Abstract
The focus of this study was to explore phage display systems employing bacteriophage lambda (λ) gene fusions to its capsid decoration protein gpD as reagent tools for tackling disease. The biological activity of gpD-fusions was examined by testing for the retained antimicrobial toxicity of cathelicidins or defensins fused to gpD. Our previous finding that only COOH fusions of either cathelicidins or defensins to gpD were toxigenic was expanded to show that only the reduced form of fused defensin antimicrobial polypeptides was found to be toxigenic. Compared in review are gene-fusion lytic display systems (where the fusion-display gene is integrated within the viral genome) with a surrogate system, employed herein, that exogenously provides the fusion-display protein for addition to phage capsid. It is easily possible to produce fully coated lambda display particles (LDP) serving as single epitope vaccines (SEV), or antimicrobials, or to produce partially coated LDP without any complex bacteriophage genetic engineering, making the system available to all. The potential to build vaccine vector phage particles (LDNAP) comprising essentially sheathed DNA vaccines encapsulated within an environmentally protective capsid is described. LDNAP are produced by introducing a cassette into the phage genome either by phage–plasmid recombination or cloning. The cassette carries a high-level eukaryotic expression promoter driving transcription of the vaccine candidate gene and is devoid of plasmid resistance elements.
1. Introduction
1.1. Phages
Phages are bacterial viruses with either double-stranded (ds) or single-stranded DNA or RNA genomes. Phage particles, representing a genome encased within a capsid of protein, represent the predominant form of genetic biomass on Earth. Those with ds DNA genomes are estimated at >1031 [1], or by simplistic analogy 20 billion particles per mm2 of the earth’s surface. Overall, many phage types represent an enormous and diverse gene pool constantly changin, attributable to recombinational events arising within an infected host cell, for example by exchanging parts (a) with prophage (or prophage fragments) integrated within the chromosome of the infected cell, (b) with extrachromosomal elements (as plasmids or phage genomes) within the host cell, or (c) with coinfecting phage genomes injected in parallel into the host cell. Phage can encode genetic traits (lysogenic traits) that allow the phage genome to stably coexist within its host cell without killing it (i.e., the temperate phage). These temperate phage genomes undergo a decision, upon entering a host cell, to either stably associate within the host or undergo a lytic cycle to kill the host and release progeny as phage particles. Other phage types (the lytic phage) lack lysogenic traits. They may encode highly toxic substances causing death/lysis of the infected cell and only undergo a lytic cycle. Humans have evolved in the constant presence of this biomass and yet there is no known human disease linked to direct phage exposure, even though phages clearly play a role in the evolution of bacterial pathogens [2,3,4,5,6,7,8].
1.2. Use of Phages as Therapeutic Agents
Basic approaches include phage therapy (PT) and phage display (PD). The rationale for PT assumes (a) that predator phages (perhaps a cocktail of different infectious types) that infect a form of pathogenic bacterium can be selected and purified from natural sources (e.g., from sewage) and concentrated, and (b) a cocktail of phages (with differing infective mechanisms) can be introduced within a complex milieu (animal infected by pathogenic bacterium) and effect a reduction or elimination of the pathogen. PT was first employed in 1919, shortly after the discovery of bacteriophages [9,10,11,12,13,14,15,16,17,18], and a few recent strategies for PT in wound care and infection, which is beyond the scope of this manuscript, may be explored further in references [19,20,21,22]. PD requires strategies for fusing the coding region for a protein, polypeptide, or peptide with a phage surface capsid gene and then displaying the hybrid protein on a virus capsid. The display of peptides and proteins on the capsids of filamentous or lytic phages is a powerful technique for the identification or targeting of specific ligands and can be adapted to generate vaccine reagents [23,24,25,26,27]. Gupta et al. [24] have suggested the use of display vehicles for targeted gene delivery, in vivo diagnostics, isolation of tissue-specific peptides, tissue imaging agents (in vitro and in vivo), cell targeting [28], studying the specificities of immune responses of patients with various diseases, cancer therapy, autoimmune disorders and age-related conditions, design of diagnostic assays and therapeutic vaccines, as bio-detectors for a variety of threat agents (viruses, bacteria, spores, and toxins), as drug and vaccine delivery to specific cells, and for applications involving the use of surface display vehicles as biocatalysts.
1.3. Phage Display (PD) Systems
In PD the fused polypeptide, perhaps with activity as a ligand, or antigen, can theoretically be designed to join either the NH2- or COOH- terminal ends of a capsid protein. Display density refers to the extent to which the fused capsid protein can be added to the capsid. Virus particles with only one or a few gene-fusion display proteins per capsid have low display density, whereas particles displaying perhaps hundreds of fusion proteins have high display density; thus, display density can vary from less than one per collection of particles to greater than hundreds per particle. Display densities as low as one or fewer per particle are important in isolating binder particles with very high affinities for a target ligand; displays of three to ten copies of the fusion-display protein are sufficient to allow isolation of binders to bait when the strength of interaction is in nanomolar to micromolar range. However, for the study of weaker interactions (micromolar to millimolar range), the display needs to be on the order of a few hundred fusion proteins per particle [24]. “Landscape vehicles” [24], i.e., those which have a very dense display of the candidate peptide on the capsid surface of the phage particle, may be better immunogens for use in live vaccine development and as vehicles for biocatalysis and bioadsorption.
The two main types of PD systems are filamentous and lytic as reviewed in [29]. Filamentous PD was introduced by Smith in 1985 with the typically low density display of peptides fused to one of the low copy capsid proteins at the ends of linear filamentous phage, e.g., M13 [30]. This method of PD, referenced many thousands of times, and with numerous clever variations for either cloning the fusion protein within the vector genome or the use of surrogate systems to produce the fusion protein, is based on M13 and related filamentous phages that are released from infected cells by extrusion of phage particles through the cell wall without producing cell lysis. An M13-encoded fusion capsid protein must traverse the bacterial cytoplasmic membrane and then be assembled on the phage coat/capsid within the periplasm. This can hinder the display of highly hydrophilic proteins. In contrast, lytic PD employs either temperate phages, as λ (the focus of this study), or true lytic phages as T4 [27,31,32,33,34] and T7 [35] that release (burst) from infected cells by lysing them in terminal stage of the phage growth cycle. Phage particles released by cell lysis can theoretically display hundreds of capsid fusion proteins per particle and in some systems the capsid fusion display protein, prepared separately, can be added to a procapsid structure in vitro, e.g., both the hoc and soc genes encoding T4 capsid proteins are dispensable and can be added exogenously [31,32,33,36,37].
