Association Between FOXP3 and OX40 Expression in Adult T-Cell Leukemia Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Cell Culture
2.3. Flow Cytometric Analysis
2.4. Determination of FOXP3+ Group
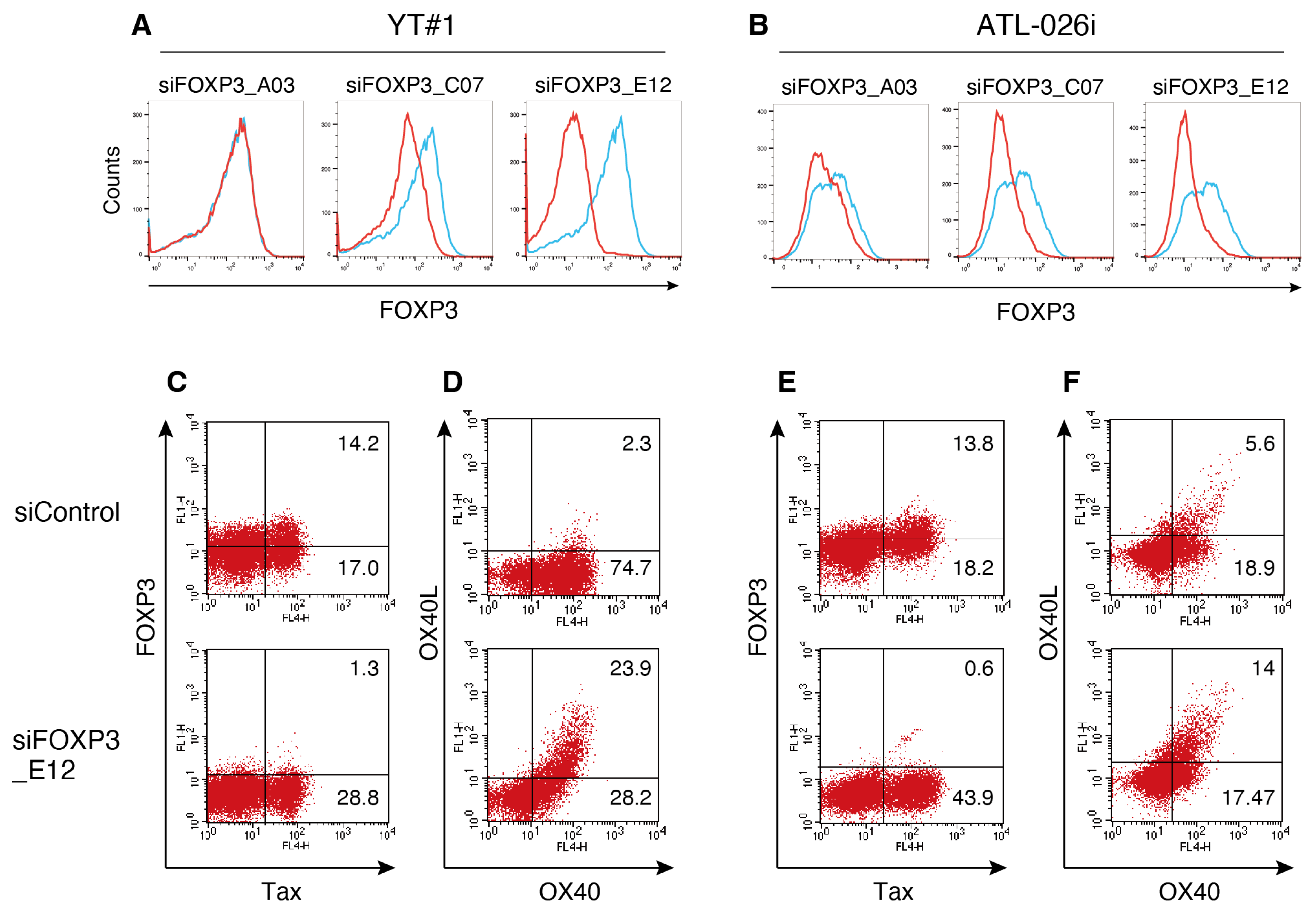
2.5. Transfection and Small Interfering RNA Treatment
2.6. Statistical Analysis
3. Results
3.1. Patients with ATL and an Increased Number of FOXP3+ Cells Exhibit Higher OX40 Expression
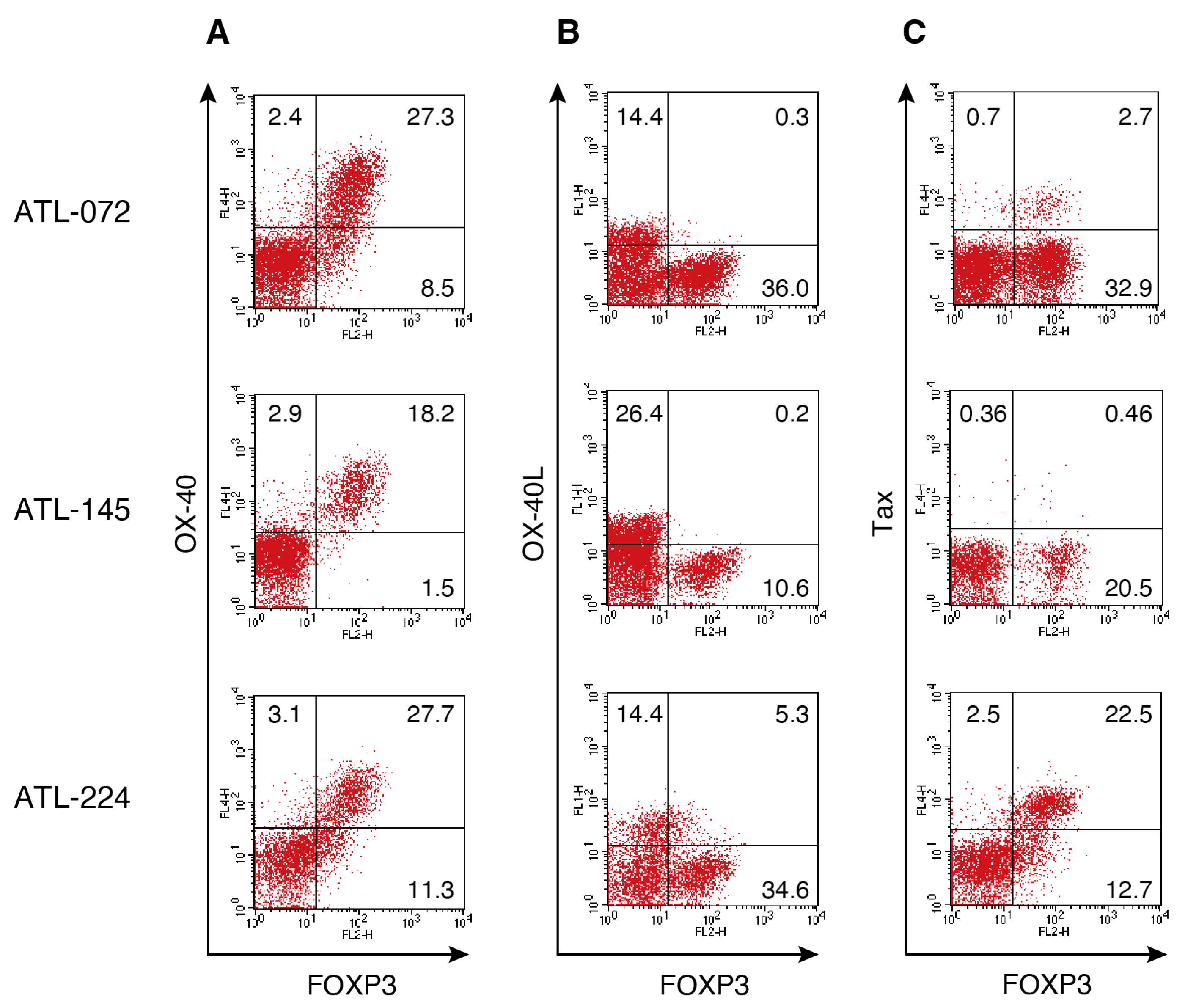
3.2. FOXP3 Expression Associates with OX40 but Not OX40L in PBMCs Cultured for 1 Day from Patients with ATL
3.3. FOXP3 Suppresses the Expression of OX40L in HTLV-1-Infected Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef]
- Hinuma, Y.; Nagata, K.; Hanaoka, M.; Nakai, M.; Matsumoto, T.; Kinoshita, K.I.; Shirakawa, S.; Miyoshi, I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 1981, 78, 6476–6480. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Iwanaga, M.; Watanabe, T.; Yamaguchi, K. Adult T-cell leukemia: A review of epidemiological evidence. Front. Microbiol. 2012, 3, 322. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Yamagishi, M.; Kubokawa, M.; Kuze, Y.; Suzuki, A.; Yokomizo, A.; Kobayashi, S.; Nakashima, M.; Makiyama, J.; Iwanaga, M.; Fukuda, T.; et al. Chronological genome and single-cell transcriptome integration characterizes the evolutionary process of adult T cell leukemia-lymphoma. Nat. Commun. 2021, 12, 4821. [Google Scholar] [CrossRef]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Schallenberg, S.; Tsai, P.Y.; Riewaldt, J.; Kretschmer, K. Identification of an immediate Foxp3(-) precursor to Foxp3(+) regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J. Exp. Med. 2010, 207, 1393–1407. [Google Scholar] [CrossRef]
- Shevach, E.M.; Thornton, A.M. tTregs, pTregs, and iTregs: Similarities and differences. Immunol. Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef]
- Abbas, A.K.; Benoist, C.; Bluestone, J.A.; Campbell, D.J.; Ghosh, S.; Hori, S.; Jiang, S.; Kuchroo, V.K.; Mathis, D.; Roncarolo, M.G.; et al. Regulatory T cells: Recommendations to simplify the nomenclature. Nat. Immunol. 2013, 14, 307–308. [Google Scholar] [CrossRef]
