Molecular Responses to Avian Reovirus Inoculation In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. S1133 Inoculum Preparation and Dose Determination
2.3. Virus Inoculation
2.4. Quantitative PCR for Viral Load
2.5. RNA Sequencing
2.6. Read Processing and Differential Expression Analyses
2.7. Pathway Annotation and Comparison
2.8. Protein–Protein Interaction (PPI) Analysis
2.9. Isoform Switch Analysis
3. Results
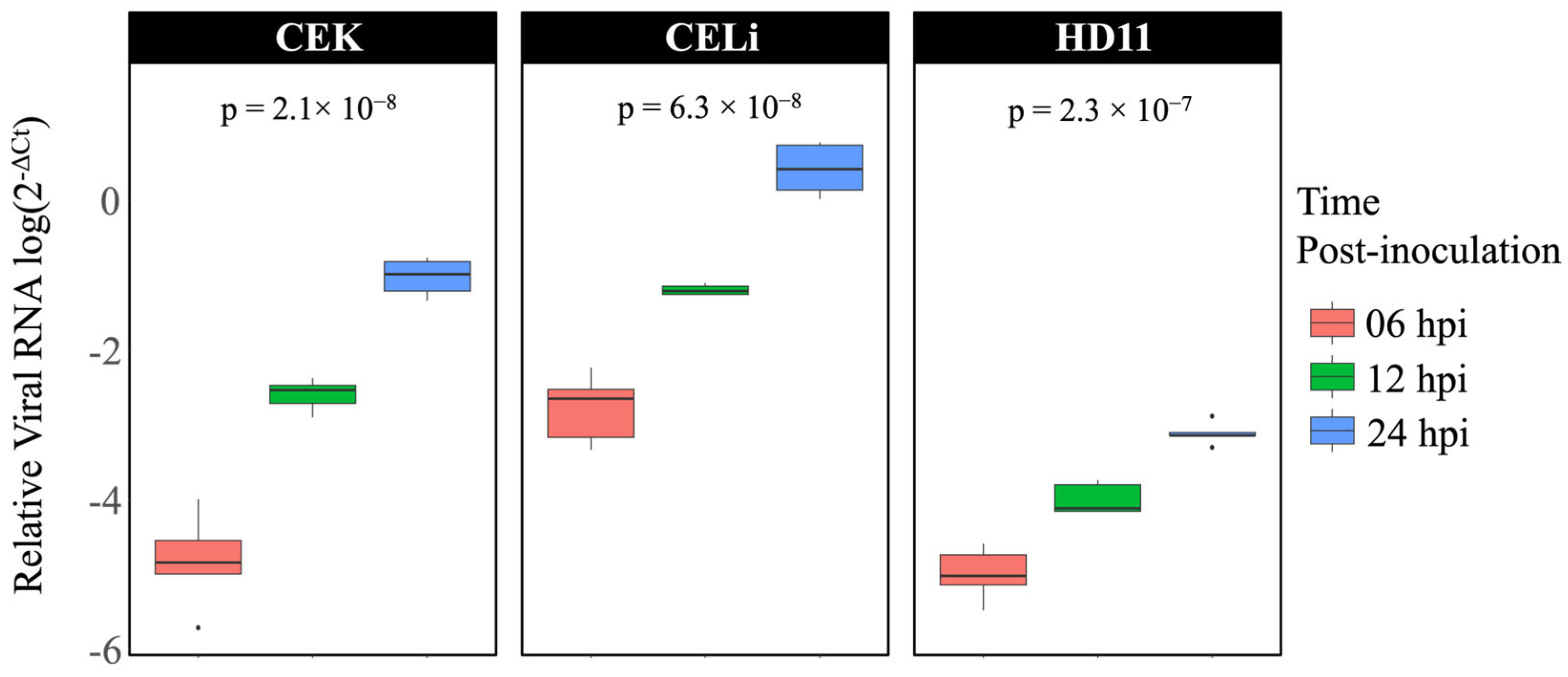
3.1. Avian Reovirus Replication in Different Cell Types
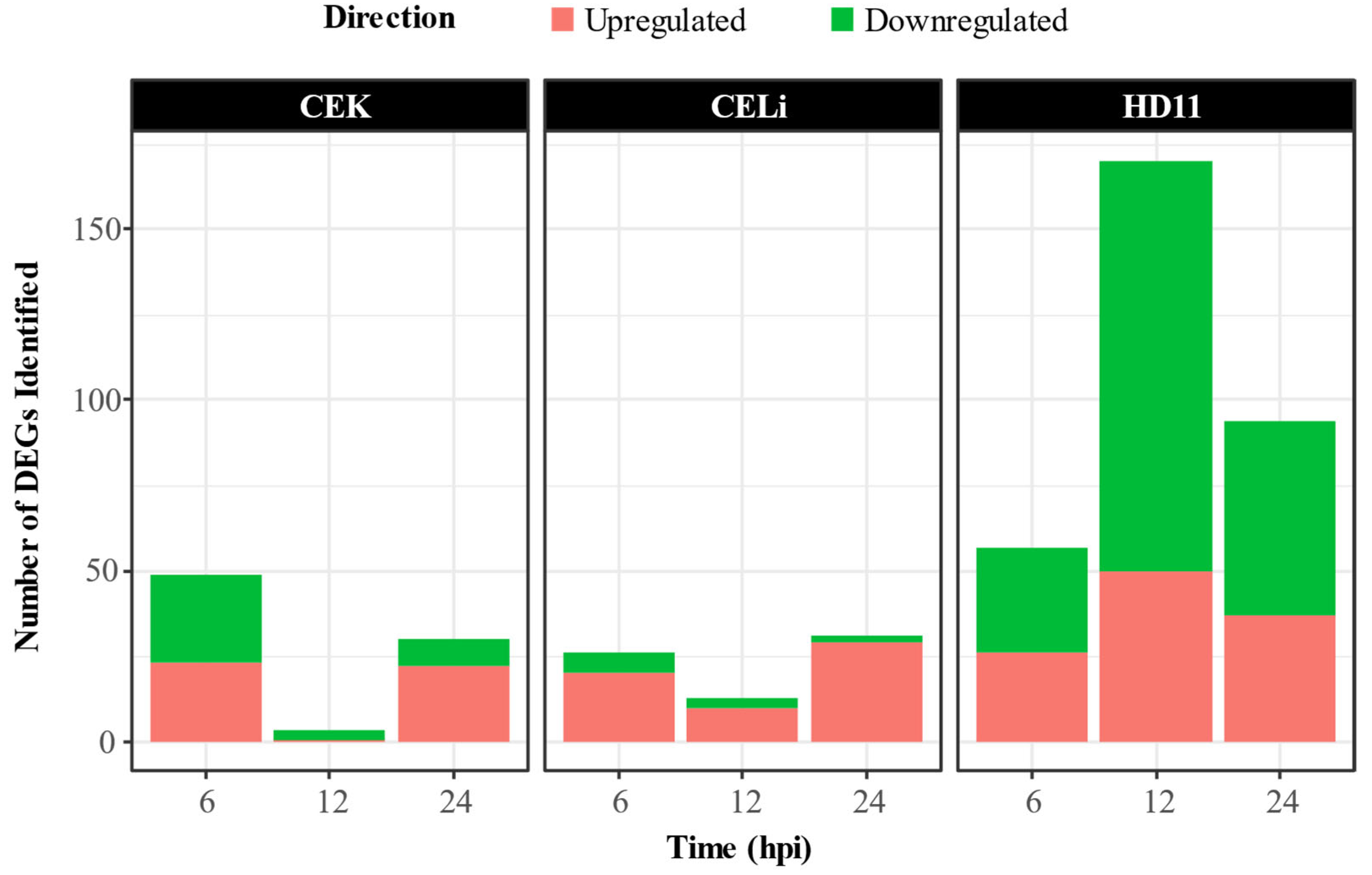
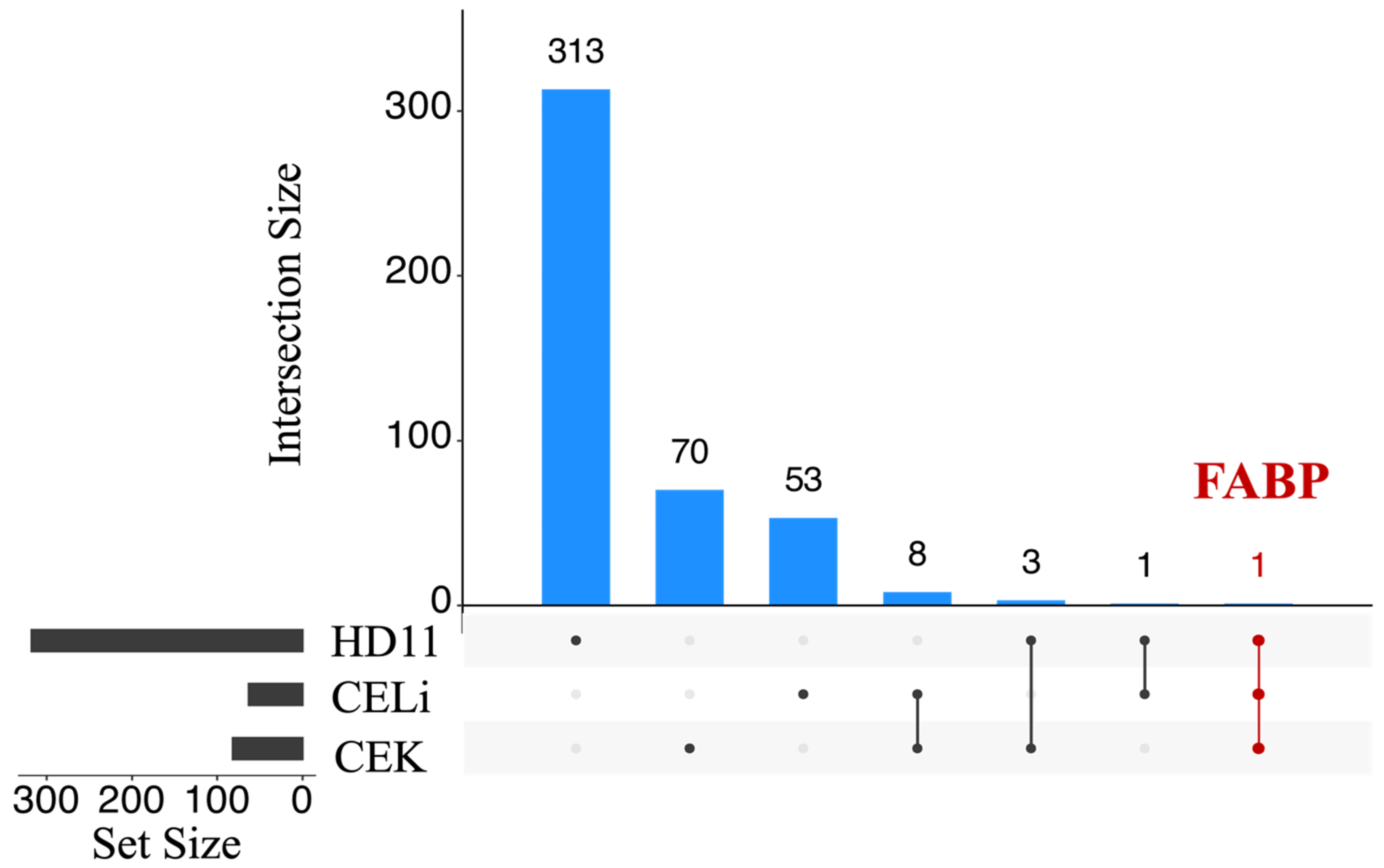
3.2. Differential Gene Expression Analysis
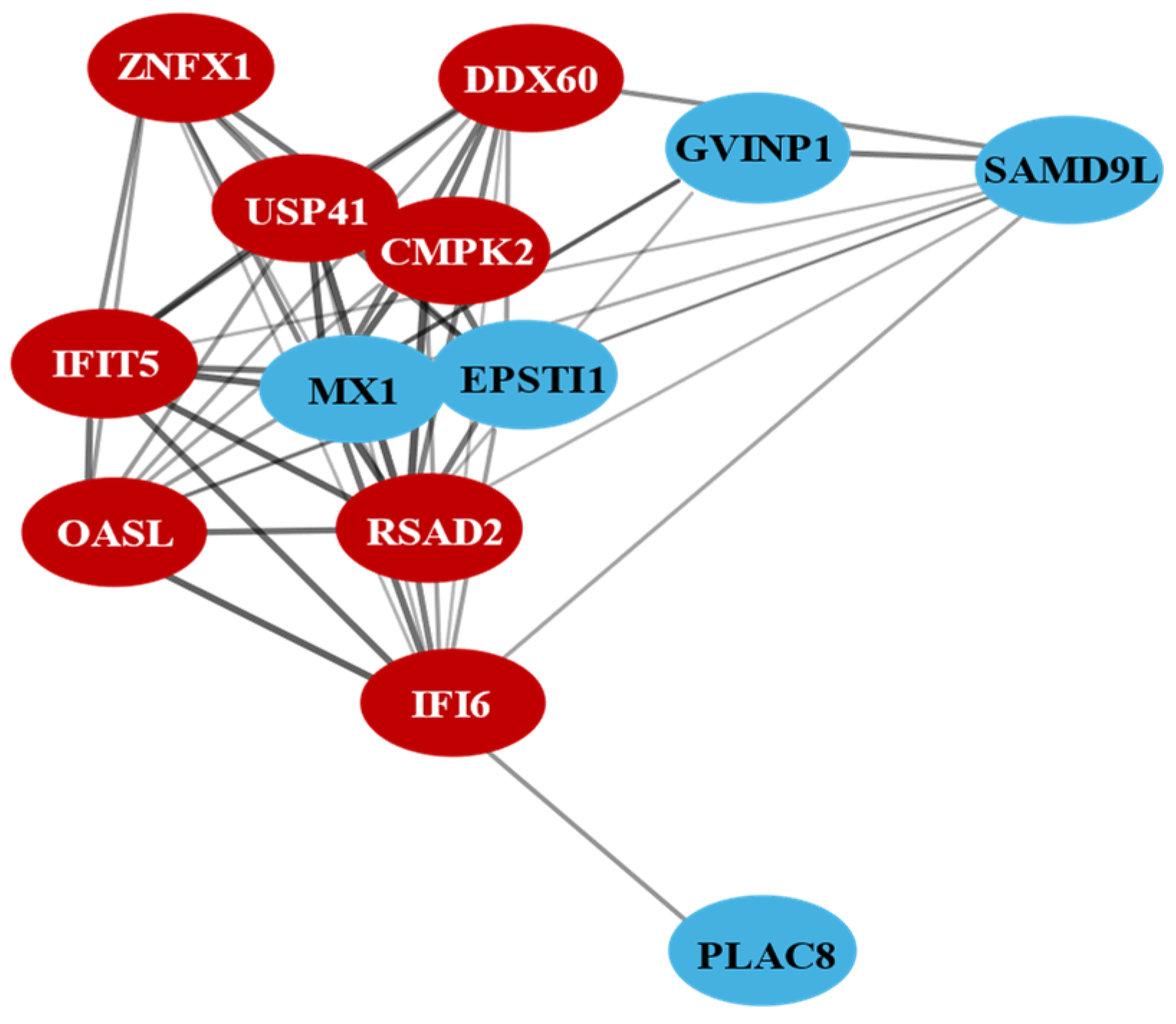
3.3. Protein–Protein Interaction Network
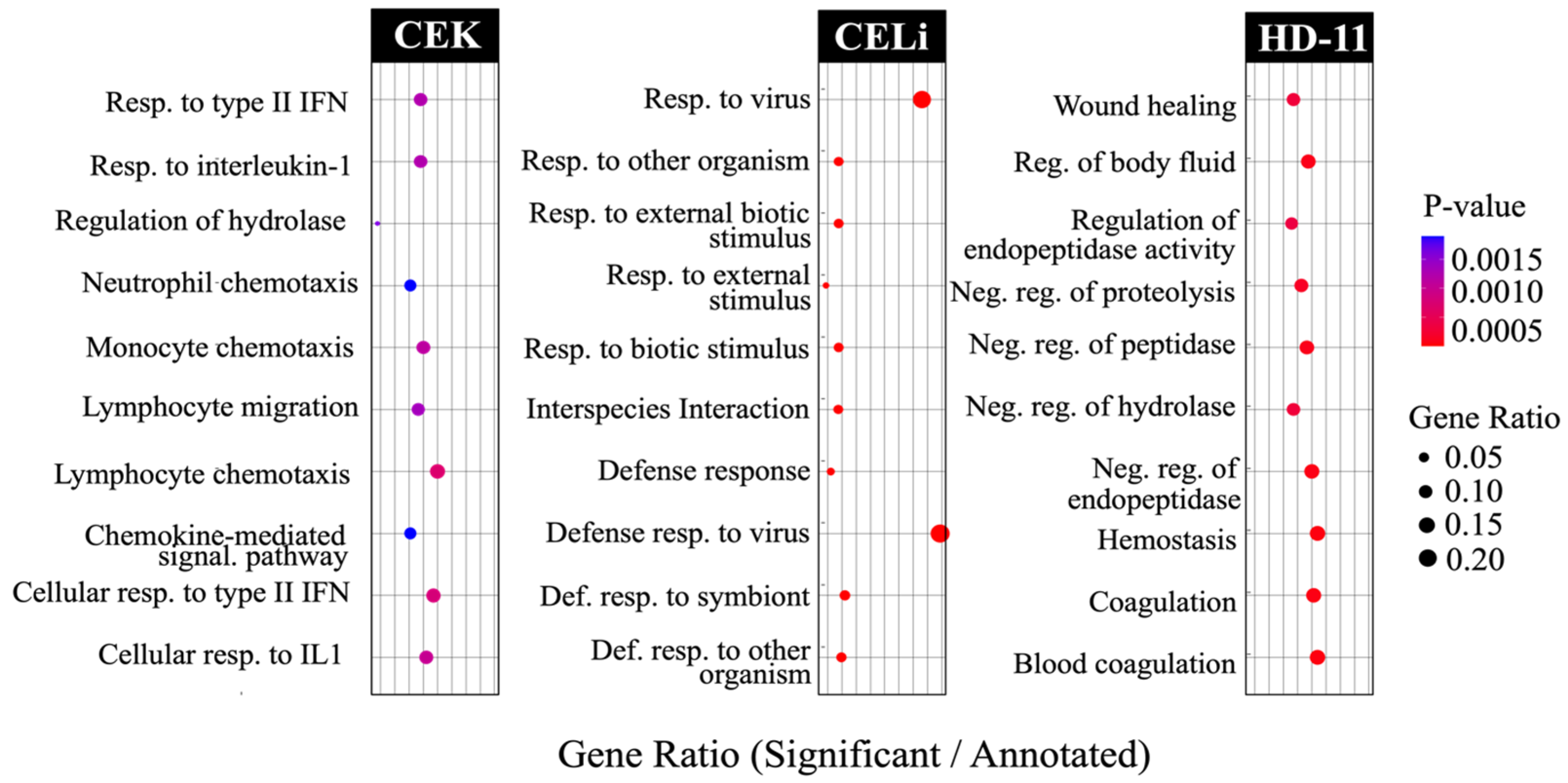
3.4. Pathway Enrichment: Gene Ontology Analysis
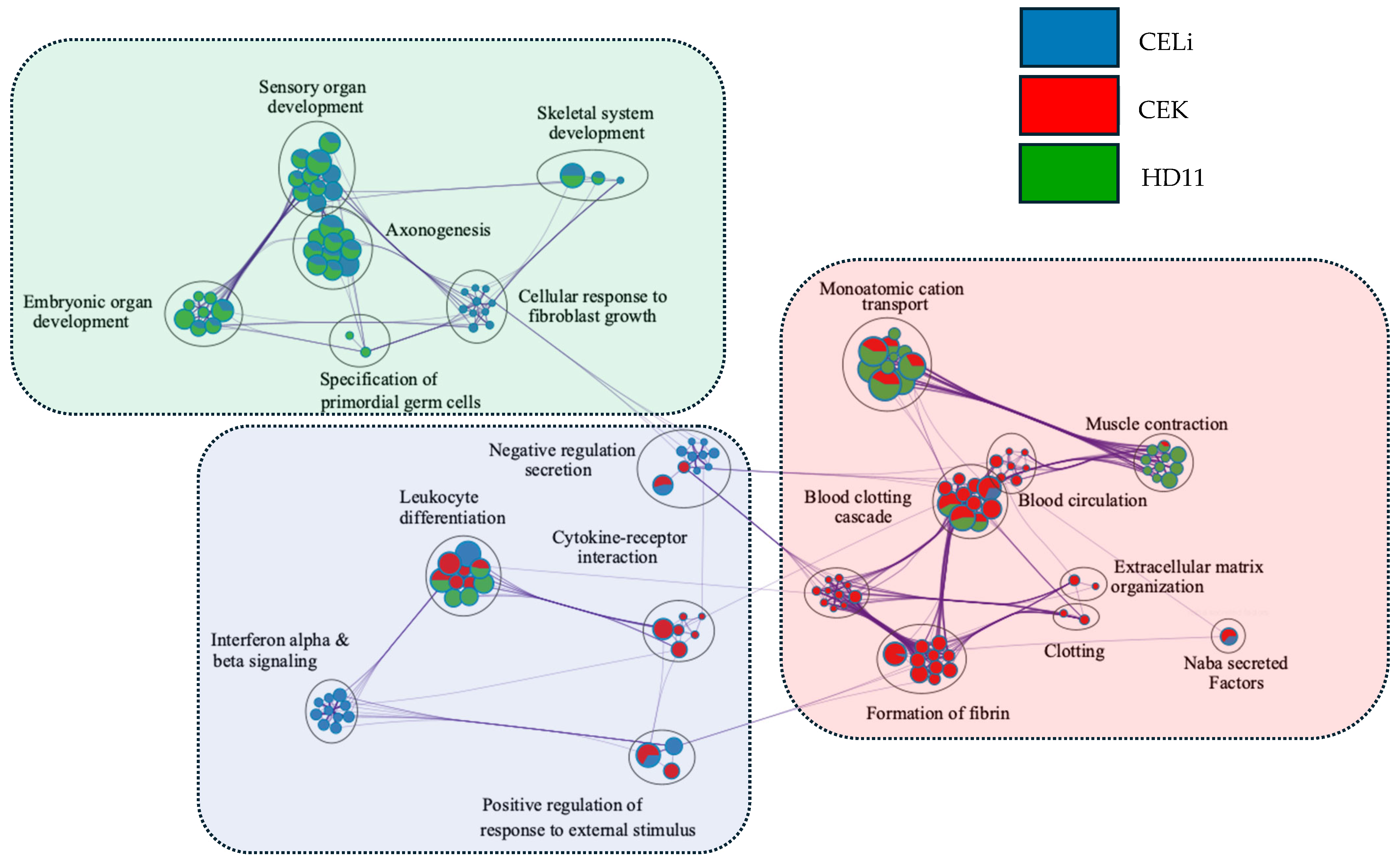
3.5. Network Analysis of Pathways
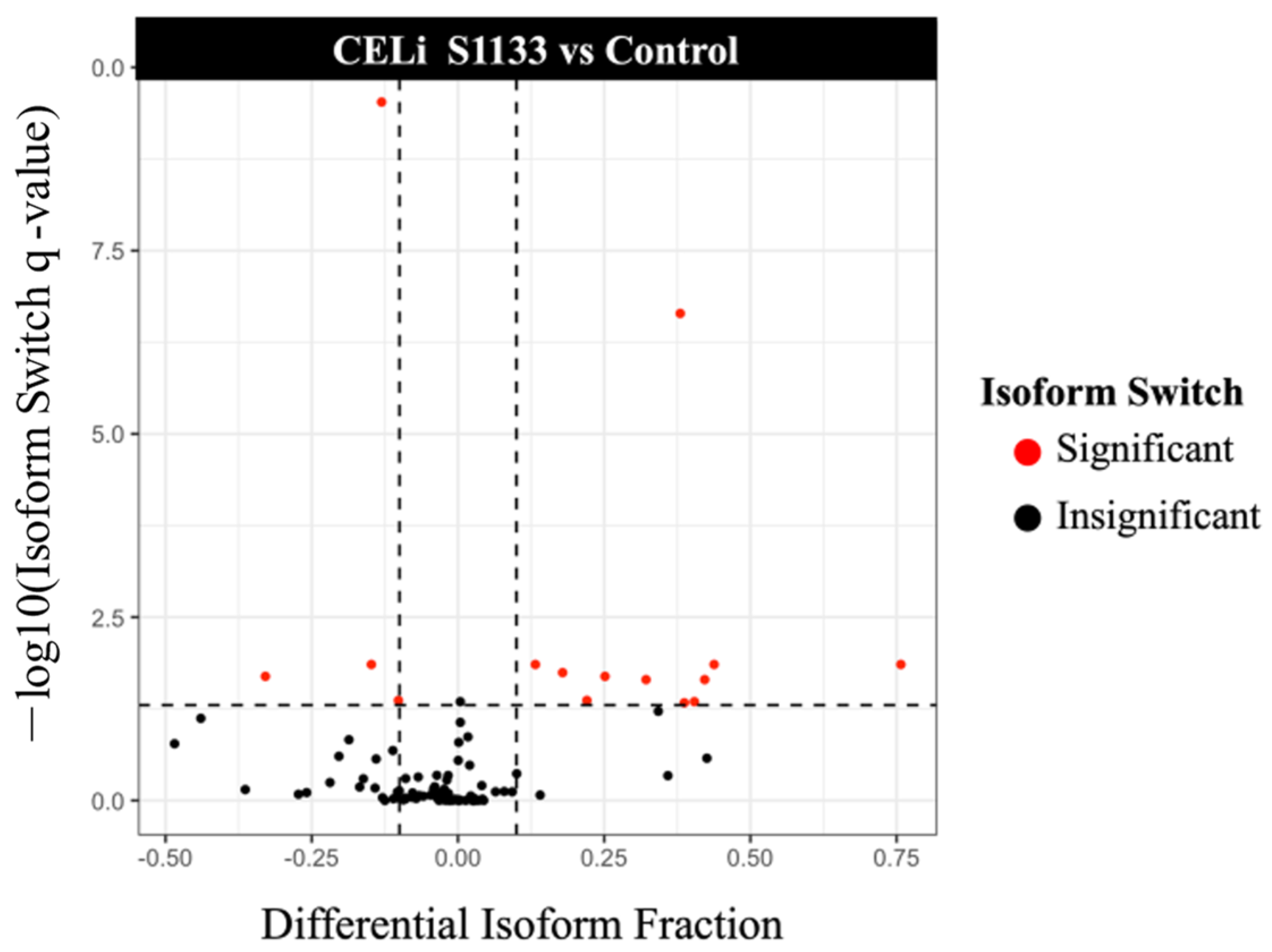
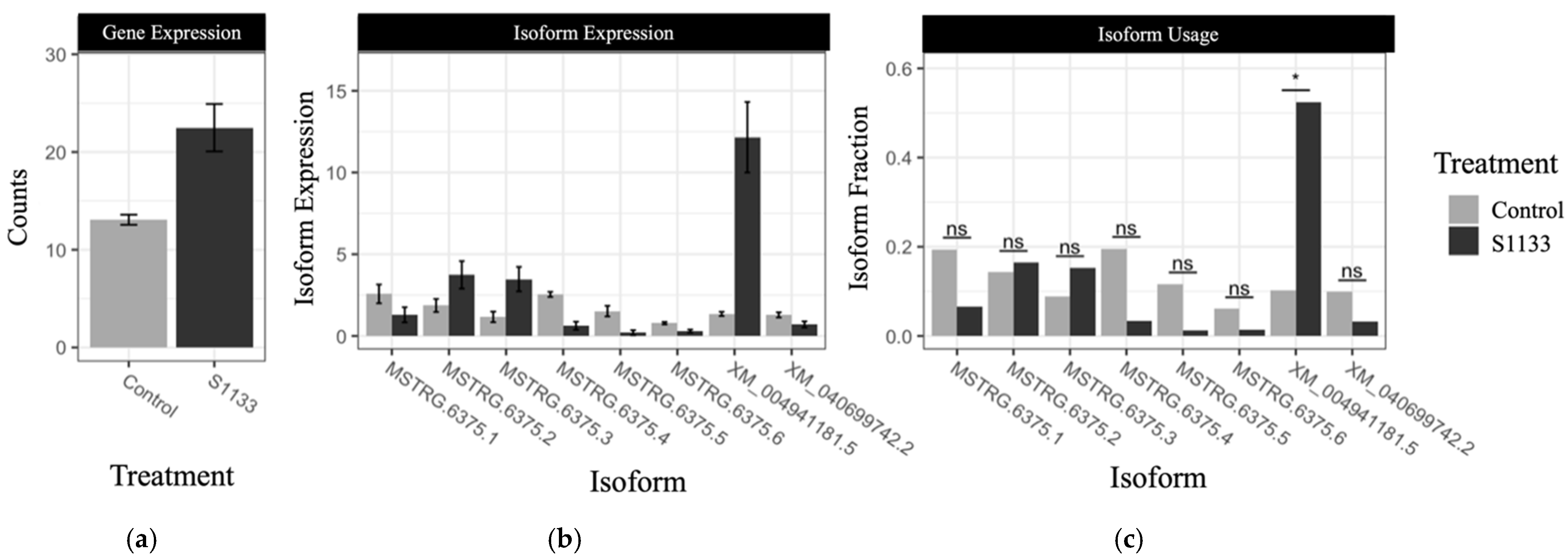
3.6. Isoform Switching Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovács, E.; Varga-Kugler, R.; Mató, T.; Homonnay, Z.; Tatár-Kis, T.; Farkas, S.; Kiss, I.; Bányai, K.; Palya, V. Identification of the main genetic clusters of avian reoviruses from a global strain collection. Front. Vet. Sci. 2023, 9, 1094761. [Google Scholar] [CrossRef]
- French, D. Incidence and economic impact of reovirus in the poultry industries in the United States. Avian Dis. 2022, 66, 432–434. [Google Scholar] [CrossRef]
- Olson, N.O.; Solomon, D.P. A natural outbreak of synovitis caused by the viral arthritis agent. Avian Dis. 1968, 12, 311–316. [Google Scholar] [CrossRef]
- Rosenberger, J.K.; Sterner, F.J.; Botts, S.; Lee, K.P.; Margolin, A. In vitro and in vivo characterization of avian reoviruses. I. Pathogenicity and antigenic relatedness of several avian reovirus isolates. Avian Dis. 1989, 33, 535. [Google Scholar] [CrossRef]
