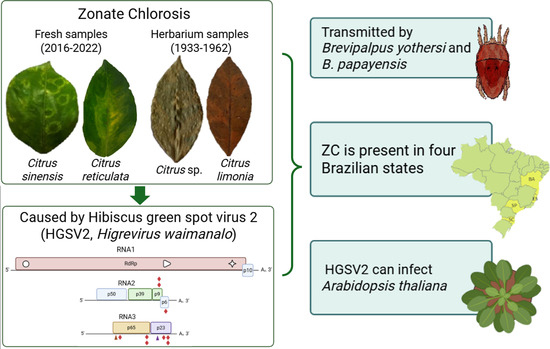
Historical and Contemporary Evidence Confirms a Higrevirus as the Causal Agent of Citrus Zonate Chlorosis in Brazil
Abstract
1. Introduction
2. Materials and Methods
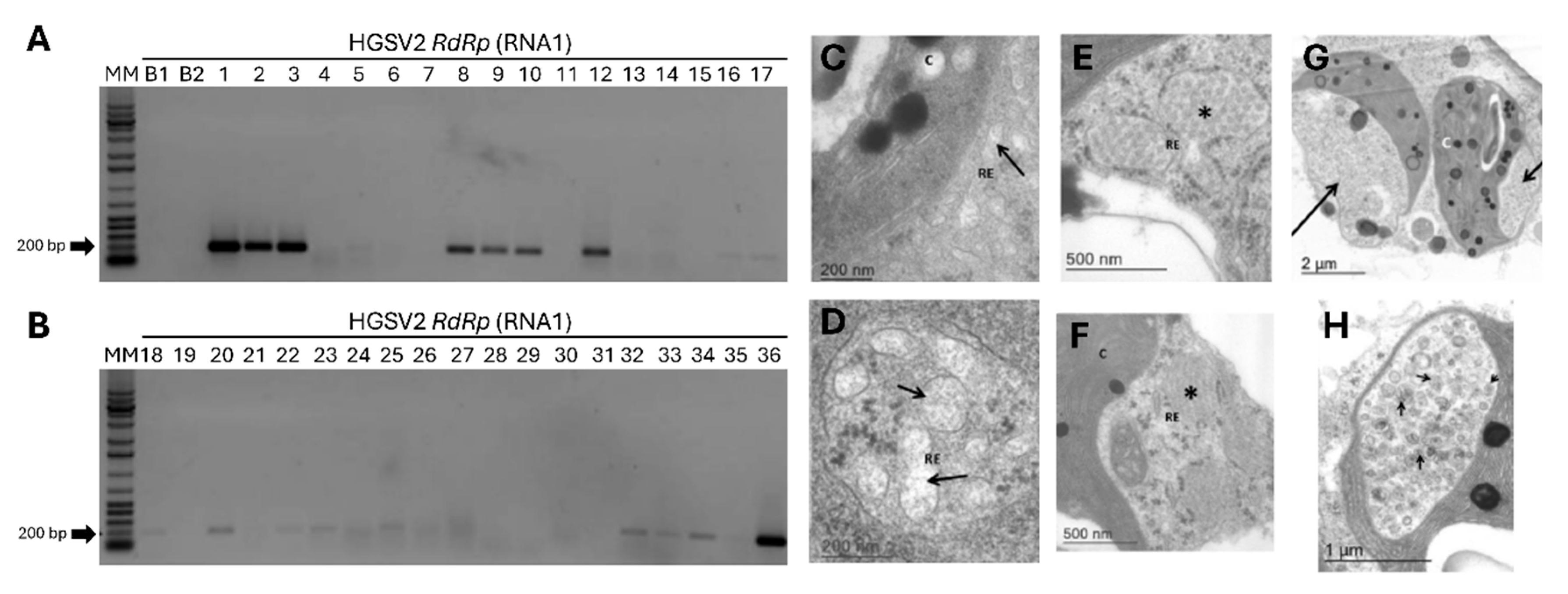
2.1. Plant Material and Transmission Electron Microscopy
2.2. RNA Extraction and HTS Preparation
2.3. Bioinformatic Analysis
2.4. Phylogenetic Analysis
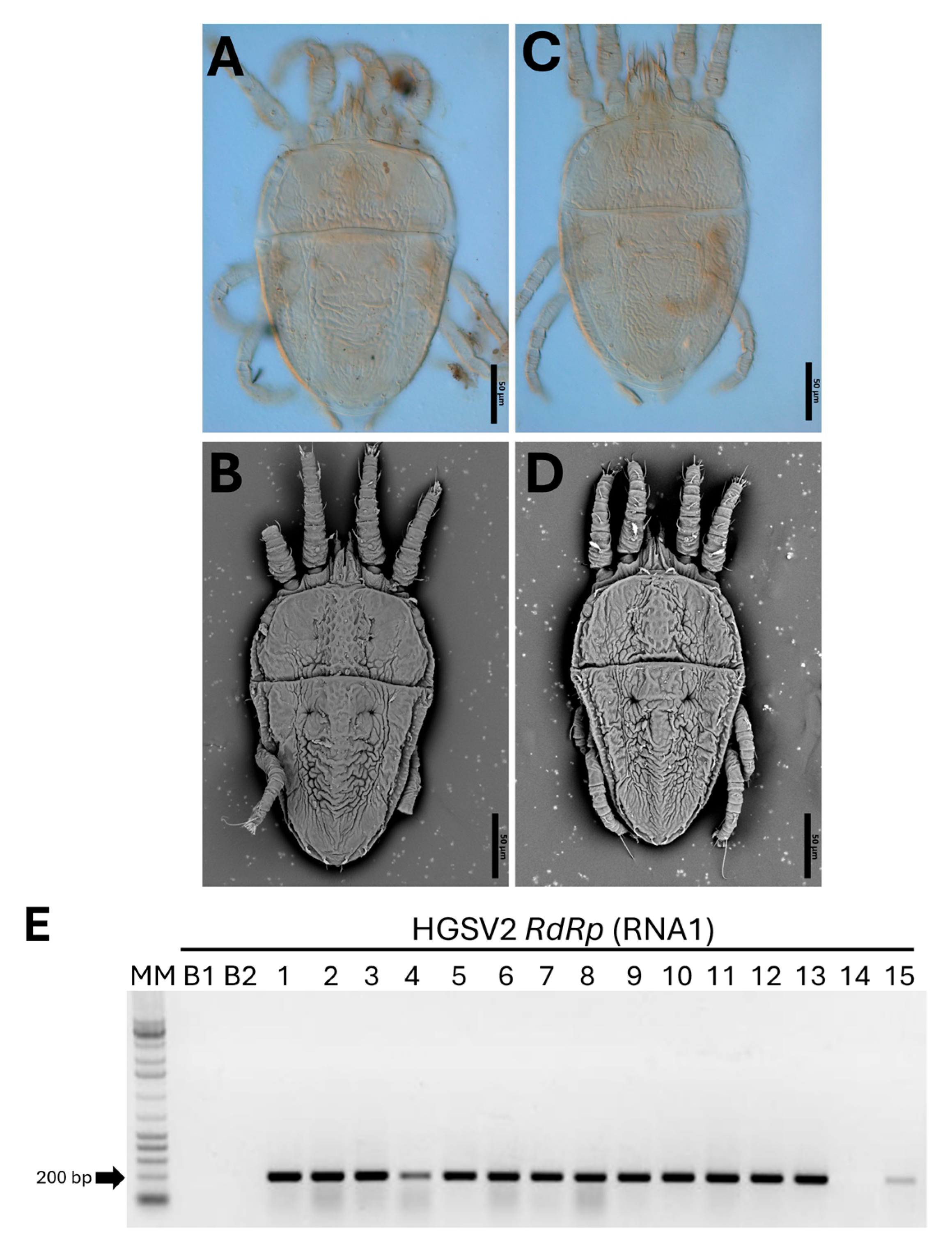
2.5. Morphological Identification of Brevipalpus Mites and Transmission Assays
3. Results
3.1. Symptom Characterization and Microscopy Suggest a Viral Agent Is Associated with Zonate Chlorosis
3.2. HTS Reveals HGSV2 as the Causal Agent of Zonate Chlorosis in Fresh and Herbarium Specimens
3.3. In Silico Analysis of the Brazilian Isolate of HGSV2
3.4. Brazilian Isolate of HGSV2 Clusters with Hawaiian Isolate in the Genus Higrevirus
3.5. HGSV2 Can Be Found in Diverse Citrus spp. Samples from Coastal Regions of Brazil, Associated with Brevipalpus Yothersi and B. papayensis
3.6. The Causal Agent of Zonate Chlorosis Is Transmitted by Brevipalpus Yothersi and B. papayensis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO/USDA Oranges|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/production/commodity/0571120 (accessed on 15 September 2025).
- FAO/USDSA Tangerines/Mandarins|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/production/commodity/0571220 (accessed on 15 September 2025).
- IBGE Produção de Tangerina No Brasil|IBGE. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/tangerina/br (accessed on 15 September 2025).