- Satou, Y.; Utsunomiya, A.; Tanabe, J.; Nakagawa, M.; Nosaka, K.; Matsuoka, M. HTLV-1 modulates the frequency and phenotype of FoxP3+CD4+ T cells in virus-infected individuals. Retrovirology 2012, 9, 46. [Google Scholar] [CrossRef]
- Satou, Y.; Yasunaga, J.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef]
- Shimazu, Y.; Shimazu, Y.; Hishizawa, M.; Hamaguchi, M.; Nagai, Y.; Sugino, N.; Fujii, S.; Kawahara, M.; Kadowaki, N.; Nishikawa, H.; et al. Hypomethylation of the Treg-Specific Demethylated Region in FOXP3 Is a Hallmark of the Regulatory T-cell Subtype in Adult T-cell Leukemia. Cancer Immunol. Res. 2016, 4, 136–145. [Google Scholar] [CrossRef]
- Imura, A.; Hori, T.; Imada, K.; Kawamata, S.; Tanaka, Y.; Imamura, S.; Uchiyama, T. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: Implication for the possible involvement of OX40 in leukemic cell infiltration. Blood 1997, 89, 2951–2958. [Google Scholar] [CrossRef]
- Kato, M.; Imaizumi, N.; Tanaka, R.; Mizuguchi, M.; Hayashi, M.; Miyagi, T.; Uchihara, J.; Ohshiro, K.; Todoroki, J.; Karube, K.; et al. Elevation of the Plasma Levels of TNF Receptor 2 in Association with Those of CD25, OX40, and IL-10 and HTLV-1 Proviral Load in Acute Adult T-Cell Leukemia. Viruses 2022, 14, 751. [Google Scholar] [CrossRef]
- Ohtsuka, E.; Kikuchi, H.; Nasu, M.; Takita-Sonoda, Y.; Fujii, H.; Yokoyama, S. Clinicopathological features of adult T-cell leukemia with CD30 antigen expression. Leuk. Lymphoma 1994, 15, 303–310. [Google Scholar] [CrossRef]
- Higuchi, M.; Matsuda, T.; Mori, N.; Yamada, Y.; Horie, R.; Watanabe, T.; Takahashi, M.; Oie, M.; Fujii, M. Elevated expression of CD30 in adult T-cell leukemia cell lines: Possible role in constitutive NF-kappaB activation. Retrovirology 2005, 2, 29. [Google Scholar] [CrossRef]
- Uchiyama, T.; Hori, T.; Tsudo, M.; Wano, Y.; Umadome, H.; Tamori, S.; Yodoi, J.; Maeda, M.; Sawami, H.; Uchino, H. Interleukin-2 receptor (Tac antigen) expressed on adult T cell leukemia cells. J. Clin. Investig. 1985, 76, 446–453. [Google Scholar] [CrossRef]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef]