- Kibenge, F.S.B.; Wilcox, G.E. Tenosynovitis in chickens. Avian Dis. 1983, 53, 431–444. [Google Scholar]
- Mandelli, G.; Rampin, T.; Finazzi, M. Experimental reovirus hepatitis in newborn chicks. Vet. Pathol. 1978, 15, 531–543. [Google Scholar] [CrossRef]
- Davis, J.F.; Kulkarni, A.; Fletcher, O. Myocarditis in 9- and 11-day-old broiler breeder chicks associated with a reovirus infection. Avian Dis. 2012, 56, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C. Reoviruses from chickens with hydropericardium. Vet. Rec. 1976, 99, 458. [Google Scholar] [CrossRef] [PubMed]
- Neelima, S.; Ram, G.; Kataria, J.; Goswami, T. Avian reovirus induces an inhibitory effect on lymphoproliferation in chickens. Vet. Res. Commun. 2003, 27, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Z.; Sun, A.; Sun, S.-H. Influence of avian reovirus infection on the bursa and immune-reactions in chickens. Wei Sheng Wu Xue Bao [Acta Microbiol. Sin]. 2007, 47, 492–497. [Google Scholar]
- Meng, S.; Jiang, K.; Zhang, X.; Zhang, M.; Zhou, Z.; Hu, M.; Yang, R.; Sun, C.; Wu, Y. Avian reovirus triggers autophagy in primary chicken fibroblast cells and Vero cells to promote virus production. Arch. Virol. 2012, 157, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Labrada, L.; Bodelón, G.; Viñuela, J.; Benavente, J. Avian reoviruses cause apoptosis in cultured cells: Viral uncoating, but not viral gene expression, is required for apoptosis induction. J. Virol. 2002, 76, 7932–7941. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wang, Y.; Li, M.; Zhang, X.; Wu, Y. Transcriptome analysis of avian reovirus-mediated changes in gene expression of normal chicken fibroblast DF-1 cells. BMC Genom. 2017, 18, 911. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, R.; Luo, D.; Li, K.; Qi, X.; Liu, C.; Zhang, Y.; Cui, H.; Wang, S.; Gao, Y.; et al. Avian reovirus σA protein inhibits type I interferon production by abrogating interferon regulatory factor 7 activation. J. Virol. 2022, 97, e01785-22. [Google Scholar] [CrossRef]