- Fundecitrus PES—Fundecitrus. Available online: https://www.fundecitrus.com.br/pesquisar/pes/#relatorios (accessed on 15 September 2025).
- Ramos-González, P.L.; Chabi-Jesus, C.; Arena, G.D.; Tassi, A.D.; Kitajima, E.W.; Freitas-Astúa, J. Citrus Leprosis: A Unique Multietiologic Disease. Citrus Am. 2018, 1, 4–19. [Google Scholar] [CrossRef]
- Bitancourt, A. Relação Das Doenças e Fungos Parasitas Observados Na Secção de Fitopatologia Durante Os Anos de 1931 e 1932. Arq. Do Inst. Biológico 1934, 5, 185–196. [Google Scholar]
- Bitancourt, A.; Grillo, H.V.S. A Clorose Zonada: Uma Nova Doença de Citrus. Arq. Inst. Biol. 1934, 5, 245–247. [Google Scholar]
- Bitancourt, A.A. A Clorose Zonada No Estado de São Paulo. Arq. Inst. Biol. 1934, 5, 247–249. [Google Scholar]
- Fawcett, H.S.; Bitancourt, A.A. Doenças Dos Citrus de Pernambuco. Boletim (SAIC) 1937, 2, 317–326. [Google Scholar]
- Rossetti, V.; Nakadaira, J.T.; Calza, R.; Miranda, C.A.B. Estudos Sobre a Clorose Zonada Dos Citros. Arq. Inst. Biol. 1965, 32, 111–125. [Google Scholar]
- Rossetti, V.; Nakadaira, J.T.; Calza, R.; Bonfanti de Miranda, C.A. A Propagação Da Clorose Zonada Dos Citros Pelo Ácaro Brevipalpus Phoenicis. O Biológico 1965, 31, 113–116. [Google Scholar]
- Melzer, M.J.; Sether, D.M.; Borth, W.B.; Hu, J.S. Characterization of a Virus Infecting Citrus Volkameriana with Citrus Leprosis-like Symptoms. Phytopathology 2012, 102, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Ramos-González, P.L.; Arena, G.D.; Tassi, A.D.; Chabi-Jesus, C.; Kitajima, E.W.; Freitas-Astúa, J. Kitaviruses: A Window to Atypical Plant Viruses Causing Nonsystemic Diseases. Annu. Rev. Phytopathol. 2023, 61, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, E.A.; Lezzhov, A.A.; Komarova, T.V.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. A Novel Block of Plant Virus Movement Genes. Mol. Plant Pathol. 2017, 18, 611–624. [Google Scholar] [CrossRef]