- Godfrey, W.R.; Fagnoni, F.F.; Harara, M.A.; Buck, D.; Engleman, E.G. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J. Exp. Med. 1994, 180, 757–762. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takahashi, Y.; Tanaka, R.; Miyagi, T.; Saito, M.; Fukushima, T. Association of high levels of plasma OX40 with acute adult T-cell leukemia. Int. J. Hematol. 2019, 109, 319–327. [Google Scholar] [CrossRef]
- Pankow, R.; Durkop, H.; Latza, U.; Krause, H.; Kunzendorf, U.; Pohl, T.; Bulfone-Paus, S. The HTLV-I tax protein transcriptionally modulates OX40 antigen expression. J. Immunol. 2000, 165, 263–270. [Google Scholar] [CrossRef]
- Tanaka, Y.; Inoi, T.; Tozawa, H.; Yamamoto, N.; Hinuma, Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I). Int. J. Cancer 1985, 36, 549–555. [Google Scholar] [CrossRef]
- Miura, S.; Ohtani, K.; Numata, N.; Niki, M.; Ohbo, K.; Ina, Y.; Gojobori, T.; Tanaka, Y.; Tozawa, H.; Nakamura, M.; et al. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol. Cell Biol. 1991, 11, 1313–1325. [Google Scholar]
- Ohtani, K.; Tsujimoto, A.; Tsukahara, T.; Numata, N.; Miura, S.; Sugamura, K.; Nakamura, M. Molecular mechanisms of promoter regulation of the gp34 gene that is trans-activated by an oncoprotein Tax of human T cell leukemia virus type I. J. Biol. Chem. 1998, 273, 14119–14129. [Google Scholar] [CrossRef]
- Kumar, P.; Alharshawi, K.; Bhattacharya, P.; Marinelarena, A.; Haddad, C.; Sun, Z.; Chiba, S.; Epstein, A.L.; Prabhakar, B.S. Soluble OX40L and JAG1 Induce Selective Proliferation of Functional Regulatory T-Cells Independent of canonical TCR signaling. Sci. Rep. 2017, 7, 39751. [Google Scholar] [CrossRef]
- Lee, B.; Tanaka, Y.; Tozawa, H. Monoclonal antibody defining tax protein of human T-cell leukemia virus type-I. Tohoku J. Exp. Med. 1989, 157, 1–11. [Google Scholar] [CrossRef]
- Tanaka, Y.; Zeng, L.; Shiraki, H.; Shida, H.; Tozawa, H. Identification of a neutralization epitope on the envelope gp46 antigen of human T cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J. Immunol. 1991, 147, 354–360. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tanaka, Y.; Yamashita, A.; Koyanagi, Y.; Nakamura, M.; Yamamoto, N. OX40 stimulation by gp34/OX40 ligand enhances productive human immunodeficiency virus type 1 infection. J. Virol. 2001, 75, 6748–6757. [Google Scholar] [CrossRef]