- Lostalé-Seijo, I.; Martínez-Costas, J.; Benavente, J. Interferon induction by avian reovirus. Virology 2016, 487, 104–111. [Google Scholar] [CrossRef]
- Wang, S.; Xie, L.; Xie, Z.; Wan, L.; Huang, J.; Deng, X.; Xie, Z.Q.; Luo, S.; Zeng, T.; Zhang, Y.; et al. Dynamic changes in the expression of interferon-stimulated genes in joints of SPF chickens infected with avian reovirus. Front. Vet. Sci. 2021, 8, 618124. [Google Scholar] [CrossRef]
- Wang, S.; Huang, T.; Xie, Z.; Wan, L.; Ren, H.; Wu, T.; Xie, L.; Luo, S.; Li, M.; Xie, Z.; et al. Transcriptomic and translatomic analyses reveal insights into the signaling pathways of the innate immune response in the spleens of SPF chickens infected with avian reovirus. Viruses 2023, 15, 2346. [Google Scholar] [CrossRef]
- Ellis, M.N.; Eidson, C.S.; Fletcher, O.J.; Kleven, S.H. Viral tissue tropisms and interferon production in White Leghorn chickens infected with two avian reovirus strains. Avian Dis. 1983, 27, 644–651. [Google Scholar] [CrossRef]
- Ellis, M.N.; Eidson, C.S.; Brown, J.; Kleven, S.H. Studies on interferon induction and interferon sensitivity of avian reoviruses. Avian Dis. 1983, 27, 927–936. [Google Scholar] [CrossRef]
- Khalid, Z.; Alvarez-Narvaez, S.; Harrell, T.L.; Chowdhury, E.U.; Conrad, S.J.; Hauck, R. Retention of viral heterogeneity in an avian reovirus isolate despite plaque purification. Preprints 2025. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, H.G. Whole genome alignment based one-step real-time RT-PCR for universal detection of avian orthoreoviruses of chicken, pheasant and turkey origins. Infect. Genet. Evol. 2016, 39, 120–126. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general-purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of intersecting sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2024. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Zhou, Y.Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kucera, M.; Isserlin, R.; Arkhangorodsky, A.; Bader, G.D. AutoAnnotate: A Cytoscape app for summarizing networks with semantic annotations. F1000Res. 2016, 5, 1717. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Frazee, A.C.; Pertea, G.; Jaffe, A.E.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef]
- Vitting-Seerup, K.; Sandelin, A. IsoformSwitchAnalyzeR: Analysis of changes in genome-wide patterns of alternative splicing and its functional consequences. Bioinformatics 2019, 35, 4469–4471. [Google Scholar] [CrossRef]
- Buckley, I.K.; Walton, J.R. A simple method for culturing chick embryo liver and kidney parenchymal cells for microscopic studies. Tissue Cell 1974, 6, 641–652. [Google Scholar] [CrossRef]
- Wang, H.M.; Liu, J.Y.; Zhu, P.H.; Shi, L.; Liu, Y.L.; Yang, X.J.; Yang, X. Single-nucleus transcriptome reveals cell dynamic response of liver during the late chick embryonic development. Poult. Sci. 2024, 103, 103979. [Google Scholar] [CrossRef]
- Chen, Y.S.; Shen, P.C.; Su, B.S.; Liu, T.C.; Lin, C.C.; Lee, L.H. Avian reovirus replication in mononuclear phagocytes in chicken footpad and spleen after footpad inoculation. Can. J. Vet. Res. 2015, 79, 87–94. [Google Scholar]
- Mills, J.N.; Wilcox, G.E. Replication of four antigenic types of avian reovirus in subpopulations of chicken leukocytes. Avian Pathol. 1993, 22, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Pertile, T.L.; Karaca, K.; Walser, M.M.; Sharma, J.M. Suppressor macrophages mediate depressed lymphoproliferation in chickens infected with avian reovirus. Vet. Immunol. Immunopathol. 