- Olmedo-Velarde, A.; Larrea-sarmiento, A.; Wang, X.; Hu, J.; Melzer, M. A Breakthrough in Kitavirids: Genetic Variability, Reverse Genetics, Koch’s Postulates, and Transmission of Hibiscus Green Spot Virus 2. Phytopathology 2024, 114, 282–293. [Google Scholar] [CrossRef]
- Kitajima, E.W.; Nome, C.F. Microscopia Electrónica Em Virologia Vegetal. In Métodos Para Detectar Patógenos Sistémicos; Docampo, D.M., Lenardón, S.L., Eds.; IFFIVE/INTA-JICA: Cordova, Argentina, 1999; pp. 59–87. [Google Scholar]
- Ramos-González, P.L.; Chabi-Jesus, C.; Guerra-Peraza, O.; Tassi, A.D.; Kitajima, E.W.; Harakava, R.; Salaroli, R.B.; Freitas-Astúa, J. Citrus Leprosis Virus N: A New Dichorhavirus Causing Citrus Leprosis Disease. Phytopathology 2017, 107, 963–976. [Google Scholar] [CrossRef]
- Chabi-Jesus, C.; Ramos-González, P.L.; Postclam-Barro, M.; Fontenele, R.S.; Harakava, R.; Bassanezi, R.B.; Moreira, A.S.; Kitajima, E.W.; Varsani, A.; Freitas-Astúa, J. Molecular Epidemiology of Citrus Leprosis Virus C: A New Viral Lineage and Phylodynamic of the Main Viral Subpopulations in the Americas. Front. Microbiol. 2021, 12, 641252. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 16 September 2025).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Cox, E.; Holmes, J.B.; Anderson, W.R.; Falk, R.; Hem, V.; Tsuchiya, M.T.N.; Schuler, G.D.; Zhang, X.; Torcivia, J.; et al. Exploring and Retrieving Sequence and Metadata for Species across the Tree of Life with NCBI Datasets. Sci. Data 2024, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- The Galaxy Community. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res. 2024, 52, W83–W94. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Armenteros, J.J.A.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM Predicts Alpha and Beta Transmembrane Proteins Using Deep Neural Networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of Protein Subcellular Localization Using Deep Learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]