The display of peptides and proteins on the capsids of filamentous or lytic phages is a powerful technique for the identification or targeting of specific ligands and can be adapted to generate vaccine reagents [23,24,25,26,27].
1.4. λPD
During phage lambda (λ) morphogenesis its genomic ds DNA is packaged into a preformed prohead which expands during packaging, exposing sites for increased binding of the capsid head decoration protein gpD (11.4 kDa) to the underlying molecules of protein gpE making up the prohead [38,39,40]. In the process of prohead expansion into a stable mature λ head, about 420 gpD monomers are enzymatically added to the prohead via conversion into 140 gpD3 trimers, which are affixed to 20 icosahedral faces of the mature particle. The gpD3 trimer was shown to stabilize the capsid head shell during packaging of the lambda genome [41,42]. The addition of gpD to the prohead is dispensable when the 48.5 Kb λ genome includes a deletion of at least 18% [43]. Cryo-electron microscopy and image reconstruction have shown that the side of the gpD3 trimer that binds to the capsid is the side on which both the NH2- and COOH- terminal ends reside. Despite this orientation of the gpD trimer, fusion proteins connected by linker peptides to either terminus bind to the capsid, allowing protein and peptide display [44].
While fusions to gpV, the tail tube protein have been reported [45,46], most λ PD reports involve engineered recombination into the λ genome fusing the coding region of a foreign protein/peptide/polypeptide (<) with gene D, making D< [47,48,49,50,51,52,53,54]. Each of these studies anticipate the formation of a viable phage where the chimeric gpD< protein retains gpD functionality and “<“ has been fused either to the NH2- or COOH- terminal ends of gpD [55], or internally within the D coding sequence [46]. For gpD< to function similarly to gpD it should not interfere with (i) the interaction of gpD or gpD< monomers to form a trimer or (ii) the incorporation of gpD3 or gpD<3 trimers on the surface of the λ particle capsid head. If the binding of gpD< is challenged, the expansion of the head is blocked, negating full packaging of the lambda genome, stabilization of lambda particles, and the formation of viable phage particles. Diverse proteins have been fused at the N- or C-terminal ends of gpD, including β-galactosidase protein [55], Cry1Ac toxin from Bacillus thuringiensis [56], and the ligand-binding domain of the human peroxisome proliferator-activated receptor gamma [LBDPPARγ] [57]. In the latter example, the solubility of the LBD of PPARγ, which is very prone to protein aggregation, was dramatically increased, indicating that generating a gpD-fusion may improve the solubility of proteins which normally clump and precipitate in E. coli. (See [58] on using lambda display as a powerful tool for antigen discovery). It is likely that the extent to which genomically encoded gpD< fusion proteins can form gpD<3 trimers on lambda capsid heads is limited/varies [59,60]. The numbers of fusion proteins displayed by λ appear somewhat size-dependent: small peptides of about 10 residues were present at as many as 405 copies per particle, with lower display densities seen for larger gpD< fusions; however, overall, phage display on lambda was reported two to three orders of magnitude higher than was displayed by pIII and pVIII fusions on M13 [59].
From the late 1970s the mechanism for packaging of phage λ DNA was beginning to be understood, including recognizing the components of the phage terminase that cleaved λ DNA at its cos site needed for initiating phage DNA packaging into the procapsid. This information led to strategies for packaging λ DNA in vitro [61,62,63,64,65], permitting the manipulation and packaging of genetically engineered λ DNA into mature phage particles. Ansuini et al., 2002 [47] engineered a λ phage with two copies of D for the purpose of circumventing the presence of having only a single marginally functional gpD< fusion product available for phage display, by provisionally being able to supplement capsid formation dependent on gpD< with wild-type gpD. They developed a clever licensed vector λD-bio containing two copies of capsid gene D. One copy of D included a suppressible nonsense (amber) mutation, where gpD would only be produced when the phage was grown in E. coli cells encoding a nonsense suppressor tRNA, and the gpD produced would only be changed from wild-type gpD by the substitution of the suppressor amino acid at the site of the nonsense mutation. The λD-bio contained a second D encoding the gpD< fusion gene. This gpD fusion gene included after its D coding sequence the in-frame cloning sites SpeI and NotI with an included ochre termination codon followed by a 13-amino-acid sequence recognized by the biotin holoenzyme synthetase BirA, and then a second termination signal. Cloning of out-of-frame DNA fragments between SpeI and NotI nullified synthesis of the downstream peptide recognized by BirA, whereas selection for clones with open reading frames inserted between SpeI and NotI was made possible by streptavidin affinity chromatography binding BirA. This system and its derivatives [46,66,67] enabled phage display by co-expression of functional gpD and gpD< gene fusions; however, the recombinant phage tended to accumulate mutations able to block gpD< fusion protein expression, resulting in better phage particle production [46] by omitting synthesis of the gpD fusion. Recalcitrant gene fusion combinations of gpD< blocking phage display were identified to be incompatible with the assembly of stable phage particles [68,69], yet the recalcitrant property of gpD< was found suppressible, permitting its display, if a surrogate plasmid system that simultaneously produced gpD was added.