1996, 53, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Guneratne, J.R.M.; Jones, R.C.; Georgiou, K. Some observations on the isolation and cultivation of avian reoviruses. Avian Pathol. 1982, 11, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Lukert, P.D. Comparative sensitivities of embryonating chicken’s eggs and primary chicken embryo kidney and liver cell cultures to infectious bronchitis virus. Avian Dis. 1965, 9, 308–316. [Google Scholar] [CrossRef]
- Haffer, K. In vitro and in vivo studies with an avian reovirus derived from a temperature-sensitive mutant clone. Avian Dis. 1984, 28, 669–676. [Google Scholar] [CrossRef]
- von Bülow, V.; Klasen, A. Effects of avian viruses on cultured chicken bone-marrow-derived macrophages. Avian Pathol. 1983, 12, 179–198. [Google Scholar] [CrossRef]
- Swanson, G.J.; Meyers, J.; Huang, D.D. Restricted growth of avirulent avian reovirus strain 2177 in macrophage-derived HD11 cells. Virus Res. 2001, 81, 103–111. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Li, S.; Li, S.-H.; Yu, S.X.; Wang, Q.H.; Zhang, K.H.; Qu, L.; Sun, Y.; Bi, Y.H.; Tang, F.C.; et al. Transcriptome profiling in swine macrophages infected with African swine fever virus at single-cell resolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2201288119. [Google Scholar] [CrossRef]
- Sun, X.Q.; Wang, Z.; Shao, C.H.; Yu, J.; Liu, H.Y.; Chen, H.J.; Li, L.; Wang, X.R.; Ren, Y.D.; Huang, X.D.; et al. Analysis of chicken macrophage functions and gene expressions following infectious bronchitis virus M41 infection. Vet. Res. 2021, 52, 14. [Google Scholar] [CrossRef]
- Khalid, Z.; Fathima, S.; Hauck, R. Tissue-specific transcriptomic responses to avian reovirus inoculation in ovo. Viruses 2025, 17, 646. [Google Scholar] [CrossRef]
