) | 820 (375–1500) | 0.012 * |
| Treatment characteristics | |||
| Antiviral therapy (oseltamivir-molnupiravir), n (%): | 150 (86.2) | 50 (32.9) | <0.001 ** |
| Antiviral initiation ≤48 h, n (%): | 146/150 (97.3) | 47/50 (94.0) | 1.000 ** |
| Clinical severity and mortality | |||
| Respiratory support (highest level during stay), n (%) | |||
| - Oxygen requirement (any) | 93 (53.4) | 98 (64.5) | 0.044 ** |
| - Low-flow (nasal cannula + simple mask) | 44 (25.3) | 40 (26.3) | 0.832 ** |
| - High-flow/reservoir mask | 8 (4.6) | 15 (9.9) | 0.064 ** |
| - Mechanical ventilation (IMV or NIMV) | 41 (23.6) | 43 (28.3) | 0.330 ** |
| ICU admission, n (%) | 72 (41.4) | 86 (56.6) | 0.006 ** |
| In-hospital mortality (overall), n (%) | 45 (25.9) | 62 (40.8) | 0.004 ** |
| Death by day 7 | 7 (4.0) | 8 (5.3) | 0.594 ** |
| Death by day 14 | 14 (8.0) | 19 (12.5) | 0.184 ** |
| Death by day 28 | 28 (16.1) | 43 (28.3) | 0.008 ** |
| Total length of hospital stay (days), median (IQR) | 14.0 (9.0–26.0) | 17.5 (9.0–28.5) | 0.297 * |
| Variable | All Patients (n = 518) | Survivors (n = 355) | Non-Survivors (n = 163) | p-Value |
|---|---|---|---|---|
| Male sex, n (%) | 282/518 (54.4) | 193/355 (54.4) | 89/163 (54.6) | 1.000 ** |
| Age, years, median (IQR) [min–max] | 66.0 (51.0–76.0) [18–94] | 64.0 (46.5–74.0) [18–94] | 70.0 (59.0–79.5) [24–93] | <0.001 * |
| CCI, median (IQR) [min–max] | 5.0 (2.0–7.0) [0–15] | 4.0 (2.0–6.0) [0–13] | 6.0 (4.0–7.5) [0–15] | <0.001 * |
| Immunosuppression, n (%) | 223/518 (43.1) | 137/355 (38.6) | 86/163 (52.8) | 0.003 ** |
| Oxygen requirement at sampling, n (%) | 262/518 (50.6) | 129/355 (36.3) | 133/163 (81.6) | <0.001 ** |
| In ICU at sampling, n (%) | 157/518 (30.3) | 60/355 (16.9) | 97/163 (59.5) | <0.001 ** |
| Virus group (monoinfections), n (%) | 0.026 ** | |||
| Influenza (A + B) | 174 (36.3) | 129 (74.1) | 45 (25.9) | |
| SARS-CoV-2 | 152 (31.7) | 90 (59.2) | 62 (40.8) | |
| Rhinovirus | 58 (12.1) | 50 (86.2) | 8 (13.8) | |
| RSV | 34 (7.1) | 21 (61.8) | 13 (38.2) | |
| Other viruses † | 61 (12.7) | 42 (68.9) | 19 (31.1) | |
| Mixed infections (≥2 pathogens), n (%) | 39/518 (7.5) | 23/355 (59.0) | 16/163 (41.0) | 0.247 ** |
| WBC (cells/µL) at sampling, median (IQR) [min–max] | 7500 (4600–11,350) [0–125,910] | 7200 (4500–10,405) [20–52,930] | 8770 (5080–13,180) [0–125,910] | 0.017 * |
| ALC (cells/µL) at sampling, median (IQR) [min–max] | 870 (520–1400) [0–114,170] | 960 (552–1510) [0–33,600] | 690 (355–1190) [0–114,170] | <0.001 * |
| CRP (mg/L) at sampling, median (IQR) [min–max] | 74.0 (33.0–166.0) [0.6–622.0] | 61.0 (23.0–145.0) [0.6–213.0] | 117.0 (54.4–197.0) [8.0–622.0] | <0.001 * |