Heat-induced λ capsid disassembly yields only soluble gpD monomers, not gpD trimers, suggesting the trimer rapidly dissociates when not attached to the λ head [70] and gpD remains soluble (does not aggregate) in high molar concentrations [44]. In contrast, in similar treatment, the major capsid protein gpE is easily precipitated and likely exists as aggregates [70]. Data from phage display by co-expression of functional gpD and gpD< gene fusions tend to suggest that gpD2gpD< or gpD<2gpD, or gpD<3 trimers can likely form on the λ head [46]. In vitro complementation has been employed to determine if N-terminal or C-terminal gpD protein fusions will add to a lambda capsid head devoid of gpD. In this system, 108 purified λ Dam15 phage particles (devoid of gpD), with 79.5% of the wild-type genome size, are mixed with purified gpD or gpD fusion proteins, to permit addition to the capsid, and then subsequently treated with EDTA (results in expansion of the phage heads devoid of gpD), chelating away Mg2+. The particle reaction mixture is then mixed with E. coli cells able to suppress the Dam15 mutation and resulting phage plaques are counted. Just about any type of N- or C-terminal gpD fusion has been shown to complement for gpD in this assay [44,46], where “complementation refers to rendering the phage resistant against EDTA treatment by binding the protein in question to the [purified] λD− phage” particles [44]. Though I may be missing something, these data omitted that the purified λ Dam15 phage particles (devoid of gpD) with 79.5% λ genomes are already resistant to EDTA, and when plated on an E. coli, suppressor strain will form viable phage plaques (whether or not they were pretreated with EDTA). The critical argument for complementation was that treatments with gpD deleted for 14 N-terminal amino acids complement very poorly. However, this could also mean that the NΔ14-gpD protein negatively complements in a reaction that does not require any gpD.
2. Materials and Methods
2.1. Microbiological Techniques
The methods utilized can also be found described in detail in the Section 2 of [71,72,73,74,75,76]. Cultures and phage lysates were prepared in Lysogeny Broth (LB) (10 g Bacto tryptone, 5 g Bacto yeast extract, 5 g NaCl per liter), and made 50 ug/mL with ampicillin. The bacterial and bacteriophage strains employed are described in [71], except for strain C3026H available from New England Biolabs, with reported genotype F’ lac, pro, lacIq/Δ(ara-leu)7697 araD139 fhuA2 lacZ::T7 gene1 Δ(phoA)PvuII phoR ahpC* galE (or U) galK λatt::pNEB3-r1-cDsbC (SpecR, lacIq) ΔtrxB rpsL150(StrR) Δgor Δ(malF)3. What is also described is the following: in vivo infection-complementation methodology/assays using transformed cells, considerations relating to complementation versus reversion assays, versus D-amber marker rescue, and considerations relative to assay temperature for complementation assays.
2.2. Phage Lysate Preparations Methodology for Preparing gpD< Display Phages
2.2.1. Single Phage Infections
Cells (grown overnight to stationary phase) were pelleted and resuspended in TM buffer [0.01 M Tris, 0.01M MgCl2, pH 7.8]), mixed and incubated 30 min at 35 °C at MOI of 0.04 with λimm434cI#5Dam123 (reversion frequency = 4.2 × 10−6). The mixtures were then pipetted into LB-Amp50 medium (held at the intended lysate incubation temperature) made 0.001M in MgCl2, and 0.01M in Tris, pH 7.8, and shaken until culture lysis in a heated water bath.
2.2.2. Trivalent Infections
This comprised a mix (0.34 mL of each) of the three expression cell types grown at 30 °C overnight, pelleted, concentrated two-fold, and suspended in TM buffer. Each separate cell aliquot was infected at MOI of 0.04 with λimm434cI#5Dam123, held at 35 °C for 30 min, and the mixture pipetted into a liter of LB broth (done in duplicate, yielding two liters of lysate). The cell titers for the culture cells were determined simultaneously as follows: 594[gpD-RBD#1] (1.14 × 109 cfu/mL), 594[gpD-RBD#2] (5.95 × 108 cfu/mL), and 594[gpD-RBD#3] (fusion of Dcoe at C-terminal to spacer and 11 amino acids (#496–506) from 2019-nCoV capsid receptor binding domain) at 1.38 × 109 cfu/mL.
2.2.3. Single Burst Infections
Single colonies of the host cells are inoculated into four two-liter flasks, each with one-liter LB-Amp50 culture medium. The cultures are incubated with shaking to reach stationary phase and the cells from each flask are pelleted and resuspended in 1/10th volume of fresh LB-Amp50 medium (about 1.5 × 1010 colony-forming units (CFU) per ml). The new cultures are incubated with shaking at 30 °C for 30 min and then infected with λimm434cI#5 at a multiplicity of infection of 3 to 5. The phage cell mixture is held at room temperature for 15–30 min without shaking. Then, the infected cells are transferred into four six-liter flasks, each with two liters of LB-Amp50 culture medium prewarmed to 39 °C. Raising the cell temperature from 30° to 39 °C permits expression of the D-fusion gene from the plasmids present in the cells. The culture absorbance is monitored at A575nm for 60–90 min, and when it drops to about 0.05 the flasks are moved from shaker to a cold room, the cells are pelleted, and the pellets are discarded. Of note, normal phage lysates are made by a two-step procedure, where initial culture cells outnumber infecting phage by ten-or-more fold. However, in preparing display phage, an objective is to avoid (as much a possible) released display phage from reinfecting growing cells and consequently being lost in the lysate by having the phage capsid affixed to the outside of the growing cells.
