The Relationship Between Hemodynamic Responses During Head-Up Tilt Testing and Parameters of Infection in Post-COVID Syndrome, Chronic Fatigue Syndrome, and Late-Stage Lyme Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Head-Up Tilt Test (HUTT)
2.3. Serology Tests
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QASAT | Quantitative Scale for Grading of Cardiovascular Autonomic Reflex Tests and Small Fibers from Skin Biopsies |
| COMPASS-31 | Composite Autonomic Symptom Score |
| SAS | Survey of Autonomic Symptoms |
| POTS | Postural Orthostatic Tachycardia Syndrome |
| ESC | European Society of Cardiology |
References
- Daroff, R.B.; Jankovic, J.; Mazziotta, J.C.; Pomeroy, S.L.; Bradley, W.G. Bradley’s Neurology in Clinical Practice; Elsevier: London, UK; New York, NY, USA, 2016. [Google Scholar]
- Fitzpatrick, A.P.; Theodorakis, G.; Vardas, P.; Sutton, R. Methodology of head-up tilt testing in patients with unexplained syncope. J. Am. Coll. Cardiol. 1991, 17, 125–130. [Google Scholar] [CrossRef]
- Ewing, D.J.; Clarke, B.F. Diagnosis and management of diabetic autonomic neuropathy. Br. Med. J. (Clin. Res. Ed.) 1982, 285, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Novak, P. Quantitative autonomic testing. J. Vis. Exp. 2011, 53, e2502. [Google Scholar] [CrossRef] [PubMed]
- Novak, P. Quantitative Scale for Grading of Cardiovascular Autonomic Reflex Tests and Small Fibers from Skin Biopsies (QASAT). J. Neurol. Disord. 2015, 3, 226. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Zilliox, L.; Peltier, A.C.; Wren, P.A.; Anderson, A.; Smith, A.G.; Singleton, J.R.; Feldman, E.L.; Alexander, N.B.; Russell, J.W. Assessing autonomic dysfunction in early diabetic neuropathy: The Survey of Autonomic Symptoms. Neurology 2011, 76, 1099–1105. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Rocha, E.A.; Mehta, N.; Távora-Mehta, M.Z.P.; Roncari, C.F.; Cidrão, A.A.L.; Elias Neto, J. Dysautonomia: A Forgotten Condition—Part 1. Arq. Bras. Cardiol. 2021, 116, 814–835, (In English and Portuguese). [Google Scholar] [CrossRef]
- Hovaguimian, A. Dysautonomia: Diagnosis and Management. Neurol. Clin. 2023, 41, 193–213. [Google Scholar] [CrossRef]
- Naschitz, J.E.; Yeshurun, D.; Rosner, I. Dysautonomia in chronic fatigue syndrome: Facts, hypotheses, implications. Med. Hypotheses 2004, 62, 203–206. [Google Scholar] [CrossRef]
- Nicolini, P.; Ciulla, M.M.; Malfatto, G.; Abbate, C.; Mari, D.; Rossi, P.D.; Pettenuzzo, E.; Magrini, F.; Consonni, D.; Lombardi, F. Autonomic dysfunction in mild cognitive impairment: Evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS ONE 2014, 9, e96656. [Google Scholar] [CrossRef]
- Koralnik, I.J.; Tyler, K.L. COVID-19: A Global Threat to the Nervous System. Ann. Neurol. 2020, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Haloot, J.; Bhavaraju-Sanka, R.; Pillarisetti, J.; Verduzco-Gutierrez, M. Autonomic Dysfunction Related to Postacute SARS-CoV-2 Syndrome. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 563–572. [Google Scholar] [CrossRef]
- Słomko, J.; Estévez-López, F.; Kujawski, S.; Zawadka-Kunikowska, M.; Tafil-Klawe, M.; Klawe, J.J.; Morten, K.J.; Szrajda, J.; Murovska, M.; Newton, J.L.; et al. Autonomic Phenotypes in Chronic Fatigue Syndrome (CFS) Are Associated with Illness Severity: A Cluster Analysis. J. Clin. Med. 2020, 9, 2531. [Google Scholar] [CrossRef]
- Jason, L.A.; Dorri, J.A. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol. Int. 2023, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front. Pediatr. 2019, 7, 12. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)—A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef]
- Shor, S.; Green, C.; Szantyr, B.; Phillips, S.; Liegner, K.; Burrascano, J.J., Jr.; Bransfield, R.; Maloney, E.L. Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group. Antibiotics 2019, 8, 269. [Google Scholar] [CrossRef]
- Adler, B.L.; Chung, T.; Rowe, P.C.; Aucott, J. Dysautonomia following Lyme disease: A key component of post-treatment Lyme disease syndrome? Front. Neurol. 2024, 15, 1344862. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.M.; Kluger, J. A tale of two syndromes: Lyme disease preceding postural orthostatic tachycardia syndrome. Ann. Noninvasive Electrocardiol. 2015, 20, 82–86. [Google Scholar] [CrossRef]
- Berghoff, W. Chronic Lyme Disease and Co-infections: Differential Diagnosis. Open Neurol. J. 2012, 6, 158–178. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chang, Z.; Wang, Y.; Wu, M.; Zhang, W.; Zhou, G.; Zou, X.; Tian, H.; Xiao, T.; Xing, J.; et al. Clinical characteristics and reasons for differences in duration from symptom onset to release from quarantine among patients with COVID-19 in Liaocheng, China. Front. Med. 2020, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Myalgic Encephalomyelitis (or Encephalopathy)/Chronic Fatigue Syndrome: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2021; Available online: https://www.nice.org.uk/guidance/ng206 (accessed on 20 June 2025).