- Ramos-González, P.L.; Alexandre, M.A.V.; Potsclam-Barro, M.; Duarte, L.M.L.; Michea Gonzalez, G.L.; Chabi-Jesus, C.; Ramos, A.F.; Harakava, R.; Lorenzi, H.; Freitas-Astúa, J.; et al. Two Novel Betarhabdovirins Infecting Ornamental Plants and the Peculiar Intracellular Behavior of the Cytorhabdovirus in the Liana Aristolochia Gibertii. Viruses 2024, 16, 322. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Ramos-González, P.L.; Chabi-Jesus, C.; Banguela-Castillo, A.; Tassi, A.D.; da Rodrigues, M.C.; Kitajima, E.W.; Harakava, R.; Freitas-Astúa, J. Unveiling the Complete Genome Sequence of Clerodendrum Chlorotic Spot Virus, a Putative Dichorhavirus Infecting Ornamental Plants. Arch. Virol. 2018, 163, 2519–2524. [Google Scholar] [CrossRef]
- Beard, J.J.; Ochoa, R.; Braswell, W.E.; Bauchan, G.R. Brevipalpus Phoenicis (Geijskes) Species Complex (Acari: Tenuipalpidae)—A Closer Look. Zootaxa 2015, 3944, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.D.; Ramos-González, P.L.; Nunes, M.A.; Jesus, C.C.; Calegario, R.F.; Kitajima, E.W.; Novelli, V.M.; Freitas-Astúa, J. Arabidopsis thaliana as a Modelo Host for Brevipalpus Mite-Transmitted Viruses. Sci. Agric. 2017, 74, 85–89. [Google Scholar] [CrossRef]
- Chabi-Jesus, C.; Ramos-González, P.L.; Tassi, A.D.; Rossetto Pereira, L.; Bastianel, M.; Lau, D.; Canale, M.C.; Harakava, R.; Novelli, V.M.; Kitajima, E.W.; et al. Citrus Bright Spot Virus: A New Dichorhavirus, Transmitted by Brevipalpus Azores, Causing Citrus Leprosis Disease in Brazil. Plants 2023, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Vergani, A.R. Transmisión y Naturaleza de La “Lepra Explosiva” Del Naranjo. Buenos Aires 1945, 3, 11. [Google Scholar]
- González-García, H.E.; Santillán-Galicia, M.T.; Ortega-Arenas, L.D.; Valdovinos-Ponce, G.; Becerril-Román, A.E.; Robles-García, P.L.; Guzmán-Franco, A.W.; Sánchez-Villarreal, A. Distribution and Host Range of Viruses Associated with the Citrus Leprosis Disease Complex in Mexico. PeerJ 2025, 13, e19889. [Google Scholar] [CrossRef] [PubMed]