2.3. Banding Phage in CsCl
The supernatant from a cell lysate (after pelleting cell debris) is pooled into a carboy, and solid polyethylene glycol (PEG, average molecular weight 6000–7000) is added to 4% along with solid NaCl to 0.5M. The additions are shaken into solution and the container is held overnight at about 4 °C. The treated lysate is spun at 8000 revolutions per minute for 15 to 20 min in a 6-liter capacity JA 9.1000 Avanti rotor and the supernatant is discarded. The pellets are each resuspended at 4 °C in about 1 mL phage buffer, adjusted to a refractive index of 1.382 with solid CsCl, and spun at 30,000 in a Ti70 ultracentrifuge rotor (Coulter-Beckman) overnight at 4 °C. The collected bands are pooled and re-banded in a Ti60 rotor. The banded phages used for protein gel analysis or other immunological assays are dialyzed in phage dialysis buffer (0.3 to 0.5M NaCl, 0.01M Tris, 0.05M MgCl2, pH 7.8) and then twice against 3000× volume of PBS (0.0036M KCl, 0.0014M KH2PO4, 0.136 M NaCl, 0.004MNa2HPO4, pH 7.4). When only one liter (or less) of a lysate from each infection in needed the PEG step is omitted, lysate is subjected at differential centrifugation, first at 8000 rpm to remove remaining nonlysed cells and large cell debris and then spun in large polycarbonate tubes in a 60Ti rotor (Coulter-Beckman) for 24 hrs at 30,000 rpm to pellet phage (without PEG). The pellets are resuspended by covering them with 0.5 mL of buffer (0.3 NaCl, 0.01M Tris, 0.01M MgCl2, pH 7.8), allowing them to soften overnight at 4 °C. The resuspended pellets are adjusted with solid CsCl to a refractive index of 1.382 and the phages are banded in an ultracentrifuge as described above. Any remaining free protein in the phage pellet is excluded from the gradient and forms a thin band above the CsCl solution.
2.4. Determining the Toxicity of the gpD-Fusion Constructs by Measuring EOP
The toxicity of gpD< fusion protein expression from pcIpR[GOI]-timm plasmids is readily determined by growing transformed E. coli cultures at 30 °C, then diluting and spotting aliquots on LB or LB-Amp50 agar plates, and incubating the inverted plates in incubators with temperatures held at, e.g., at 30, 35, 37, 38, 39, 41, or 42 °C. The EOP represents the average number (of four or five aliquots) of colonies formed per plate per applied aliquot spot, divided by that obtained for parallel assay plates incubated at 30 °C. Some of the gpD< proteins expressed at 42 °C are highly toxic (EOP’s of <0.0001), compared to cell growth at 30 °C (no gpD< expression), base EOP = 1.0.
2.5. Plaque and Colony PCR Techniques
A phage lysate or cell aliquot is stripped out on an agar overlay plate with fresh host cells, or a culture aliquot is streaked onto agar plates to obtain isolated plaques or colonies. Either a single plaque is removed using a sterile wide-tipped (Aardvark) micropipette tip or a tiny amount of a fresh colony is picked (a barely visible amount) with a sterile 10 μL micropipette tip. The plaque material, or bit of the single colony is transferred into 50 μL TE* buffer (10 mM Tris + 0.1 mM Na2EDTA) in a 0.5 mL microcentrifuge tube. The solution is gently mixed and then incubated at 37 °C for 1 h. The tube with sample is heated in a thermocycler at 96 °C for five minutes to disrupt contents and then centrifuged for one minute at 12K rpm in Eppendorf microcentrifuge to pellet any debris. An amount of 10 μL of the supernatant is mixed with 10X PCR buffer (if it does not contain MgCl2, 3 μL of 50 mM, MgCl2 wasis added) along with 16 μL of a 1.25 mM solution of the four deoxynucleotide triphosphates, plus 100 picomoles of each of the 2 primers, and sufficient sterile HO to make 100 μL total, plus 0.5 μL of Taq DNA polymerase at 5 units/μL. (For 50 μL reactions decrease amounts by half). The tubes are placed in a thermocycler and run for 25 cycles of denaturation/annealing/primer extension. Many of the phage- and colony-handling genetic techniques are described in [76]. DNA preparations from either plaques, plasmid-transformed colonies, or DNA bands (extracted from acrylamide gels) were submitted to DNA sequencing services and the sequences obtained from four to six representative clones of plaques, colonies, or gel bands were scrutinized for accuracy before the phage, plasmid, or colony was used in an experiment.
2.6. Nonradioactive DNA Probe Labeling and Hybridization to Identify Recombinant Phage Plaques
The notched nitrocellulose blots showing plaque recombinants hybridizing to an EGFP probe were placed over the original blotted agar overlay plate. The regions with dark spots were pierced with a large bore needle, then the soft agar overlay region under the pierced spot was sucked out with a large bore hypodermic needle (or/followed with Aardvark large bore plastic micropipette tip). The tiny contents were transferred to 0.3 mL phage buffer (0.01M Tris HCl, pH 7,8, 0.01M NaCl, 0.001M MgCl2), in a 1.5 mL micro centrifuge tube, incubated 30 min, pelleted 1 min at 12K rpm in an Eppendorf micro centrifuge, and aliquots of the supernatant were stripped or overlaid on agar overlay plates with fresh E. coli host cells. Individual plaques arising were picked again into 0.5 mL phage buffer, and 0.1 mL aliquots were mixed with 2.5 mL top agar with 0.1 mL of fresh E. coli host cells poured over fresh LB-Amp50 agar plates. These plates were then blotted with nitrocellulose filters and hybridized with a DNA hybridization probe. Some single plaque isolates (membrane on right side) showed that all the plaques derived from a single plaque isolate included the recombinant EGFP cassette.
In these assays, phage plaques (with released DNA) on agar overlay plates were blotted to nitrocellulose filters and then hybridized with digoxigenin (DIG)-tagged EGFP PCR DNA. We used the DIG System for nonradioactive labeling and detection of nucleic acids. The randon-primed DNA probe labeling produced digoxigen-dUTP tagged EGFP DNA probe. It is bound during hybridization to plaque DNA, bound to the nitrocellulose membrane, and the bound probe is detetected as a purple color precipitate linked to anti-DIG alkaline phosphatase conjugate antibodies. (All DIG reagents for probe labeling and detection of hybridization of labeled probe to membrane were obtained as a commercially available kit from Roche Applied Sciences, #11093657910).
2.7. E. coli Strains, Phages, and Plasmids Employed
The plasmids utilized herein are described in Table 1. The sequence and details regarding the backbone prokaryotic expression plasmid pcIpR-timm = pcIpR[GOI]-timm from which all the gpD< fusions were made is described in detail in [73]. Considerations related to making the eukaryotic expression plasmids by this laboratory, borrowing from pMZS3F, a mammalian expression vector, (6309 bp) [77] received from J.F. Greenblatt, University of Toronto (which contains several parts derived from pCI-neo (Promega)), are described in the Supplement: Plasmid Construction Example.