- Vernino, S.; Bourne, K.M.; Stiles, L.E.; Grubb, B.P.; Fedorowski, A.; Stewart, J.M.; Arnold, A.C.; Pace, L.A.; Axelsson, J.; Boris, J.R.; et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Auton. Neurosci. 2021, 235, 102828. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef]
- Pence, R.; Johnston, B. SYNCOPE: A RARE PRESENTATION OF LYME DISEASE. J. Emerg. Med. 2023, 65, e23–e26. [Google Scholar] [CrossRef]
- Ivey, M.C.; Kooshkabadi, M. Third-Degree Atrioventricular Block Secondary to Lyme Disease: A Case Report. Cureus 2023, 15, e49803. [Google Scholar] [CrossRef]
- Jameson, J.L.; Fauci, A.; Kasper, D.; Hauser, S.; Longo, D.; Loscalzo, J. Harrison’s Principles of Internal Medicine 19th Edition and Harrison’s Manual of Medicine 19th Edition VAL PAK; McGraw-Hill Medical: New York, NY, USA, 2017. [Google Scholar]
- Tanking, C.; Lakkananurak, C.; Srisakvarakul, C.; Jitpreeda, A.; Threechod, K.; Sukitpunyaroj, D. Postural orthostatic tachycardia syndrome and other autonomic dysfunctions following COVID-19: Incidence, characteristics, and associated factors. J. Arrhythm. 2024, 40, 230–236. [Google Scholar] [CrossRef]
- Kenny, R.A.; Graham, L.A. Chronic fatigue syndrome symptoms common in patient with vasovagal syncope. Am. J. Med. 2001, 110, 242–243. [Google Scholar] [CrossRef]
- Bou-Holaigah, I.; Rowe, P.C.; Kan, J.; Calkins, H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA 1995, 274, 961–967. [Google Scholar] [CrossRef]
- Timmers, H.J.; Wieling, W.; Soetekouw, P.M.; Bleijenberg, G.; Van Der Meer, J.W.; Lenders, J.W. Hemodynamic and neurohumoral responses to head-up tilt in patients with chronic fatigue syndrome. Clin. Auton. Res. 2002, 12, 273–280. [Google Scholar] [CrossRef]
- van Campen, C.M.C.; Visser, F.C. Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare 2022, 10, 2058. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Arhi, C.; Chandra, N.; Franzen-McManus, A.-C.; Meyer, A.; Sutton, R. Blood pressure oscillations during tilt testing as a predictive marker of vasovagal syncope. Europace 2009, 11, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.L.M.C.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef]
- Biswal, B.; Kunwar, P.; Natelson, B.H. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J. Neurol. Sci. 2011, 301, 9–11. [Google Scholar] [CrossRef]