- León, M.G.; Campos, J.C.P.; Guevara, Y.A.; Roy, A. Distribución, Plantas Hospederas y Ácaros Vectores Del Virus de La Leprosis de Los Cítricos En Colombia; AGROSAVIA: Bogotá, Colombia, 2023; ISBN 978-958-740-642-9. [Google Scholar] [CrossRef]
- Hartung, J.S.; Roy, A.; Fu, S.; Shao, J.; Schneider, W.L.; Brlansky, R.H. History and Diversity of Citrus Leprosis Virus Recorded in Herbarium Specimens. Phytopathology 2015, 105, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Astúa, J.; Ramos-González, P.L.; Arena, G.D.; Rassi, A.D.; Kitajima, E.W. Brevipalpus-Transmitted Viruses: Parallelism beyond a Common Vector or Convergent Evolution of Distantly Related Pathogens? Curr. Opin. Virol. 2018, 33, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tassi, A.D. Diversidade Morfológica e Genética de Diferentes Espécies de Brevipalpus (Acari: Tenuipalpidae) e suas Competências como Vetores de Vírus. Doctoral Dissertation, ESALQ/USP, Piracicaba, Brazil, 2018. [Google Scholar]
- Sánchez-Velázquez, E.J.; Santillán-Galicia, M.T.; Novelli, V.M.; Nunes, M.A.; Mora-Aguilera, G.; Valdez-Carrasco, J.M.; Otero-Colina, G.; Freitas-Astúa, J. Diversity and Genetic Variation among Brevipalpus Populations from Brazil and Mexico. PLoS ONE 2015, 10, e0133861. [Google Scholar] [CrossRef]
- Chabi-Jesus, C.; Ramos-González, P.L.; Tassi, A.D.; Guerra-Peraza, O.; Kitajima, E.W.; Harakava, R.; Beserra, J.E.A.; Salaroli, R.B.; Freitas-Astúa, J. Identification and Characterization of Citrus Chlorotic Spot Virus, a New Dichorhavirus Associated with Citrus Leprosis-like Symptoms. Plant Dis. 2018, 102, 1588–1598. [Google Scholar] [CrossRef]
- Tassi, A. Transmissão Diferencial de Estirpes de Citrus Leprosis Virus C Por Subpopulações de Brevipalpus Yothersi. In Proceedings of the Anais do Congresso Brasileiro de Fitopatologia, Online, 24 August 2021. [Google Scholar]
- Nakasu, E.Y.T.; Nagata, T.; Inoue-Nagata, A.K. First Report of Tomato Fruit Blotch Virus Infecting Tomatoes in Brazil. Plant Dis. 2022, 106, 2271. [Google Scholar] [CrossRef]