Table 1.
E. coli K12 and bacteriophage λ strains and plasmids utilized.
2.8. Genetic Techniques
Each of the plasmids constructed were made ordering Gene-blocks of the designed sequences from Integrated DNA Technologies (IDT), Coralville, ID, or were prepared by PCR amplifications employing primer combinations that included requisite terminal restriction site(s). These were also received from IDT. Recombinant plasmid preparations were transformed into E. coli [85] and then individual colonies of the unique isolates (usually about five) were subjected to colony PCR and the results for each clonal isolate were sent for DNA sequence analysis. Each of the isolated plasmid constructs from clones employed were verified for DNA sequence integrity. The techniques used for colony PCR, plaque PCR, DNA sequence analysis, and acrylamide gel electrophoresis were previously described [76,77,85]. The mutation imm434cI#5 in phage λimm434cI#5 (defective in lysogeny) was recombined into λimm434Dam123 (capable of lysogeny) and single plaque lysates of the recombinant phage λimm434cI#5Dam123 (A2a, A2b, G1a, G1b), each defective for lysogeny, were employed in future infections (Figure 5 tubes 3–6) to improve lysate titer.
2.9. Protein Gels and Western Blots
The SDS denaturing gels and staining methods used for protein analysis steadily improved during course of this study. An early 12% acrylamide SDS denaturing gel, running out an E. coli protein extract was stained with Coomassie Blue. The remaining protein gels employ Invitrogen Novex Pre-cast 10–20% Tricine-SDS slab gels (Thermo Scientific) run about one hour at 130 V. The gels were stained with Bio-Rad Oriole fluorescent gel stain and copied in a Bio-Rad Chem Doc using UV light excitation specific for Oriole stain, which proved both easier, more sensitive, and more reliable than an attempted silver stain (SilverQuest Silver Staining Kit) in our hands. The standards employed included Bio-Rad Precision Plus Protein Standards (10 to 250 kDa) and ThermoScientific Low Range Protein Ladder (2, 6, 10, 15, 25, 40 kDa). The gels were electrophoretically blotted overnight at 4 °C to PVDF membrane using a Bio-Rad transfer cell, and then blocked and incubated with primary anti-His-gpD mouse antibody (1:1000) [72], rinsed, incubated with secondary rabbit anti-mouse IgG (1:1000) conjugated with horseradish peroxidase, and developed with Bio-Rad enhanced chemiluminescence (ECL, luminol and H2O2) exactly as described (Protein Gels and Western blots, [73]). The developed gel blots reacting with His-gpD mouse antibody were recorded using Bio-Rad Chem Doc. Note that the Western blot for expression of gpD-CAP gene fusion from pcIpR-D-CAP-timm plasmid shows the gpD-CAP fusion protein expressed in E. coli cells reacting with anti-Porcine Circovirus 2 polyclonal antisera obtained from a gnotobiotic pig, using procedures described in [74,75].
2.10. Fluorescence Assays for Expressed gpD-GFPuv
Cultures were inoculated from single CFU of 594 into LB and 594 E.coli [pcIpR-D-GFP-timm] into LB made 50 ug/mL with ampicillin and grown overnight (ON) at 30 °C. Larger parallel subcultures (150 mL LB) were made by inoculating 7 mL from each ON culture to LB and then shaken at 30 °C until A575 = 0.2, whereupon they were moved into shaking water baths at 37, 39, or 42 °C. Aliquots were withdrawn prior to transfer and at 20 through 180 min post-induction (3 mL to measure absorbance and 5 mL was transferred and held on ice). Following the last sample removal, all the aliquots were centrifuged at 8000 rpm for five min, decanted, and the cell pellets were resuspended in 5 mL of 1X PBS (10X = 80 g NaCl, 2 g KCl, 14.4 g Na2HPO4.2H2O, 2.4 g KH2PO4 per liter distilled H2O, pH 7.4), pelleted, and resuspended in 1 mL PBS (to 5-fold concentration). Three aliquots (100 μL) of each of the resuspended cell pellets were assayed for fluorescence using a Photon Technology International, Model LPS 100, Serial No:0177, MFG 01/11) spectrophotometer. The excitation wavelength was set at 395 nm and emission fluorescence measured at 509 nm. Triplicate assays for each time point were made and the results averaged. Values for plasmid cells represent the average of triplicate assays subtracting the triplicate average for cultures of 594 transferred in parallel to 37, 39, or 42 °C. (We appreciate that the fluorescence of cells grown in minimal medium is lower, but the fluorescent assays for cells grown in LB were undertaken to parallel with the other assay procedures employed herein). To prepare cell lysates, the cell pellets in 1 mL PBS were pelleted at 4500 rpm for 10 min and the supernatant was decanted. The pellet was resuspended in 0.15 mL lysing mix (minus EDTA) made by adding 1.5 mL 1M Tris.HCl, pH7.8, 45 mL 1M sucrose, 44.7 mL of distilled H2O, and 0.8 mL of 10 mg/mL solution of lysozyme made in 10 mM Tris.HCl, pH 7.8. The cell suspension was incubated on ice for 30 min and the spheroplasts pelleted at 4500 rpm for 10 min and supernatant decanted. The pellet was suspended in 1 mL of 0.01M Tris.HCl, pH 7.8, and allowed to sit 15 min at ambient temperature (~21 °C). The cell lysate was transferred into another tube and three 100 uL aliquots were measured for GFP activity as for the intact cell preps.