- Lopes, T.S.B.; Nankemann, J.; Breedlove, C.; Pietruska, A.; Espejo, R.; Cuadrado, C.; Hauck, R. Changes in the transcriptome profile in young chickens after infection with LaSota Newcastle disease virus. Vaccines 2024, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.M. Chemotactic activities of avian lymphocytes. Dev. Comp. Immunol. 1999, 23, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.W.; Kemp, M.C. A comparative study of avian reovirus pathogenicity: Virus spread and replication and induction of lesions. Avian Dis. 1995, 39, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.; Liu, X.; Han, Z.; Shao, Y.; Kong, X.; Liu, S. Transcriptome analysis of chicken kidney tissues following coronavirus avian infectious bronchitis virus infection. BMC Genom. 2013, 14, 743. [Google Scholar] [CrossRef]
- Jayasekera, J.P.; Moseman, E.A.; Carroll, M.C. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007, 81, 3487–3494. [Google Scholar] [CrossRef]
- Aasa-Chapman, M.M.I.; Holuigue, S.; Aubin, K.; Wong, M.; Jones, N.A.; Cornforth, D.; Pellegrino, P.; Newton, P.; Williams, I.; Borrow, P.; et al. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 2005, 79, 2823–2830. [Google Scholar] [CrossRef]
- Hirsch, R.L.; Griffin, D.E.; Winkelstein, J.A. The effect of complement depletion on the course of Sindbis virus infection in mice. J. Immunol. 1978, 121, 1276–1278. [Google Scholar] [CrossRef]
- Avirutnan, P.; Malasit, P.; Seliger, B.; Bhakdi, S.; Husmann, M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 1998, 161, 6338–6346. [Google Scholar] [CrossRef]
- Ebert, D.H.; Deussing, J.; Peters, C.; Dermody, T.S. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 2002, 277, 24609–24617. [Google Scholar] [CrossRef]