- He, J.; Hollingsworth, K.G.; Newton, J.L.; Blamire, A.M. Cerebral vascular control is associated with skeletal muscle pH in chronic fatigue syndrome patients both at rest and during dynamic stimulation. Neuroimage Clin. 2013, 2, 168–173. [Google Scholar] [CrossRef]
- Manoharan, S.; Ying, L.Y. Epstein Barr Virus Reactivation during COVID-19 Hospitalization Significantly Increased Mortality/Death in SARS-CoV-2(+)/EBV(+) than SARS-CoV-2(+)/EBV(−) Patients: A Comparative Meta-Analysis. Int. J. Clin. Pract. 2023, 2023, 1068000. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djajic, V.; Bajic, D.; Djokovic, A.; Krajnovic, T.; Jovanovic, S.; Verhaz, A.; Kovacevic, P.; Ostojic, M. Assessment of Autonomic Nervous System Dysfunction in the Early Phase of Infection With SARS-CoV-2 Virus. Front. Neurosci. 2021, 15, 640835. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A.; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef] [PubMed]
- Gravelsina, S.; Vilmane, A.; Svirskis, S.; Rasa-Dzelzkaleja, S.; Nora-Krukle, Z.; Vecvagare, K.; Krumina, A.; Leineman, I.; Shoenfeld, Y.; Murovska, M. Biomarkers in the diagnostic algorithm of myalgic encephalomyelitis/chronic fatigue syndrome. Front. Immunol. 2022, 13, 928945. [Google Scholar] [CrossRef]
- Perlejewski, K.; Radkowski, M.; Pawełczyk, A.; Rydzanicz, M.; Dzieciątkowski, T.; Makowiecki, M.; Paciorek, M.; Welc-Falęciak, R.; Horban, A.; Laskus, T. Enteroviral central nervous system infections in patients with Lyme neuroborreliosis. Ticks Tick Borne Dis. 2023, 14, 102253. [Google Scholar] [CrossRef] [PubMed]
- Koester, T.M.; Meece, J.K.; Fritsche, T.R.; Frost, H.M. Infectious Mononucleosis and Lyme Disease as Confounding Diagnoses: A Report of 2 Cases. Clin. Med. Res. 2018, 16, 66–68. [Google Scholar] [CrossRef]
- Gylfe, A.; Wahlgren, M.; Fahlén, L.; Bergström, S. Activation of latent Lyme borreliosis concurrent with a herpes simplex virus type 1 infection. Scand. J. Infect. Dis. 2002, 34, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.K.; Abdelbaky, M.; Lahoti, L. Diagnostic Challenges of Lyme Co-infections: Lessons From a Lyme and Herpes Simplex Virus-1 (HSV-1) Cocktail. Cureus 2024, 16, e60213. [Google Scholar] [CrossRef]
- Hannestad, U.; Apostolou, E.; Sjögren, P.; Bragée, B.; Polo, O.; Bertilson, B.C.; Rosén, A. Post-COVID sequelae effect in chronic fatigue syndrome: SARS-CoV-2 triggers latent adenovirus in the oral mucosa. Front. Med. 2023, 10, 1208181. [Google Scholar] [CrossRef] [PubMed]
