Simultaneous ASFV and Haptoglobin Detection by Duplex qPCR Enables Pre-Viremia Diagnosis of African Swine Fever
Abstract
1. Introduction
2. Materials and Methods
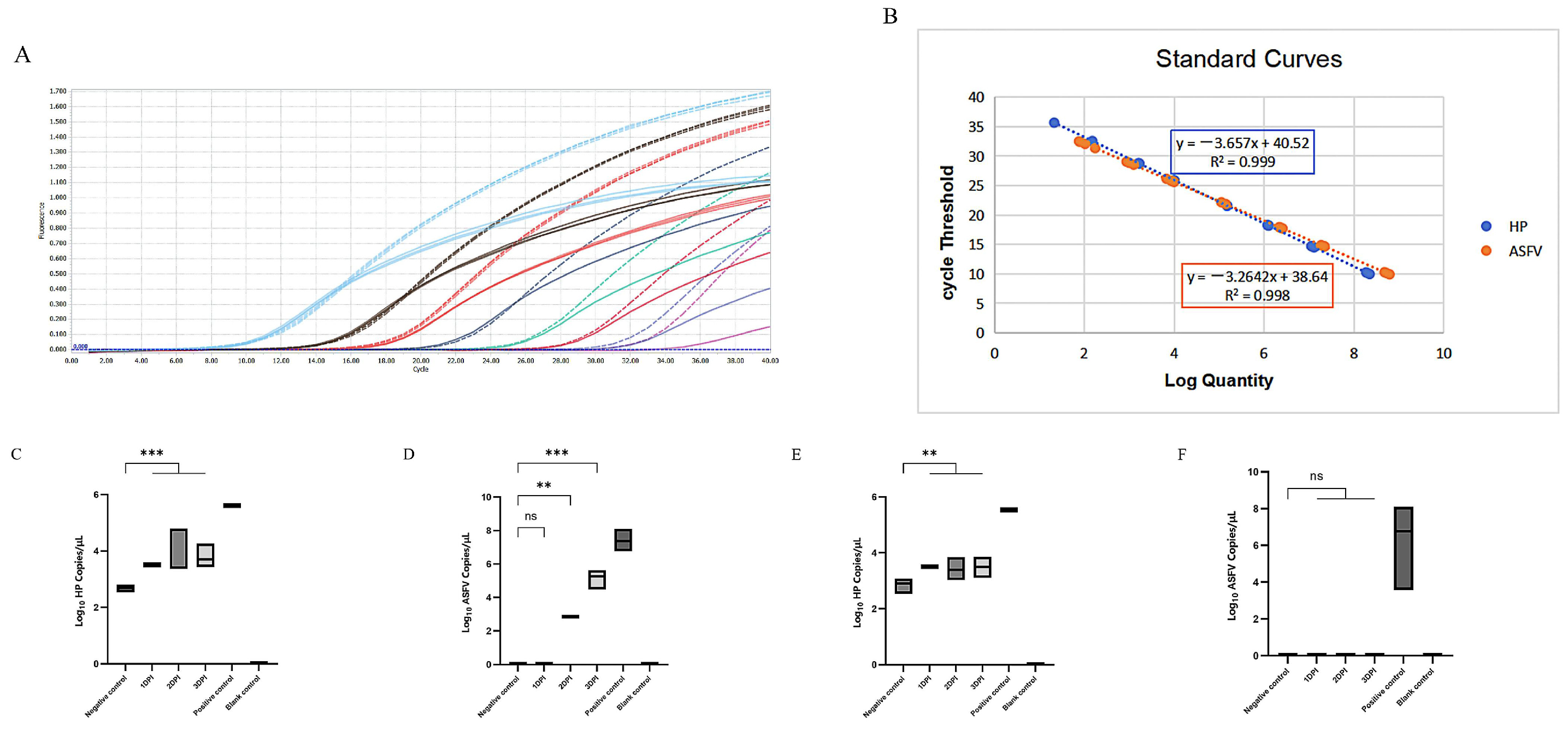
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, K.; Qian, X.; Shi, Y.; Wei, H.; Pan, Y.; Long, F.; Zhou, Q.; Mo, S.; Hu, L.; Li, Z. A triplex crystal digital PCR for the detection of genotypes I and II African swine fever virus. Front. Vet. Sci. 2024, 11, 1351596. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Li, M.; Zheng, H. Insights and progress on epidemic characteristics, pathogenesis, and preventive measures of African swine fever virus: A review. Virulence 2025, 16, 2457949. [Google Scholar] [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.L.; Ren, T.; Huang, L.Y.; Weldu, T.; Zhu, Y.M.; Li, F.; Sun, E.C.; Bu, Z.G.; Zhao, D.M. Developing a duplex ARMS-qPCR method to differentiate genotype I and II African swine fever viruses based on their B646L genes. J. Integr. Agric. 2023, 22, 1603–1607. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.J.; Tesfagaber, W.; Zhang, J.W.; Li, F.; Sun, E.C.; Tang, L.J.; Bu, Z.G.; Zhu, Y.M.; Zhao, D.M. Establishment of an indirect immunofluorescence assay for the detection of African swine fever virus antibodies. J. Integr. Agric. 2024, 23, 228–238. [Google Scholar] [CrossRef]
- Auer, A.; Cattoli, G.; Padungtod, P.; Lamien, C.E.; Oh, Y.; Jayme, S.; Rozstalnyy, A. Challenges in the Application of African Swine Fever Vaccines in Asia. Animals 2024, 14, 2473. [Google Scholar] [CrossRef] [PubMed]
- Zeller, L.; Tyrrell, P.N.; Wang, S.; Fischer, N.; Haas, J.P.; Hügle, B. α2-fraction and haptoglobin as biomarkers for disease activity in oligo- and polyarticular juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2022, 20, 66. [Google Scholar] [CrossRef]
- Kohansal-Nodehi, M.; Swiatek-de Lange, M.; Tabarés, G.; Busskamp, H. Haptoglobin polymorphism affects its N-glycosylation pattern in serum. J. Mass. Spectrom. Adv. Clin. Lab. 2022, 25, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, L.; Carvajal, A.; Puente, H.; Peres Rubio, C.; Cerón, J.J.; Rubio, P.; Argüello, H. New insights into swine dysentery: Faecal shedding, macro and microscopic lesions and biomarkers in early and acute stages of Brachyspira hyodysenteriae infection. Porc. Health Manag. 2024, 10, 24. [Google Scholar] [CrossRef]