- Golden, J.W.; Bahe, J.A.; Lucas, W.T.; Nibert, M.L.; Schiff, L.A. Cathepsin S supports acid-independent infection by some reoviruses. J. Biol. Chem. 2004, 279, 8547–8557. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.I.; Kermani, N.Z.; Neerincx, A.H.; Vijverberg, S.J.H.; Guo, Y.; Howarth, P.; Dahlen, S.; Djukanovic, R.; Sterk, P.J.; Kraneveld, A.D.; et al. Association of endopeptidases, involved in SARS-CoV-2 infection, with microbial aggravation in sputum of severe asthma. Allergy 2021, 76, 1917–1921. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, A.Y.W.; Kot, C.H.; Yung, P.S.H.; Chen, S.-C.; Lui, P.P.Y. Upregulation of FABP4 induced inflammation in the pathogenesis of chronic tendinopathy. J. Orthop. Transl. 2024, 47, 105–115. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Chiu, K.Y.; Wen, C.; Xu, A.; Yan, C.H. FABP4 as a biomarker for knee osteoarthritis. Biomarkers Med. 2018, 12, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Baazim, H.; Koyuncu, E.; Tuncman, G.; Burak, M.F.; Merkel, L.; Bahour, N.; Karabulut, E.S.; Lee, G.Y.; Hanifehnezhad, A.; Karagoz, Z.F.; et al. FABP4 as a therapeutic host target controlling SARS-CoV-2 infection. EMBO Mol. Med. 2025, 17, 414–440. [Google Scholar] [CrossRef] [PubMed]
- Boudreault, S.; Martenon-Brodeur, C.; Caron, M.; Garant, J.-M.; Tremblay, M.-P.; Armero, V.E.S.; Durand, M.; Lapointe, E.; Thibault, P.; Tremblay-Létourneau, M.; et al. Global profiling of the cellular alternative RNA splicing landscape during virus-host interactions. PLoS ONE 2016, 11, e0161914. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.F.; Meistermann, H.; Tzouros, M.; Baker, A.; Golling, S.; Polster, J.S.; Ledwith, M.P.; Gitter, A.; Augustin, A.; Javanbakht, H.; et al. Alternative splicing liberates a cryptic cytoplasmic isoform of mitochondrial MECR that antagonizes influenza virus. PLoS Biol. 2022, 20, e3001934. [Google Scholar] [CrossRef]
- Li, M.; Qi, W.; Chang, Q.; Chen, R.; Zhen, D.; Liao, M.; Wen, J.; Deng, Y. Influenza A virus protein PA-X suppresses host ANKRD17-mediated immune responses. Microbiol. Immunol. 2021, 65, 48–59. [Google Scholar] [CrossRef]