3. Results and Discussion
3.1. In Vivo Complementation Assay for gp< Activity
In testing whether gpD< fusions will add to head formation in the presence of gpD, or can complement a deficiency in gpD, it is essential to undertake in vivo complementation assays where the possibility for negative complementation by gpD< is examined. These assays are easily accomplished by (a) assessing the efficiency of plating by gpD+ λ phage on cells where gpD< is being expressed in parallel from a surrogate plasmid, and (b) measuring complementation by surrogate expression of gpD< in non-suppressing (nonpermissive) cells infected with λDam phage, and in parallel, measuring the reversion frequency of λDam to λD+ as a control, found to be less than 10−5. Phage particles fully coated with gpD< (where gpD< = gpD-YML in Figure 1A (lanes for λcI857Dam123 and λimm434Dam123 infecting 594[pcIpR-Dcoe-YML-timm] cells) and Figure 1B (lane 7) are recovered whenever the transformed cells expressing a functional gpD< from a surrogate plasmid are infected with D-defective phage. Lanes 7 and 8 are the same as shown in (Figure 6 of reference [72]). In the examples (Figure 1A,B) the host cells are infected at 39 °C where the CI[Ts]857 repressor encoded by the pcIpR-Dcoe-YML-timm plasmid is thermally inactivated, permitting both phages to grow vegetatively. Of note, the imm434 infectin phage is not sensitive to repression by the λ immλ CI[Ts]857 repressor encoded on the pcIpR-Dcoe-YMY-timm plasmid. In an in vivo assay the influence of gpD< expression on culture viability, i.e., its intra-cellular toxicity is easily examined, as is the relative display density of gpD< on phage particles generated by complementation. The Western blot in Figure 1B and in a later figure shows that our His-gpD antibody preps bind well with gpD but bind very poorly with the gpD-YML* band.
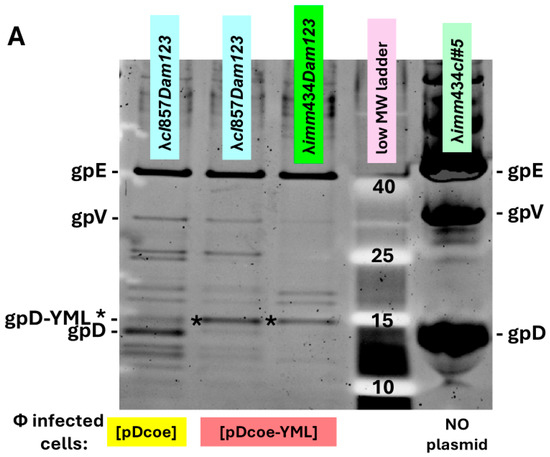
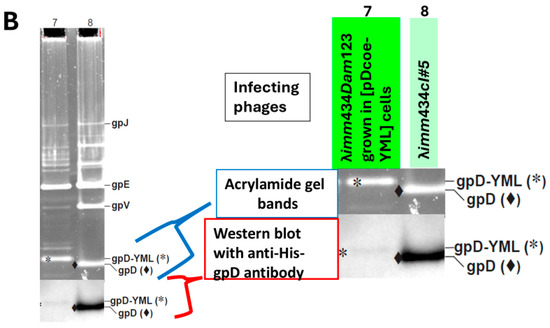
Figure 1.
Comparison of gpD and gpD< [gpD-YML] from purified phage particles. Phage particles from separate infections were concentrated by differential centrifugation (first to remove cell debris, second after PEG addition, then collecting and resuspending the pellet). Following CsCl banding (Methods 2.3) and dialysis of banded phage particles, the preps were submitted to denaturing acrylamide gel electrophoresis. The left gel lane in (A), and lane 7 in (B) show phage particles coated with gpD. The gpD band is lighter than the gpD-YML* band because gpD-YML has 17 more amino acids than gpD (i.e., a the spacer-GGSGAP joined to 11 amino acids GYMLGSAMSRP representing the disease-specific epitope, termed YML, from the sequence of cervid prion pRp protein [73]). (A,B) represent 10–20% SDS acrylamide gels stained and subjected to Western analysis as described in Methods (Methods 2.9). The lower Western blot (B) employed anti-His-gpD mouse antibody with chemiluminescence detection of secondary rabbit anti-mouse IgG (HRP conjugated).
The in vivo complementation assay sensitively detects when gpD< proteins provided by the surrogate plasmid are recalcitrant in suppressing the Dam defect coded by the infecting phage (Figure 2). Nonpermissive cells with a surrogate plasmid expressing gpD< with a 16-aminoacid N-terminal addition (NH2-YML-Dcoe-COOH = MGYMLGSAMSRP-GSGQspacer-gpD) failed to complement (absence of phage plaques) the infecting Dam phage with near wild-type genome size. In contrast, similar expression of either gpD (wild type) or gpDcoe (codon optimized) where the YML epitope is fused to the C-terminal end of gpD (=gpD-GGSGAPspacer-GYMLGSAMSRP both complemented well, permitting plaque formation by the Dam test phage.
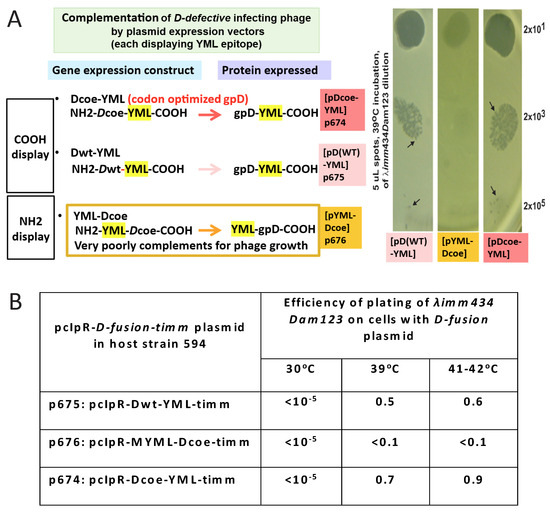
Figure 2.