- Chémali, K.; Sandefer, A. Mycoplasma pneumoniae infection associated with autoimmune small fiber neuropathy and postural orthostatic tachycardia syndrome (POTS) (P18-1.007). Neurology 2022, 98 (Suppl. 18). [Google Scholar] [CrossRef]
- Price, R.W.; Rubenstein, R.; Khan, A. Herpes simplex virus infection of isolated autonomic neurons in culture: Viral replication and spread in a neuronal network. Arch. Virol. 1982, 71, 127–140. [Google Scholar] [CrossRef]
- Price, R.W.; Katz, B.J.; Notkins, A.L. Latent infection of the peripheral ANS with herpes simplex virus. Nature 1975, 257, 686–688. [Google Scholar] [CrossRef]
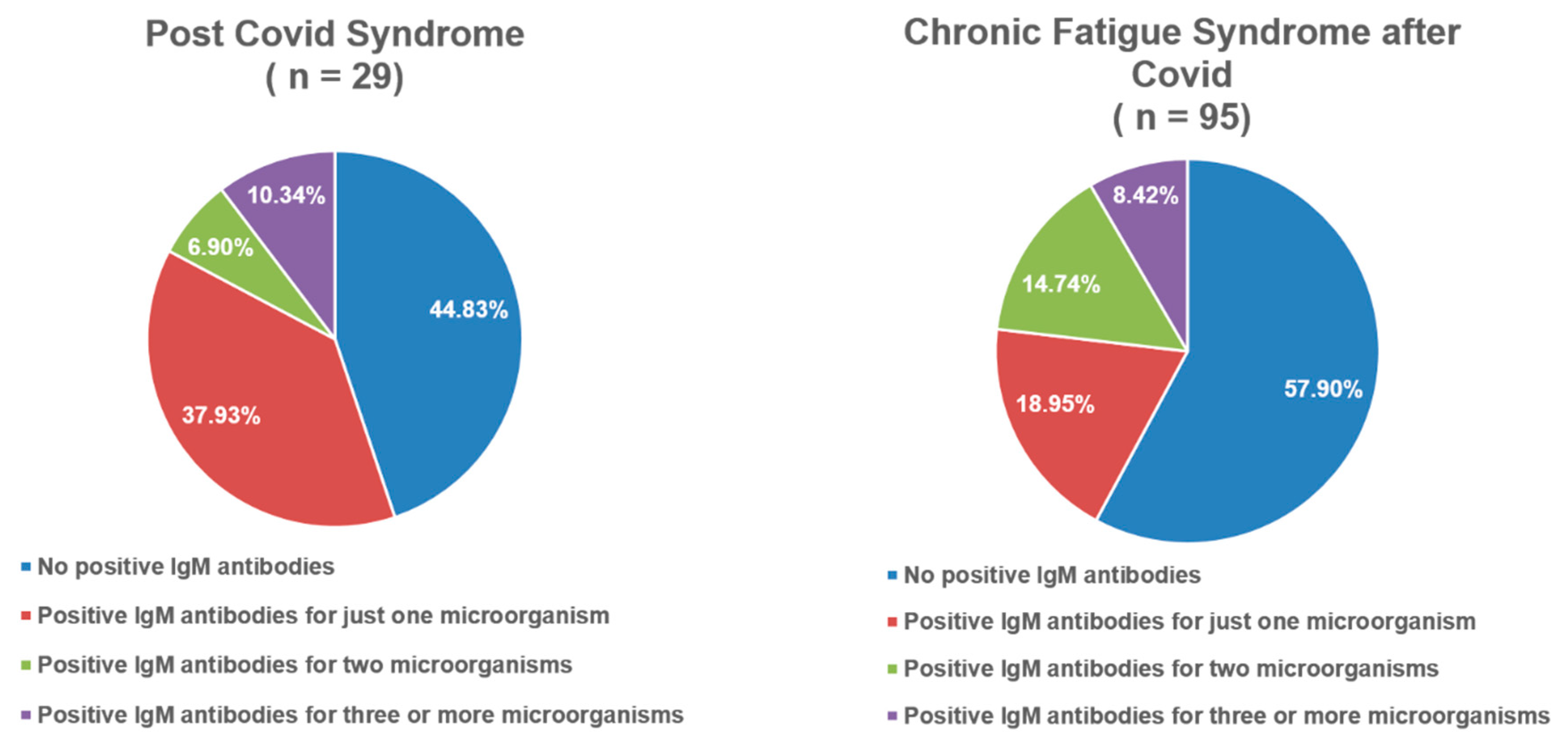
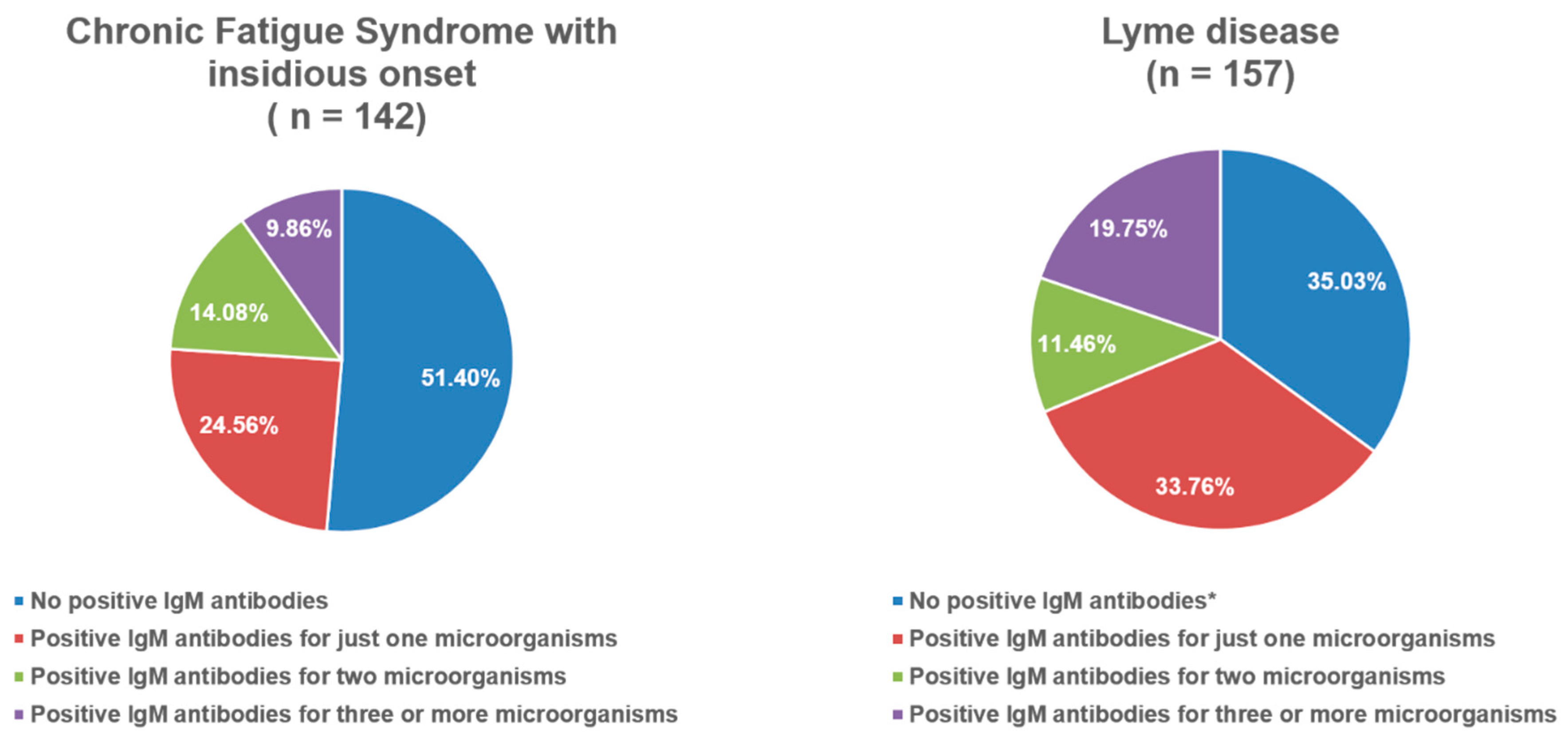
| PCS (n = 95) | CFS After Cov. (n = 285) | CFS (n = 499) | Lyme (n = 157) | p | |
|---|---|---|---|---|---|
| Male (n, %) | 37 (39.95%) | 87 (30.53%) | 157 (31.46%) | 53 (33.76%) | 0.45 1 |
| Female (n, %) | 60 (60.05%) | 195 (69.47%) | 342 (68.64%) | 104 (66.24%) | |
| Age (mean + SD) | 45.88 ± 13.60 | 46.26 ± 13.30 | 44.31 ± 12.79 | 44.76 ± 14.92 | 0.231 2 |
| History of Syncope (n, %) | 30 (31.60%) | 117 (41.10%) | 176 (35.30%) | 64 (57.10%) +; #; * | <0.001 1 |
| +Syncop. and −OH (n, %) | 19 (20.00%) | 78 (27.40%) | 109 (21.9%) | 35 (31.3%) | 0.072 1 |
| +Syncop. and +OH (n, %) | 11 (11.60%) | 39 (13.70%) | 67 (13.40%) | 29 (25.90%) #; * | 0.005 1 |
| −Syncop. And +OH (n, %) | 4 (4.20%) | 18 (6.30%) | 28 (5.60%) | 4 (3.60%) | 0.685 1 |
| PCS (n = 95) | CFS After Cov. (n = 285) | CFS (n = 499) | Lyme (n = 112) | p | |
|---|---|---|---|---|---|
| Positive HUTT (n, %) | 26 (27.40%) | 96 (33.70%) | 145 (29.10%) | 56 (50%) +; #; * | <0.001 1 |
| EVBP during HUTT (n, %) | 44 (46.30%) | 155 (54.40%) | 257 (51.50%) | 52 (46.40%) | 0.531 1 |
| SMVP during HUTT (n, %) | 9 (9.50%) | 36 (12.60%) | 98 (19.60%) | 11 (9.80%) * | 0.001 1 |
| OH during HUTT (n, %) | 15 (15.8%) | 57 (20.00%) | 95 (19.00%) | 33 (29.50%) | 0.162 1 |
| POTS during HUTT (n, %) | 13 (13.70%) | 24 (8.40%) | 32 (6.40%) | 6 (5.40%) | 0.102 1 |
| Hypertens. reaction (n, %) | 18 (18.90%) | 57 (20.00%) | 119 (23.80%) | 17 (15.20%) | 0.259 1 |
| Extrem. Hypertens. reaction (n, %) | 7 (7.40%) | 34 (11.90%) | 50 (10.00%) | 8 (7.10%) | 0.322 1 |
| Normal response (n, %) | 25 (26.30%) | 42 (14.70%) | 87 (17.40%) | 13 (11.60%) + | 0.026 1 |
| PCS (n = 29) | CFS After Cov. (n = 95) | CFS (n = 142) | Lyme (n = 157) | p | |
|---|---|---|---|---|---|