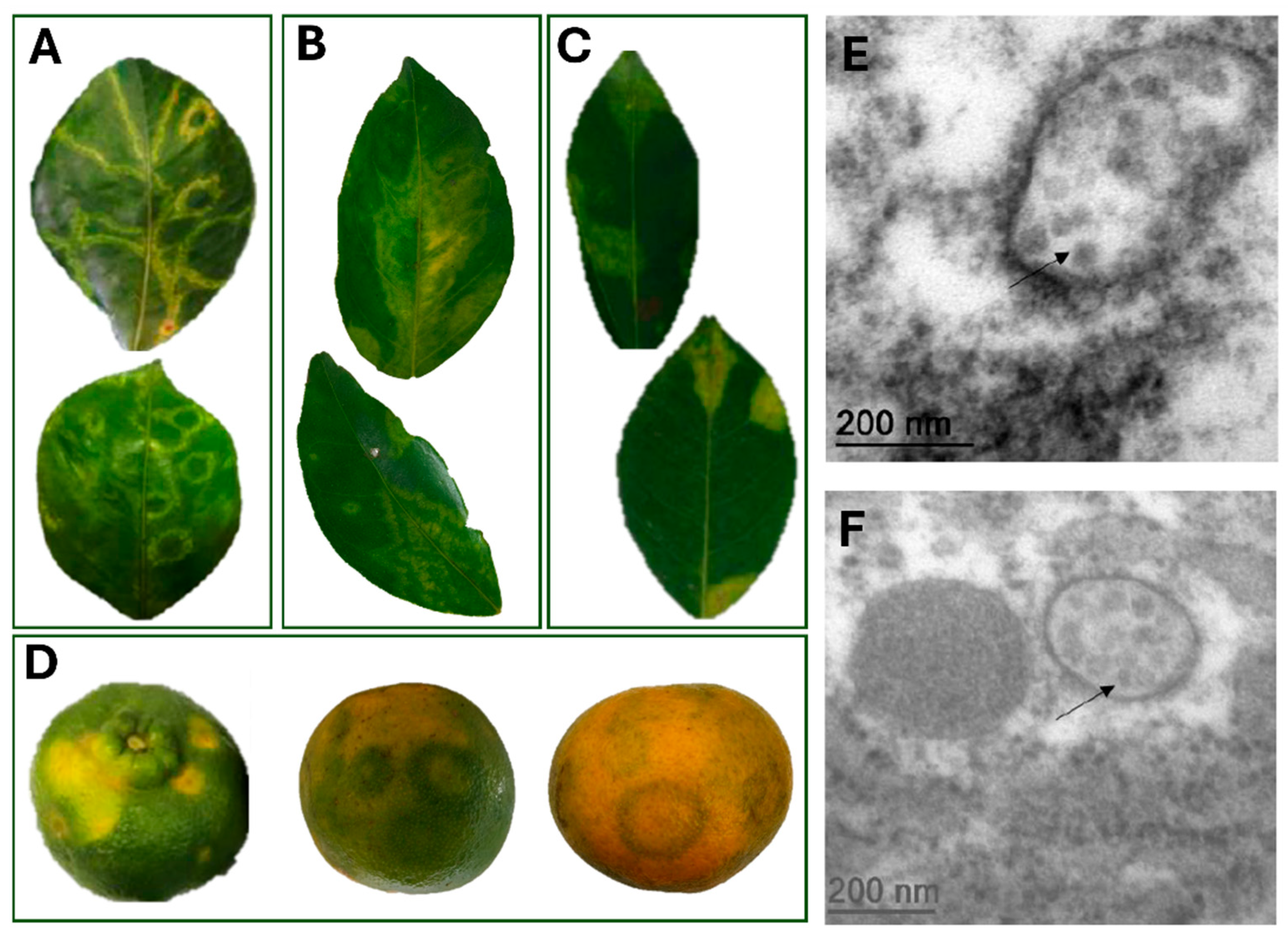
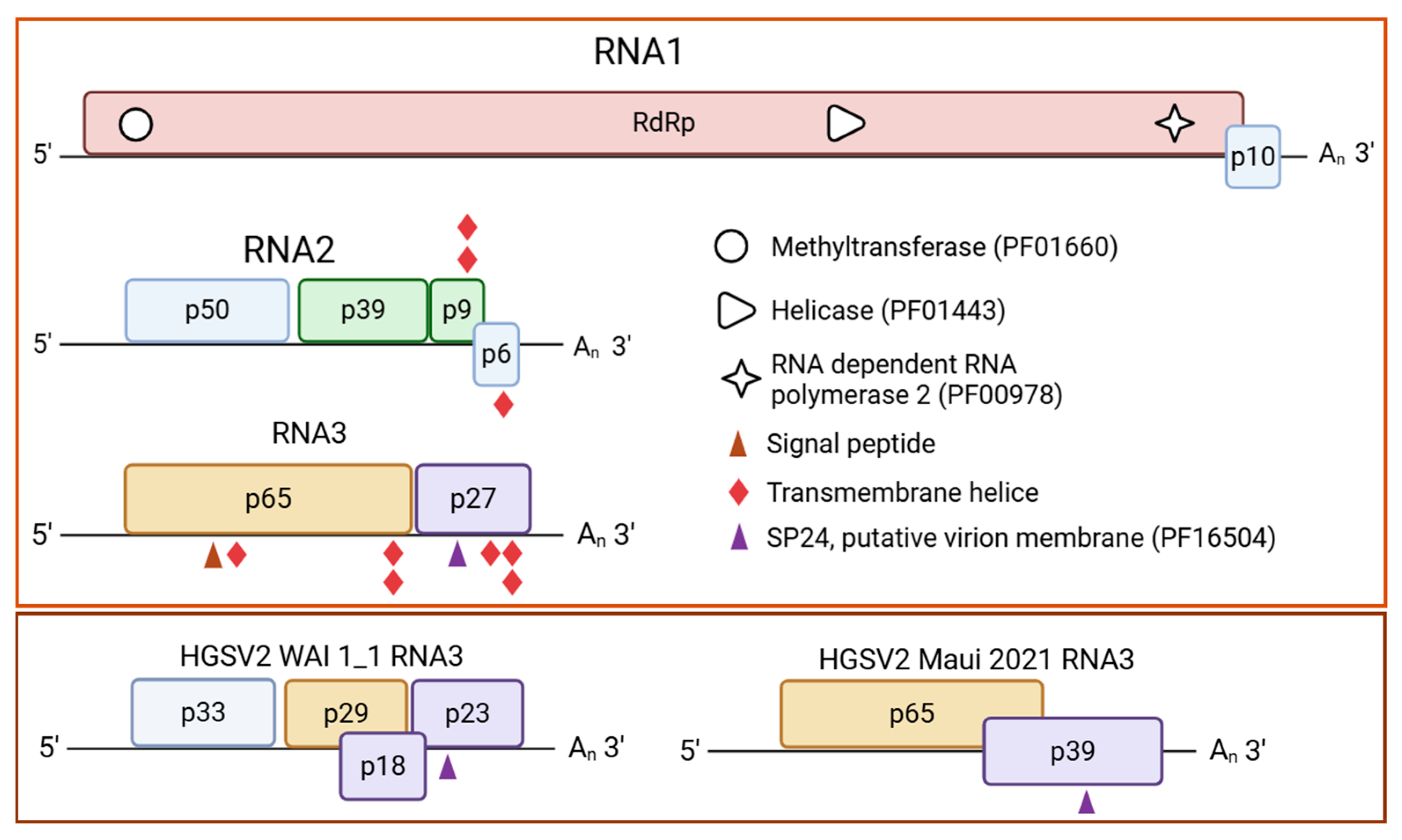
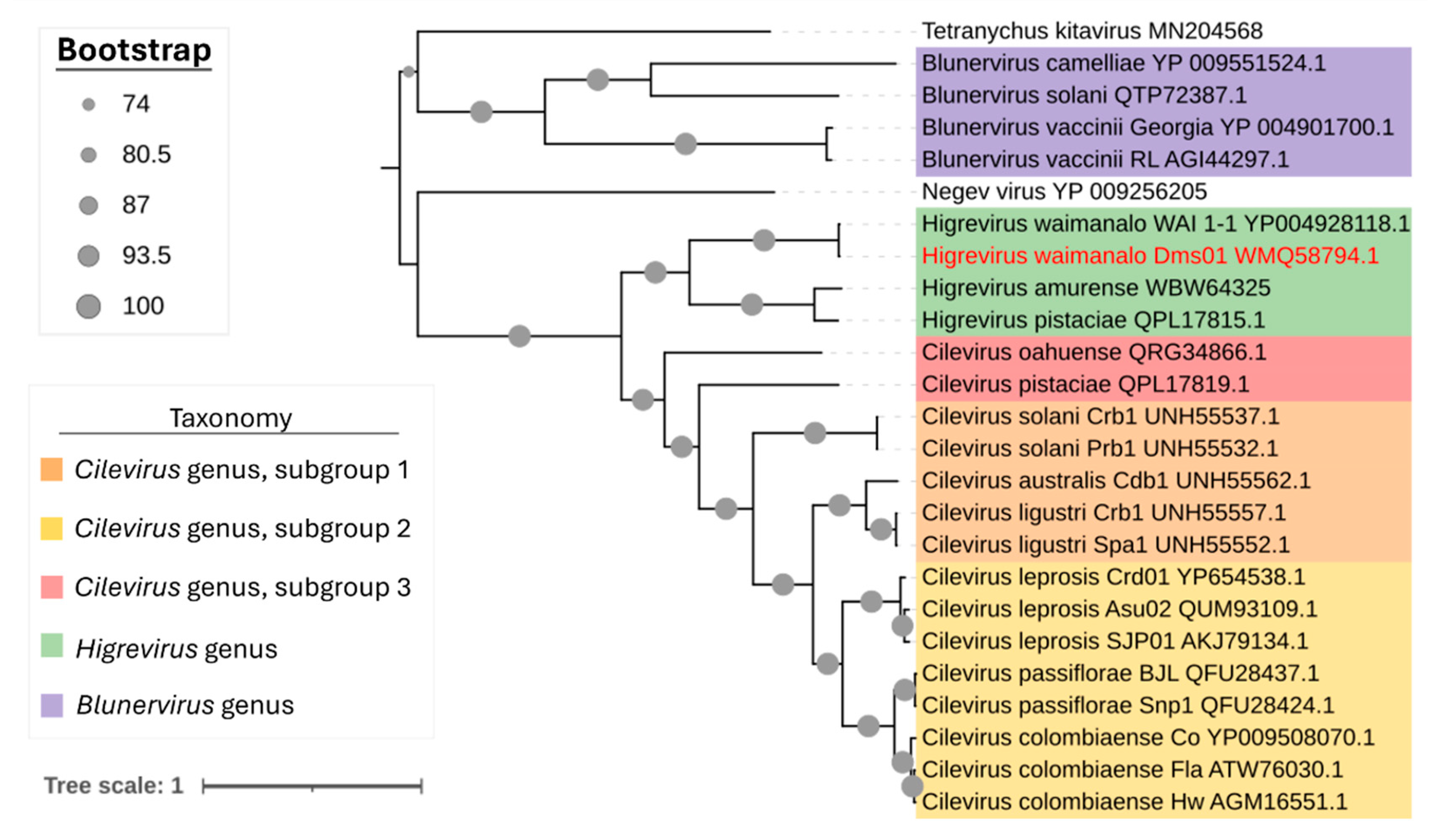
| Year of Collection | Host Species | Isolate Identification | Place of Collection a | Experimental Use |
|---|---|---|---|---|
| Fresh samples | ||||
| 2016 | C. sinensis | CdA_01 | Cruz das Almas, BA | HTS (mRNA) |
| 2016, 2016, 2021 | C. sinensis | CdA_03, CdA_04, CdA_12 | Cruz das Almas, BA | RT-PCR, mite collection and identification |
| 2016 | C. deliciosa | CdA_07 | Cruz das Almas, BA | RT-PCR |
| 2016, 2021 | C. reticulata | CdA_08, CdA_15 | Cruz das Almas, BA | RT-PCR, inoculum for mite transmission (Experiment II and III) |
| 2016 | C. clementina | CdA_09 | Cruz das Almas, BA | RT-PCR |
| 2016 | C. aurantium | CdA_10 | Cruz das Almas, BA | TEM, RT-PCR |
| 2016 | Citrus sp. | VNI_01 | Venda Nova do Imigrante, ES | RT-PCR |
| 2017, 2017 | C. reticulata | DMs_01, DMs_02 | Domingo Martins, ES | HTS (mRNA) |
| 2017 | C. sinensis | DMs_03 | Domingos Martins, ES | RT-PCR, mite collection and identification |
| 2021 | C. sinensis × C. paradisi | CdA_14 | Cruz das Almas, BA | RT-PCR |
| 2021 | C. limon | CdA_17 | Cruz das Almas, BA | RT-PCR |
| 2022 | C. reticulata | Itj_01, Itj_02 | Itajaí, SC | RT-PCR, mite identification and viral transmission (Experiment I) |
| Herbarium samples | ||||
| 1933 | C. reticulata | Ubt_01 | Ubatuba, SP | HTS (small RNA) |
| 1936 | Citrus sp. | Cmp_01 | Campinas, SP | HTS (small RNA) |
| 1937 | Citrus sp. | Itn_02 | Itanháem, SP | HTS (small RNA) |
| 1962 | C. limonia | SPa_01 | São Paulo, SP | HTS (small RNA) |
| Virus | GenBank Accession Number | Genomic Segments | ||
|---|---|---|---|---|
| HGSV2 DMs_01 | OR161045, OR161046 and OR161047 | RNA1 (%) | RNA2 (%) | RNA3 (%) |
| HGSV2 DMs_02 | PX437934, PX437935 and PX437936 | 100 | 99.9 | 99.9 |
| HSGV2 CdA_01 | PX437943, PX437944 and PX437945 | 94.9 * | 87.5 * | 92.1 * |