In vivo complementation assay for either N- or C-terminal gpD< fusions ability to substitute for gpD defect on infecting phage. Complementation was evaluated for plasmids that express three different D-fusions. The results for 594[pcIpR-Dcoe-timm] and 594[pcIpR-D wild type-timm] are not shown but are identical to the results shown for 594[pcIpR-Dcoe-YML-timm] = strain 594[p674]. Each of the D-fusions include an 11-amino-acid (GYMLGSAMSRP, termed YML) single epitope from the sequence of the cervid PrP protein [73]. Plaque formation represents positive complementation, indicating that the expressed gpD< in the infected cells can substitute for the null mutation in gene D on the infecting phage’s genome. (A) The nonpermissive E. coli strain 594 was transformed with plasmids p674: pcIpR-Dcoe-YML-timm expressing gpD-spacer[GGSGAP]-YML; p675: pcIpR-Dwt-YML-timm expressing gpD-spacer [GGSGAP]-YML epitope; and p676: pcIpR-MYML-Dcoe-timm, expressing M-YML-spacer [GSGQ]-gpD. The plating results on 594[p613]=pcIpR-Dcoe-timm expressing only gpD are not shown but they were identical to those shown for 594[p674] and 594[p675]. Part A is revision of Figure 5 in reference [72]. (B) Efficiency of plating λ imm434 Dam123 on cells blocked (30 °C) or derepressed for gpD-YML fusion protein constructs. In parallel, the assessed complementation by 594[p676] of λ Dam123 was less than 10−5 at 30, 39, and 41 °C. (Note that λ imm434 Dam123 is insensitive to the imm λ-CI[TS]857 repressor encoded by the plasmids and would be complemented if the p674 or p675 plasmids could express the gpD< fusion at 30 °C.) The efficiency of plating of phage λ imm434 Dam123 was determined on various host strains with gpD< expressing plasmids by diluting the phage stock 0.5, 10−1, 10−2, and 10−3 and spotting 5 μL aliquots (i.e., 1/200th of phage lysate dilutions) in quadruplicate on agar overlay plates [2.5 mL top agar plus 0.1 mL of stationary phase overnight plasmid transformed cell culture (at about 2 × 109 cells/mL) poured over solidified 30 mL LB-Amp50 agar plates]. After the spots dried (about 15 min) on the overlay plates, they were inverted and incubated in separate incubators held at the assay temperature(s) for 24 h. The plaque forming units per spot per dilution per incubated agar plate were counted and averaged. The efficiency of plating represented the highest average titer per quadruplicate dilution spots giving well separate plaques divided by the original titer of the λ imm434 Dam123 phage lysate determined on an amber-suppressing E. coli host, i.e., on TC600 supE cells. The arrows indicate equivalent names for plasmid-encoded gpD-fusion constructs transformed into E. coli cells. The complementation assays are color coded: all assays represent one lambda Dam phage spotted on agar plates overlaid with cells transformed, and expressing, the different -color-coded- plasmids.
The synthetic plasmid pcIpR-GOI-timm expression vector [74] positions the cloned gene of interest (GOI) in the position of λ gene cro (on the phage) and is placed just upstream from a powerful λ terminator tImm and downstream from λ promoter pR (regulated by the plasmid-encoded temperature sensitive repressor CI[Ts]857). Numerous variations of this expression plasmid have been employed [73,74,75,76,85,86] to vary thermally the expression of the cloned GOI from fully off (culture incubation temperatures 25–30 °C) to fully expressed (at 42 °C). When cells with this plasmid are grown at 25 or 30 °C the plasmid-encoded CI repressor can prevent the expression of extremely toxic proteins, peptides, or gpD fusions [72,85]. The utility of expressing gene fusions with gpD is that the fusion protein does not aggregate within the cells or on purification. An example is illustrated in Figure 3 with the 207 amino acid fusion between gpD and epitopes from the Porcine Circovirus 2 capsid protein. We have seen similar results (no aggregation at high concentration) for the 354 amino acid gpD-GFP and similar large fusion proteins.
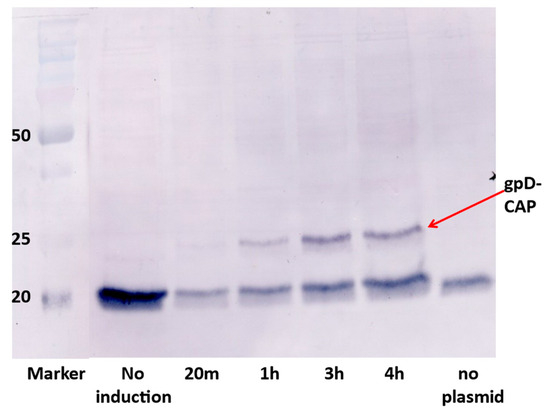
Figure 3.
Expression of gpD-CAP gene fusion from pcIpR-D-CAP-timm plasmid in E.coli W3350 cells upon shifting cells growing at 30 °C to 42 °C. The gpD-CAP fusion protein represents gpD fused to short spacer (6 amino acids) and joined to 92 amino acids including 4 epitopes from the Porcine Circovirus 2 capsid protein (CAP). E. coli cell extracts from each induction time were separated by 12% SDS PAGE. The gel blot was reacted with anti-Porcine Circovirus 2 (PCV2) polyclonal obtained from gnotobiotic pigs (see Methods), as described in detail in [74,75]. The lower band(s) of about 20 kD represents an unidentified protein made by E. coli that cross-reacts with polyclonal antibody to PCV2.
Thermal regulation of the GOI encoded by plasmid pcIpR-GOI-timm is varied by shifting the transformed cells growing at 30 °C to higher temperatures, as shown in Table 2.

Table 2.
Temperature dependence of thermally inducible intra-cellular gpD-GFP fusion expression from plasmid pcIpR-D-GFPuv-timm [flurorescence units × 10−3].
3.2. Dual Display
Once it was realized that both gpD and gpD< can be combined on a lambda particle and that in the absence of gpD some gpD< fusions are incompatible with the assembly of stable phage particles [69] it was proposed that “dual” gpD expression systems [74] that eliminated the complex cloning of recombinant D-fusions into the lambda vector genome, or into a lambda vector with both D and Dam genes, could be advantaged. The D-fusion could be induced and expressed from a surrogate plasmid in E. coli cells while simultaneously infecting the cells either with λD+ or λDam phage. In the first instance, both gpD (generated by infecting phage) and gpD< (expression induced from plasmid) would compete in decorating the capsid head of the infecting phage. In the second situation, the head of the infecting λDam phage is fully complemented by the plasmid-expressed gpD< fusion protein (Figure 1). In these assays negative complementation by a gpD< (as seen for the N-terminal addition of the YML epitope to gpD, plasmid p676, and pYML-Dcoe) is observed as a reduction in plating of λD+ on cells expressing the gpD< fusion protein. Positive complementation is measurable by observing the formation of plaque forming λDam phage on non-amber suppressing cells expressing a plasmid-encoded gpD< fusion.