| Total score of pos. IgM (Mdn + IQR) | 1 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–2) ”; & | 0.003 1 |
| Percentage of positive IgM antibodies among the groups | |||||
| Pos. IgM for Adenovirus | 0 (0%) | 5 (5.30%) | 13 (9.20%) | 21 (13.40%) | 0.047 2 |
| Pos. IgM for Parvo B19 | 1 (3.40%) | 2 (2.10%) | 2 (1.40%) | 14 (8.90%) * | 0.009 2 |
| Pos. IgM for Coxsackiae | 1 (3.40%) | 3 (3.20%) | 5 (3.50%) | 35 (22.30%) #; * | <0.001 2 |
| Percentage of positive IgG antibodies among the groups | |||||
| Pos. IgG for Adenovirus | 16 (55.20%) | 68 (71.60%) | 79 (55.60%) | 57 (36.30%) #; * | <0.001 2 |
| Pos. IgG for Coxsackiae | 4 (13.80%) | 11 (11.60%) | 9 (6.30%) | 29 (18.50%) * | 0.017 2 |
| Pos. IgG for CMV | 25 (86.20%) | 70 (73.70%) | 107 (75.40%) | 92 (58.60%) +; * | 0.001 2 |
| Pos. IgG for EBV | 24 (82.80%) | 77 (81.10%) | 106 (74.60%) | 93 (59.20%) #; * | <0.001 2 |
| Pos. IgG for Candida | 1 (3.40%) | 5 (5.30%) | 18 (12.70%) | 6 (3.80%) * | 0.016 2 |
| Pos. IgG for VZV | 25 (86.20%) | 77 (81.10%) | 112 (78.90%) | 60 (38.20%) +; #; * | <0.001 2 |
| Pos. IgG for HSV1 | 21 (72.40%) | 63 (66.30%) | 93 (63.50%) | 40 (25.50%) +; #; * | <0.001 2 |
| Pos. IgG for HSV2 | 3 (10.30%) | 14 (14.70%) | 23 (16.20%) | 9 (5.70%) * | 0.027 2 |
| Variable | Univariable Model | |
|---|---|---|
| Exp (B) (95% CI) | p | |
| Positive head-up tilt test a | ||
| Hypotension at supine | 6.09 (1.04–35.56) | 0.045 |
| EVBP during head-up tilt test b | ||
| Pos. IgG for Adenovirus | 0.15 (0.03–0.76) | 0.022 |
| SVBP during head-up tilt test c | ||
| SVBP at supine | 12.00 (1.46–98.60) | 0.021 |
| OH d | ||
| Hypotension at supine | 14.18 (2.32–86.74) | 0.008 |
| Hypertensive reaction (>130/90 mmHg) e | ||
| Pos. IgA for Helicobacter Pylori | 34.5 (2.35–505.75) | 0.01 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Exp (B) (95% CI) | p | Exp (B) (95% CI) | p | |
| Positive head-up tilt test a | ||||
| Female | 2.95 (1.60–5.44) | 0.001 | 10.03 (2.52–39.95) | 0.001 |
| SVBP at Supine | 0.23 (0.07–0.77) | 0.017 | ||
| Hypotension at Supine | 6.34 (1.25–32.02) | 0.025 | ||
| Pos. IgG for Adenovirus | 2.86 (1.07–7.64) | 0.036 | ||
| Pos. IgA for Helicobacter | 10.91 (1.29–92.66) | 0.029 | ||
| Pos. IgG for Bartonella | 9.09 (1.05–78.75) | 0.045 | ||
| SVBP pressure during head-up tilt test b | ||||
| Pos. IgG for Coxiella burnetii | 12.15 (1.03–143.85) | 0.048 | 21.00 (1.62–264.33) | 0.019 |
| Pos. IgG for SARS-CoV-2 | 3.50 (1.11–11.09) | 0.033 | 4.59 (1.36–15.57) | 0.014 |