- Tanaka, Y.; Inoi, T.; Tozawa, H.; Sugamura, K.; Hinuma, Y. New monoclonal antibodies that define multiple epitopes and a human-specific marker on the interleukin 2 receptor molecules of primates. Microbiol. Immunol. 1986, 30, 373–388. [Google Scholar] [CrossRef]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Takahashi, Y.; Tanaka, R.; Fukushima, T.; Tanaka, Y. Conservation of a Neutralization Epitope of Human T-cell Leukemia Virus Type 1 (HTLV-1) among Currently Endemic Clinical Isolates in Okinawa, Japan. Pathogens 2020, 9, 82. [Google Scholar] [CrossRef]
- Arandi, N.; Mirshafiey, A.; Abolhassani, H.; Jeddi-Tehrani, M.; Edalat, R.; Sadeghi, B.; Shaghaghi, M.; Aghamohammadi, A. Frequency and expression of inhibitory markers of CD4(+) CD25(+) FOXP3(+) regulatory T cells in patients with common variable immunodeficiency. Scand. J. Immunol. 2013, 77, 405–412. [Google Scholar] [CrossRef]
- Jiang, T.J.; Cao, X.L.; Luan, S.; Cui, W.H.; Qiu, S.H.; Wang, Y.C.; Zhao, C.J.; Fu, P. Percentage and function of CD4+CD25+ regulatory T cells in patients with hyperthyroidism. Mol. Med. Rep. 2018, 17, 2137–2144. [Google Scholar] [CrossRef]
- Niu, H.Q.; Zhao, X.C.; Li, W.; Xie, J.F.; Liu, X.Q.; Luo, J.; Zhao, W.P.; Li, X.F. Characteristics and reference ranges of CD4(+)T cell subpopulations among healthy adult Han Chinese in Shanxi Province, North China. BMC Immunol. 2020, 21, 44. [Google Scholar] [CrossRef]
- Takasawa, N.; Ishii, N.; Higashimura, N.; Murata, K.; Tanaka, Y.; Nakamura, M.; Sasaki, T.; Sugamura, K. Expression of gp34 (OX40 ligand) and OX40 on human T cell clones. Jpn. J. Cancer Res. 2001, 92, 377–382. [Google Scholar] [CrossRef]
- Hinuma, Y.; Gotoh, Y.; Sugamura, K.; Nagata, K.; Goto, T.; Nakai, M.; Kamada, N.; Matsumoto, T.; Kinoshita, K. A retrovirus associated with human adult T-cell leukemia: In vitro activation. Gan 1982, 73, 341–344. [Google Scholar]
- Umadome, H.; Uchiyama, T.; Hori, T.; Tamori, S.; Motoi, T.; Araki, K.; Uchino, H. Close association between interleukin 2 receptor mRNA expression and human T cell leukemia/lymphoma virus type I viral RNA expression in short-term cultured leukemic cells from adult T cell leukemia patients. J. Clin. Investig. 1988, 81, 52–61. [Google Scholar] [CrossRef]