| HGSV2 Itn_02 | PX437940, PX437941 and PX437942 | 87.6 * | 93.8 | 90.3 * |
| HGSV2 Cmp_01 | PX437946, PX437947 and PX437948 | 91.1 * | 95.7 * | 96.2 * |
| HGSV2 WAI_1-1 | NC016141, NC016143 and NC016142 | 97.6 | 93.3 | 92.5 |
| HGSV2 UHM2019 | OQ689785, OQ689786 and OQ689787 | 97.7 | 93.4 | 93.1 |
| HGSV2 Maui_2021 | OQ689788, OQ689789 and OQ689790 | 97.6 | 92.6 | 93.1 |
| PixVX | MT334620, MT334619 and MT334618 | 54.2 | 51.5 | 47.6 |
| PaHLV | OP324809, OP324810 and OP324811 | 53.8 | 48.7 | 43.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.R.; Rodrigues, M.C.; Chabi-Jesus, C.; Ramos-González, P.L.; Barbosa, C.J.; Santos, M.G.; Costa, H.; Maro, L.C.; Tassi, A.D.; Kitajima, E.W.; et al. Historical and Contemporary Evidence Confirms a Higrevirus as the Causal Agent of Citrus Zonate Chlorosis in Brazil. Viruses 2025, 17, 1428. https://doi.org/10.3390/v17111428
Pereira LR, Rodrigues MC, Chabi-Jesus C, Ramos-González PL, Barbosa CJ, Santos MG, Costa H, Maro LC, Tassi AD, Kitajima EW, et al. Historical and Contemporary Evidence Confirms a Higrevirus as the Causal Agent of Citrus Zonate Chlorosis in Brazil. Viruses. 2025; 17(11):1428. https://doi.org/10.3390/v17111428
Chicago/Turabian StylePereira, Laura R., Mariane C. Rodrigues, Camila Chabi-Jesus, Pedro L. Ramos-González, Cristiane J. Barbosa, Magno G. Santos, Helcio Costa, Luana C. Maro, Aline D. Tassi, Elliot W. Kitajima, and et al. 2025. "Historical and Contemporary Evidence Confirms a Higrevirus as the Causal Agent of Citrus Zonate Chlorosis in Brazil" Viruses 17, no. 11: 1428. https://doi.org/10.3390/v17111428
APA StylePereira, L. R., Rodrigues, M. C., Chabi-Jesus, C., Ramos-González, P. L., Barbosa, C. J., Santos, M. G., Costa, H., Maro, L. C., Tassi, A. D., Kitajima, E. W., Harakava, R., & Freitas-Astúa, J. (2025). Historical and Contemporary Evidence Confirms a Higrevirus as the Causal Agent of Citrus Zonate Chlorosis in Brazil. Viruses, 17(11), 1428. https://doi.org/10.3390/v17111428