| OH c | ||||
| Hypotension at Supine | 7.21 (1.67–31.14) | 0.008 | ||
| Extreme hypertensive reaction (>170/120 mmHg) d | ||||
| Male | 3.41 (1.64–7.09) | 0.001 | 17.86 (2.03–166.67) | 0.009 |
| Tachycardia at Supine | 5.98 (1.278–27.95) | 0.023 | ||
| Pos. IgG for Adenovirus | 0.20 (0.05–0.92) | 0.039 | ||
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Exp (B) (95% CI) | p | Exp (B) (95% CI) | p | |
| Positive head-up tilt test a | ||||
| Female | 3.45 (2.09–5.69) | <0.001 | 7.28 (2.50–21.18) | <0.001 |
| Pos. IgM for HSV1 | 4.93 (1.25–19.48) | 0.023 | 6.51 (1.40–30.36) | 0.017 |
| SVBP during head-up tilt test b | ||||
| Male | 1.58 (1.01–2.51) | 0.049 | ||
| POTS c | ||||
| Pos. IgM for Candida | 15.00 (1.19–189.42) | 0.036 | ||
| Orthostatic hypotension d | ||||
| Hypotension at Supine | 5.56 (1.46–21.10) | 0.012 | ||
| Female | 2.08 (1.21–3.57) | 0.008 | ||
| Pos. IgG antibodies for EBV | 0.37 (0.16–0.84) | 0.017 | 0.30 (0.12–0.73) | 0.008 |
| Pos. IgG for SARS-CoV-2 | 3.74 (1.21–11.59) | 0.022 | 3.90 (1.15–13.21) | 0.029 |
| Hypertensive reaction (>130/90 mmHg) e | ||||
| EVBP during Supine | 6.57 (1.19–36.35) | 0.031 | ||
| Pos. IgG for Adenovirus | 0.28 (0.11–0.70) | 0.007 | 0.29 (0.11–0.77) | 0.008 |
| Pos. IgG for Helicobacter | 4.04 (1.01–16.24) | 0.049 | ||
| Extreme hypertensive reaction (>170/120 mmHg) f | ||||
| Male | 2.00 (1.11–3.62) | 0.021 | ||
| HTA at Supine | 6.19 (1.01–38.00) | 0.049 | ||
| Pos. IgG for Adenovirus | 0.16 (0.03–0.75) | 0.020 | 0.138 (0.03–0.74) | 0.021 |
| Pos. IgG for Helicobacter | 7.81 (1.64–37.16) | 0.010 | ||
| Pos. IgG for Toxoplasma | 5.67 (1.45–22.18) | 0.013 | ||
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Exp (B) (95% CI) | p | Exp (B) (95% CI) | p | |
| Positive head-up tilt test a | ||||
| Pos. IgG for Toxoplasma | 11.00 (1.34–90.62) | 0.026 | ||
| EVBP during head-up tilt test b | ||||
| Male | 3.82 (1.63–8.93) | 0.002 | ||
| POTS c | ||||
| Pos. IgM for Chlamydia Pneumoniae | 34.00 (1.52–760.85) | 0.26 | ||
| Orthostatic hypotension d | ||||
| Pos. IgM for Adenovirus | 4.62 (1.36–15.72) | 0.014 | ||
| Extreme hypertensive reaction (>170/120) e | ||||
| Pos. IgM for Candida Albicans | 22.33 (1.11–450.13) | 0.043 | ||
| Pos. IgG for Candida Albicans | 33.00 (2.96–368.36) | 0.005 | ||
| Pos. IgG for HHV6 | 12.60 (1.45–109.39) | 0.022 | 19.72 (1.04–373.10) | 0.047 |
| Pos. IgG for SARS-CoV-2 | 12.60 (1.45–109.39) | 0.022 | ||