- Kinpara, S.; Hasegawa, A.; Utsunomiya, A.; Nishitsuji, H.; Furukawa, H.; Masuda, T.; Kannagi, M. Stromal cell-mediated suppression of human T-cell leukemia virus type 1 expression in vitro and in vivo by type I interferon. J. Virol. 2009, 83, 5101–5108. [Google Scholar] [CrossRef]
- Mendel, I.; Shevach, E.M. Activated T cells express the OX40 ligand: Requirements for induction and costimulatory function. Immunology 2006, 117, 196–204. [Google Scholar] [CrossRef]
- Soroosh, P.; Ine, S.; Sugamura, K.; Ishii, N. OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J. Immunol. 2006, 176, 5975–5987. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Takatori, M.; Sakihama, S.; Yoshita-Takahashi, M.; Imaizumi, N.; Takahashi, Y.; Hasegawa, H.; Karube, K.; Fukushima, T.; Nakamura, M.; et al. Acute type adult T-cell leukemia cells proliferate in the lymph nodes rather than in peripheral blood. Cancer Gene Ther. 2022, 29, 1570–1577. [Google Scholar] [CrossRef]
- Mahgoub, M.; Yasunaga, J.I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef]
- Ramanayake, S.; Moulding, D.A.; Tanaka, Y.; Singh, A.; Bangham, C.R.M. Dynamics and consequences of the HTLV-1 proviral plus-strand burst. PLoS Pathog. 2022, 18, e1010774. [Google Scholar] [CrossRef]
- Kulkarni, A.; Taylor, G.P.; Klose, R.J.; Schofield, C.J.; Bangham, C.R. Histone H2A monoubiquitylation and p38-MAPKs regulate immediate-early gene-like reactivation of latent retrovirus HTLV-1. JCI Insight 2018, 3, e123196. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Sasaki, Y.; Hara, T.; Higuchi, M.; Tanaka, Y.; Funato, N.; Tanaka, N.; Fujii, M.; Nakamura, M. Induction of Cell Death in Growing Human T-Cells and Cell Survival in Resting Cells in Response to the Human T-Cell Leukemia Virus Type 1 Tax. PLoS ONE 2016, 11, e0148217. [Google Scholar] [CrossRef]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.D.; Bopp, T.; Schmitt, E.; et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007, 5, e38. [Google Scholar] [CrossRef]
- Polansky, J.K.; Kretschmer, K.; Freyer, J.; Floess, S.; Garbe, A.; Baron, U.; Olek, S.; Hamann, A.; von Boehmer, H.; Huehn, J. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 2008, 38, 1654–1663. [Google Scholar] [CrossRef]