- Grantz, J.M.; Thirumalaikumar, V.P.; Jannasch, A.H.; Andolino, C.; Taechachokevivat, N.; Avila-Granados, L.M.; Neves, R.C. The platelet and plasma proteome and targeted lipidome in postpartum dairy cows with elevated systemic inflammation. Sci. Rep. 2024, 14, 31240. [Google Scholar] [CrossRef]
- Parra, M.D.; Fuentes, P.; Tecles, F.; Martínez-Subiela, S.; Martínez, J.S.; Muñoz, A.; Cerón, J.J. Porcine acute phase protein concentrations in different diseases in field conditions. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 488–493. [Google Scholar] [CrossRef]
- Miao, C.; Yang, S.; Shao, J.; Zhou, G.; Ma, Y.; Wen, S.; Hou, Z.; Peng, D.; Guo, H.; Liu, W.; et al. Identification of p72 epitopes of African swine fever virus and preliminary application. Front. Microbiol. 2023, 14, 1126794. [Google Scholar] [CrossRef]
- Duan, X.; Ru, Y.; Yang, W.; Ren, J.; Hao, R.; Qin, X.; Li, D.; Zheng, H. Research progress on the proteins involved in African swine fever virus infection and replication. Front. Immunol. 2022, 13, 947180. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, H.; Zhang, Y.; Luan, J.; Wang, H. Progress in African Swine Fever Vector Vaccine Development. Int. J. Mol. Sci. 2025, 26, 921. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Rai, A.; Espinoza, N.; Velazquez-Salinas, L.; Gladue, D.P. ASF Vaccine Candidate ASFV-G-∆I177L Does Not Exhibit Residual Virulence in Long-Term Clinical Studies. Pathogens 2023, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Franco-Martínez, L.; Beer, M.; Martínez-Subiela, S.; García-Manzanilla, E.; Blome, S.; Carrau, T. Impact of ASFV Detergent Inactivation on Biomarkers in Serum and Saliva Samples. Pathogens 2022, 11, 750. [Google Scholar] [CrossRef]