| (a) Reference Category: Post COVID Syndrome * | |||||
|---|---|---|---|---|---|
| Parameter Estimate | |||||
| Group | Variable | p | Exp (B) | 95% CI | |
| CFS after COVID-19 | Neg. IgG for Adenovirus a | 0.017 | 0.22 | 0.07–0.77 | |
| Pos. IgG for Adenovirus | 0 * | ||||
| CFS with insidious onset | Neg. IgG for Coxsackiae virus b | 0.013 | 8.35 | 1.58–44.25 | |
| Pos. IgG for Coxsackiae virus | 0 * | ||||
| Neg. IgG for SARS-CoV-2 c | 0.026 | 6.00 | 1.24–28.97 | ||
| Pos. IgG for SARS-CoV-2 | 0 * | ||||
| Lyme disease | Neg. WB IgM for Borrelia spp. d | <0.001 | 0.001 | 0.01–0.08 | |
| Pos. WB IgM for Borrelia spp. | 0 * | ||||
| Neg. IgG for Chlamydia Pneumoniae e | 0.044 | 0.23 | 0.06–0.96 | ||
| Pos. IgG for Chlamydia Pneumoniae | 0 * | ||||
| Neg. IgG for VZV f | 0.018 | 9.97 | 1.48–67.40 | ||
| Pos. IgG for VZV | 0 * | ||||
| Neg. IgG for HSV1 g | <0.001 | 21.70 | 3.91–120.57 | ||
| Pos. IgG for HSV1 | 0 * | ||||
| Neg. IgG for SARS-CoV-2 | 0.029 | 13.15 | 1.31–131.91 | ||
| Pos. IgG for SARS-CoV-2 | 0 * | ||||
| (b) Reference Category: CFS After COVID-19 * | |||||
| Parameter Estimate | |||||
| Group | Variable | p | Exp (B) | 95% CI | |
| CFS with insidious onset | Neg. IgG for Chlamydia Pneumoniae a | 0.030 | 2.08 | 1.07–4.01 | |
| Pos. IgG for Chlamydia Pneumoniae | 0 * | ||||
| Neg. IgG for Candida Albicans b | 0.048 | 0.29 | 0.08–0.99 | ||
| Pos. IgG for Candida Albicans | 0 * | ||||
| Neg. IgG for SARS-CoV-2 c | 0.007 | 4.03 | 1.45–11.17 | ||
| Pos. IgG for SARS-CoV-2 | 0 * | ||||
| Lyme disease | Neg. Elisa IgM for Borrelia spp. d | <0.001 | 0.05 | 0.01–0.21 | |
| Pos. Elisa IgM for Borrelia spp. | 0 * | ||||
| Neg. Elisa IgG for Borrelia spp. e | 0.005 | 0.05 | 0.01–0.41 | ||
| Pos. Elisa IgG for Borrelia spp. | 0 * | ||||
| Neg. WB IgM for Borrelia spp. f | <0.001 | 0.01 | 0.01–0.06 | ||
| Pos. WB IgM for Borrelia spp. | 0 * | ||||
| Neg. WB IgG for Borrelia spp. g | <0.001 | 0.015 | 0.01–0.08 | ||
| Pos. WB IgG for Borrelia spp. | 0 * | ||||
| Neg. IgG for VZV h | 0.02 | 5.23 | 1.30–21.08 | ||
| Pos. IgG for VZV | 0 * | ||||
| Neg. IgG for HSV1 i | 0.004 | 6.93 | 1.85–25.97 | ||
| Pos. IgG for HSV1 | 0 * | ||||
| Neg. IgG for SARS-CoV-2 | 0.026 | 8.84 | 1.30–60.02 | ||
| Pos. IgG for SARS-CoV-2 | 0 * | ||||
| (c) Reference Category: CFS with Insidious Onset * | |||||
| Parameter Estimates | |||||
| Group | Variables | p | Exp (B) | 95% CI | |