- Miyao, T.; Floess, S.; Setoguchi, R.; Luche, H.; Fehling, H.J.; Waldmann, H.; Huehn, J.; Hori, S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 2012, 36, 262–275. [Google Scholar] [CrossRef]
- Weiss, J.M.; Bilate, A.M.; Gobert, M.; Ding, Y.; Curotto de Lafaille, M.A.; Parkhurst, C.N.; Xiong, H.; Dolpady, J.; Frey, A.B.; Ruocco, M.G.; et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 2012, 209, 1723–1742. [Google Scholar] [CrossRef]
- Yang, B.H.; Hagemann, S.; Mamareli, P.; Lauer, U.; Hoffmann, U.; Beckstette, M.; Fohse, L.; Prinz, I.; Pezoldt, J.; Suerbaum, S.; et al. Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016, 9, 444–457. [Google Scholar] [CrossRef]
- Ohkura, N.; Hamaguchi, M.; Morikawa, H.; Sugimura, K.; Tanaka, A.; Ito, Y.; Osaki, M.; Tanaka, Y.; Yamashita, R.; Nakano, N.; et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 2012, 37, 785–799. [Google Scholar] [CrossRef]
- Baron, U.; Floess, S.; Wieczorek, G.; Baumann, K.; Grutzkau, A.; Dong, J.; Thiel, A.; Boeld, T.J.; Hoffmann, P.; Edinger, M.; et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur. J. Immunol. 2007, 37, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
| Group | Bank Number | Diagnosis | Sex | Age | FOXP3+/ CD4+ (%) | OX40+ CD25+/ PBMCs (%) | OX40−CD25+/ PBMCs (%) | Total CD25+/ PBMCs (%) | OX40+ /CD25+ (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FOXP3+ high | ATL-420 | Acute | M | 58 | 85.61 | 9.95 | 54.6 | 64.55 | 15.42 |
| 2 | ATL-424 | Acute | M | 87 | 82.58 | 4.9 | 76.09 | 80.99 | 6.06 | |
| 3 | ATL-400 | Acute | F | 71 | 76.19 | 59.57 | 12.43 | 72 | 82.74 | |
| 4 | ATL-428 | Chronic | M | 79 | 70.49 | 45.95 | 48.53 | 94.48 | 48.64 | |
| 5 | ATL-250 | Chronic | M | 60 | 65.38 | 17.01 | 29.96 | 46.97 | 36.22 | |
| 6 | ATL-408 | Acute | F | 79 | 49.76 | 2.17 | 77.91 | 80.08 | 2.71 | |
| 7 | ATL-414 | Acute | F | 80 | 43.09 | 2.68 | 80.86 | 83.54 | 3.21 | |
| 8 | ATL-251 | Acute | F | 64 | 39.37 | 10.78 | 25.22 | 36 | 29.95 | |
| 9 | ATL-388 | Acute | M | 61 | 22.94 | 2.64 | 52.5 | 55.14 | 4.79 | |
| 10 | FOXP+ low | ATL-421 | Acute | F | 53 | 8.11 | 2.33 | 24.23 | 26.56 | 8.78 |
| 11 | ATL-412 | Acute | M | 39 | 1.05 | 5.22 | 41.9 | 47.12 | 11.08 | |