- Menning, M.; Kufer, T.A. A role for the ankyrin repeat containing protein ANKRD17 in NOD1- and NOD2-mediated inflammatory responses. FEBS Lett. 2013, 587, 2137–2142. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Huang, J.W.; Huang, W.R.; Chen, I.C.; Chen, M.S.; Liao, T.-L.; Chang, Y.-K.; Munir, M.; Liu, H.-J. Oncolytic avian reovirus σA-modulated upregulation of the HIF-1α/c-Myc/GLUT1 pathway to produce more energy in different cancer cell lines benefiting virus replication. Viruses 2023, 15, 523. [Google Scholar] [CrossRef]
- Robles-Planells, C.; Barrera-Avalos, C.; Rojo, L.E.; Spencer, E.; Cortez-San Martin, M.; Matiacevich, S.; Pavez, J.; Milla, L.A.; Navarro, F.D.; Martínez, B.A.; et al. Chitosan-based delivery of avian reovirus fusogenic protein p10 gene: In vitro and in vivo studies towards a new vaccine against melanoma. Biomed. Res. Int. 2020, 2020, 4045760. [Google Scholar] [CrossRef]
- Chiu, H.C.; Huang, W.R.; Liao, T.L.; Chi, P.I.; Nielsen, B.L.; Liu, J.H.; Liu, H.J. Mechanistic insights into avian reovirus p17-modulated suppression of cell cycle cdk–cyclin complexes and enhancement of p53 and cyclin h interaction. J. Biol. Chem. 2018, 293, 12542–12562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, Z.; Hauck, R. Molecular Responses to Avian Reovirus Inoculation In Vitro. Viruses 2025, 17, 1489. https://doi.org/10.3390/v17111489
Khalid Z, Hauck R. Molecular Responses to Avian Reovirus Inoculation In Vitro. Viruses. 2025; 17(11):1489. https://doi.org/10.3390/v17111489
Chicago/Turabian StyleKhalid, Zubair, and Ruediger Hauck. 2025. "Molecular Responses to Avian Reovirus Inoculation In Vitro" Viruses 17, no. 11: 1489. https://doi.org/10.3390/v17111489
APA StyleKhalid, Z., & Hauck, R. (2025). Molecular Responses to Avian Reovirus Inoculation In Vitro. Viruses, 17(11), 1489. https://doi.org/10.3390/v17111489