| Radiological evidence of pneumonia, n (%) ‡ | 109/518 (21.0) | 50/355 (14.1) | 59/163 (36.2) | <0.001 ** |
| Secondary bacterial infection, n (%) | 53/518 (10.2) | 18/355 (5.1) | 35/163 (21.5) | <0.001 ** |
| Mechanical ventilation (any), n (%) | 111/518 (21.4) | 22/355 (6.2) | 89/163 (54.6) | <0.001 ** |
| ICU admission after sampling (among ward at sampling), n (%) § | 96/361 (26.6) | 40/295 (13.6) | 56/66 (84.8) | <0.001 ** |
| Total length of hospital stay, days, median (IQR) [min–max] | 16.0 (9.0–29.0) [0–176] | 13.0 (8.0–25.0) [0–158] | 23.0 (13.0–37.0) [2–176] | <0.001 * |
| Variable | Odds Ratio (OR) | 95% CI | p-Value |
|---|---|---|---|
| Age (per 10 years) | 1.25 | 1.00–1.57 | 0.048 |
| Sex (male) | 0.82 | 0.49–1.39 | 0.467 |
| Charlson Comorbidity Index (per point) | 1.08 | 0.96–1.22 | 0.207 |
| Immunosuppression | 3.67 | 1.95–6.89 | <0.001 |
| Secondary bacterial infection | 7.00 | 3.13–15.66 | <0.001 |
| ICU at sampling | 5.52 | 3.00–10.16 | <0.001 |
| Oxygen requirement at sampling | 3.39 | 1.86–6.18 | <0.001 |
| Virus group (ref = Influenza) | |||
| SARS-CoV-2 | 1.74 | 0.95–3.18 | 0.073 |
| Rhinovirus | 0.29 | 0.11–0.78 | 0.014 |
| RSV | 2.01 | 0.75–5.37 | 0.165 |
| Other viruses | 1.56 | 0.66–3.69 | 0.308 |
| White blood cell count (per 1000 cells/µL) | 1.04 | 1.00–1.09 | 0.036 |
| Absolute lymphocyte count (per 100 cells/µL) | 0.99 | 0.99–1.00 | 0.037 |
| CRP (per 10 mg/L) | 1.01 | 0.99–1.03 | 0.269 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahraman, H.; Keser, G.; Ölmezoğlu, F.S.; Altıntaş Öner, B.; Kurt, O.S.; Us, T.; Erdem, F. Comparative Clinical Outcomes of Major Respiratory Viruses in Hospitalized Adults During the Post-Pandemic Period: A Retrospective Cohort Study. Viruses 2025, 17, 1545. https://doi.org/10.3390/v17121545
Kahraman H, Keser G, Ölmezoğlu FS, Altıntaş Öner B, Kurt OS, Us T, Erdem F. Comparative Clinical Outcomes of Major Respiratory Viruses in Hospitalized Adults During the Post-Pandemic Period: A Retrospective Cohort Study. Viruses. 2025; 17(12):1545. https://doi.org/10.3390/v17121545
Chicago/Turabian StyleKahraman, Hasip, Gizem Keser, Furkan Süha Ölmezoğlu, Betül Altıntaş Öner, Onur Sedat Kurt, Tercan Us, and Fatma Erdem. 2025. "Comparative Clinical Outcomes of Major Respiratory Viruses in Hospitalized Adults During the Post-Pandemic Period: A Retrospective Cohort Study" Viruses 17, no. 12: 1545. https://doi.org/10.3390/v17121545
APA StyleKahraman, H., Keser, G., Ölmezoğlu, F. S., Altıntaş Öner, B., Kurt, O. S., Us, T., & Erdem, F. (2025). Comparative Clinical Outcomes of Major Respiratory Viruses in Hospitalized Adults During the Post-Pandemic Period: A Retrospective Cohort Study. Viruses, 17(12), 1545. https://doi.org/10.3390/v17121545