| Lyme disease | Neg. Elisa IgM for Borrelia spp. a | <0.001 | 0.03 | 0.01–0.13 | |
| Pos. Elisa IgM for Borrelia spp. | 0 * | ||||
| Neg. Elisa IgG for Borrelia spp. b | 0.003 | 0.05 | 0.01–0.36 | ||
| Pos. Elisa IgG for Borrelia spp. | 0 * | ||||
| Neg. WB IgM for Borrelia spp. c | <0.001 | 0.01 | 0.01–0.06 | ||
| Pos. WB IgM for Borrelia spp. | 0 * | ||||
| Neg. WB IgG for Borrelia spp. d | <0.001 | 0.01 | 0.01–0.03 | ||
| Pos. WB IgG for Borrelia spp. | 0 * | ||||
| Neg. IgM for Coxsackiae virus e | 0.013 | 0.06 | 0.01–0.56 | ||
| Pos. IgM for Coxsackiae virus | 0 * | ||||
| Neg. IgG for Chlamydia Pneumoniae f | 0.020 | 0.25 | 0.08–0.80 | ||
| Pos. IgG for Chlamydia Pneumoniae | 0 * | ||||
| Negative IgG for VZV g | 0.001 | 9.28 | 2.39–36.12 | ||
| Pos. IgG for VZV | 0 * | ||||
| Negative IgG for HSV1 h | 0.006 | 6.07 | 1.66–22.17 | ||
| Pos. IgG for HSV1 | 0 * | ||||
| Neg. IgM for Bartonella Henselae i | 0.048 | 17.59 | 1.02–302.39 | ||
| Pos. IgM for Bartonella Henselae | 0 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanovic, B.; Markovic, N.; Petrovic, M.; Stojanovic, S.; Zugic, V.; Ostojic, M.; Bojic, M. The Relationship Between Hemodynamic Responses During Head-Up Tilt Testing and Parameters of Infection in Post-COVID Syndrome, Chronic Fatigue Syndrome, and Late-Stage Lyme Disease. Viruses 2025, 17, 1430. https://doi.org/10.3390/v17111430
Milovanovic B, Markovic N, Petrovic M, Stojanovic S, Zugic V, Ostojic M, Bojic M. The Relationship Between Hemodynamic Responses During Head-Up Tilt Testing and Parameters of Infection in Post-COVID Syndrome, Chronic Fatigue Syndrome, and Late-Stage Lyme Disease. Viruses. 2025; 17(11):1430. https://doi.org/10.3390/v17111430
Chicago/Turabian StyleMilovanovic, Branislav, Nikola Markovic, Masa Petrovic, Smiljana Stojanovic, Vasko Zugic, Milijana Ostojic, and Milovan Bojic. 2025. "The Relationship Between Hemodynamic Responses During Head-Up Tilt Testing and Parameters of Infection in Post-COVID Syndrome, Chronic Fatigue Syndrome, and Late-Stage Lyme Disease" Viruses 17, no. 11: 1430. https://doi.org/10.3390/v17111430
APA StyleMilovanovic, B., Markovic, N., Petrovic, M., Stojanovic, S., Zugic, V., Ostojic, M., & Bojic, M. (2025). The Relationship Between Hemodynamic Responses During Head-Up Tilt Testing and Parameters of Infection in Post-COVID Syndrome, Chronic Fatigue Syndrome, and Late-Stage Lyme Disease. Viruses, 17(11), 1430. https://doi.org/10.3390/v17111430