| 12 | ATL-401 | Acute | M | 68 | 0.83 | 13.58 | 67.53 | 81.11 | 16.75 | |
| 13 | ATL-402 | Acute | M | 70 | 0.72 | 5.58 | 73.28 | 78.86 | 7.08 | |
| 14 | ATL-405 | Acute | M | 66 | 0 | 0.9 | 48.8 | 49.7 | 1.82 | |
| 15 | Carrier | ATL-427 | Carrier | F | 90 | 12.2 | 3.08 | 9.76 | 12.84 | |
| 16 | ATL-399 | Carrier | F | 63 | 11.82 | 2.48 | 5.88 | 8.36 | ||
| 17 | ATL-410 | Carrier | M | 79 | 9.81 | 1.24 | 14.75 | 15.99 | ||
| 18 | ATL-418 | Carrier | M | 67 | 8.41 | 3.99 | 15.05 | 19.04 | ||
| 19 | ATL-422 | Carrier | F | 67 | 8.28 | 3.07 | 12.52 | 15.59 | ||
| 20 | ATL-423 | Carrier | F | 58 | 5.2 | 1.84 | 8.89 | 10.73 | ||
| 21 | ATL-426 | Carrier | M | 54 | 4.08 | 2.54 | 20.92 | 23.46 | ||
| 22 | ATL-419 | Carrier | F | 79 | 3.95 | 2.13 | 10.66 | 12.79 | ||
| 23 | ATL-413 | Carrier | M | 44 | 3.04 | 2.78 | 39.07 | 41.85 | ||
| 24 | ATL-417 | Carrier | F | 87 | 1.59 | 2.8 | 20.29 | 23.09 |
| Bank Number | Diagnosis | Sex | Age | |
|---|---|---|---|---|
| 1 | ATL-072 | Acute | F | 75 |
| 2 | ATL-145 | Acute | F | 64 |
| 3 | ATL-224 | Acute | M | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuguchi, M.; Takahashi, Y.; Tanaka, R.; Imaizumi, N.; Yamashita, A.; Matsushita, N.; Fukushima, T.; Tanaka, Y. Association Between FOXP3 and OX40 Expression in Adult T-Cell Leukemia Cells. Viruses 2025, 17, 1445. https://doi.org/10.3390/v17111445
Mizuguchi M, Takahashi Y, Tanaka R, Imaizumi N, Yamashita A, Matsushita N, Fukushima T, Tanaka Y. Association Between FOXP3 and OX40 Expression in Adult T-Cell Leukemia Cells. Viruses. 2025; 17(11):1445. https://doi.org/10.3390/v17111445
Chicago/Turabian StyleMizuguchi, Mariko, Yoshiaki Takahashi, Reiko Tanaka, Naoki Imaizumi, Akio Yamashita, Nobuko Matsushita, Takuya Fukushima, and Yuetsu Tanaka. 2025. "Association Between FOXP3 and OX40 Expression in Adult T-Cell Leukemia Cells" Viruses 17, no. 11: 1445. https://doi.org/10.3390/v17111445
APA StyleMizuguchi, M., Takahashi, Y., Tanaka, R., Imaizumi, N., Yamashita, A., Matsushita, N., Fukushima, T., & Tanaka, Y. (2025). Association Between FOXP3 and OX40 Expression in Adult T-Cell Leukemia Cells. Viruses, 17(11), 1445. https://doi.org/10.3390/v17111445