- Silva, C.A.; Callegari, M.A.; Dias, C.P.; de Souza, K.L.; Romano, G.S.; Hernig, L.F.; Lippke, R.T.; Jansen, R.; Leite, F.L.; Filipe, F.; et al. Well-Being and Performance of Nursery Pigs Subjected to Different Commercial Vaccines Against Porcine Circovirus Type 2, Mycoplasma hyopneumoniae and Lawsonia intracellularis. Vaccines 2024, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, N.S.; Tegtmeier, C.; Andresen, L.O.; Piñeiro, M.; Toussaint, M.J.; Campbell, F.M.; Lampreave, F.; Heegaard, P.M. The porcine acute phase protein response to acute clinical and subclinical experimental infection with Streptococcus suis. Vet. Immunol. Immunopathol. 2006, 113, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Tor, M.; Fraile, L.; Vilaro, F.; Pena, R.N. Multiplex Assay to Determine Acute Phase Proteins in Modified Live PRRSV Vaccinated Pigs. J. Proteome Res. 2024, 23, 3515–3523. [Google Scholar] [CrossRef] [PubMed]
| Virulence Strain | Recombinant Strain | Artificial Infection | Cohabitation Natural Infection | |||||
|---|---|---|---|---|---|---|---|---|
| 1DPI | 2DPI | 3DPI | 1DPI | 2DPI | 3DPI | |||
| Hp | 6/6 | 14/14 | 6/6 | 6/6 | 6/6 | 6/6 | 4/4 | 16/20 |
| ASFV | 0/6 | 1/14 | 3/6 | 0/6 | 0/6 | 0/6 | 4/4 | 0/20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Gao, S.; Li, S.; Liu, Y.; Gao, F.; Li, L.; Tong, W.; Liu, C.; Zhou, Y.; Jiang, Y. Simultaneous ASFV and Haptoglobin Detection by Duplex qPCR Enables Pre-Viremia Diagnosis of African Swine Fever. Viruses 2025, 17, 1444. https://doi.org/10.3390/v17111444
Bao Y, Gao S, Li S, Liu Y, Gao F, Li L, Tong W, Liu C, Zhou Y, Jiang Y. Simultaneous ASFV and Haptoglobin Detection by Duplex qPCR Enables Pre-Viremia Diagnosis of African Swine Fever. Viruses. 2025; 17(11):1444. https://doi.org/10.3390/v17111444
Chicago/Turabian StyleBao, Yun, Shimin Gao, Shuang Li, Yijie Liu, Fei Gao, Liwei Li, Wu Tong, Changlong Liu, Yanjun Zhou, and Yifeng Jiang. 2025. "Simultaneous ASFV and Haptoglobin Detection by Duplex qPCR Enables Pre-Viremia Diagnosis of African Swine Fever" Viruses 17, no. 11: 1444. https://doi.org/10.3390/v17111444
APA StyleBao, Y., Gao, S., Li, S., Liu, Y., Gao, F., Li, L., Tong, W., Liu, C., Zhou, Y., & Jiang, Y. (2025). Simultaneous ASFV and Haptoglobin Detection by Duplex qPCR Enables Pre-Viremia Diagnosis of African Swine Fever. Viruses, 17(11), 1444. https://doi.org/10.3390/v17111444