The ability to vary the expression of gpD< is an important aspect of the dual display system, i.e., effectively being able to tune the expression of gpD< from the expression plasmid, pcIpR[GOI]-timm. Even quite low levels of gpD expression from pcIpR[GOI]-timm plasmids at 37 °C (compare to Table 2) can suppress the D-defect of an infecting phage, as shown in Figure 4. Previous studies where GOI was the very toxic lambda protein gpP [85] or lambda gpCII [86] showed that their level of expression, as measured by cellular toxicity or complementation, could be varied between culture incubation temperatures of 35 to 42 °C, and the levels of gpD-GFP expression [86], with time, after thermal induction (shifting cultures from growth at 30 °C to 37 °C, 39 °C, or 42 °C) showed marked differences. One might imagine that expressing more gpD (or the gpD< fusion protein) is better for complementation, but our experience suggests this would be incorrect. This conclusion may relate to our use of gpDcoe (55 poor codons of D optimized for E. coli) since lambda evolution likely conspired to limit gpD expression.
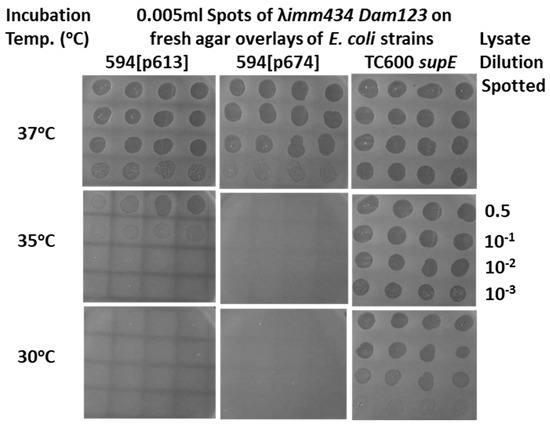
Figure 4.
Phage λ imm434 Dam123 lysate spots (quadruplicate) on nonpermissive (594) and permissive (TC600 supE) E. coli agar overlay plates as shown in Figure 2A. The objective is to determine the culture incubation temperature where expression of the partially repressed pcIpR-GOI-timm plasmid is able to provide sufficient gpD (p613) or gpD< (p674) to permit complementation of the spotted phage. The lysate dilutions spotted were identical to those shown at 35 °C.
3.3. Potential Use of Lambda Display Particles (LDP) as Vaccine Reagents
LDP can be prepared by infecting cells expressing gpD< gene fusion proteins with either λD+ or λD− phage lysates (Figure 5). When infecting only with λD− phage, only phages coated with gpD< are produced (see Figure 1). The ability to simultaneously produce multiple LDP in the same infection, as shown in Figure 5 (band in 6), allows the possibility for producing multivalent reagent preparations in the same reaction and permits the hypothesis for rapid production of multiple single epitope vaccine methodology (Figure 6).
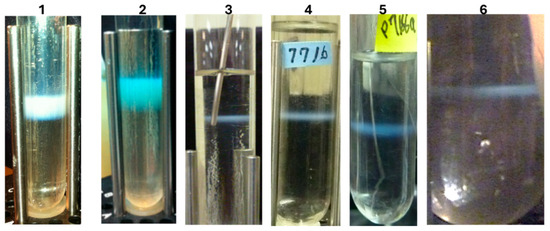
Figure 5.
CsCl banding of LDP lysate preparations made by infecting nonpermissive host cells transformed with pcIpR[ ]timm plasmids expressing gpD< gene fusions and carrying out the infections at 37 °C in a shaking water bath. The phage concentration was determined spectrophotometrically after banding, since the phage titer from lysates drops significantly after CsCl banding and storage (see [74]). The calculation was as follows: Absorbance at 260 nm × 20,000 coefficient (=10 mm/2 mm pathlength × 100 dilution × 40 μg phage/OD260 unit) × 9.4 × 109 particles per ug per ml. (The plasmids used to express and prepare the gpD< display phages are described in Methods (Table 1)). Tube 1. Display of gpD-BVDV2-E2-Ver2 (251 amino acid fusion protein); band titer 1.265 × 1010 particles/μL. Tube 2. Display of gpD-BVDV2-E2-Ver3 (241 amino acids fusion protein); band titer 1.56 × 1010 particles/μL. Tube 3. gpDcoe-YML (C-terminal fusion of gpD to 17 amino acids including YML epitope from prion protein pRp); shown second banding of phage recovered per two-liter lysate, band titer 2.63 × 1010 particles per μL. Tube 4. gpD-RBD#2 (fusion of Dcoe at C-terminal to spacer and 13 amino acids (#’s 482–494) from 2019-nCoV [87] capsid receptor binding domain for spike protein to human lung epithelial ACE2 protein, Methods Table 1); shown second banding of phage recovered per two-liter lysate: 8.93 × 1012 viable particles per 7 × 108 input phage = 1.27 × 104 amplification/input phage to infection. Tube 5. gpD-RBD#3 (Methods, Table 1), shown second banding of phage recovered per two-liter lysate: 1.08 × 1013 viable particles per 7 × 108 input phage = 1.53 × 104 amplification/input phage to infection. Tube 6. Trivalent infection: shown is first banding (larger centrifuge tube than used in lysates used to prepare Tubes 1–5) of phage output recovered per two liters of lysate: 6.47 × 1012 viable particles per 7 × 108 input phage = 9.22 × 103 amplification/input phage to infection. The phage bands seen in Tubes 1 and 2 were produced by a Single Burst procedure; the bands shown in Tubes 3 to 5 were produced by Single Phage Infection technique; and that in Tube 6 was by the Trivalent Infection method, each described in Methods.
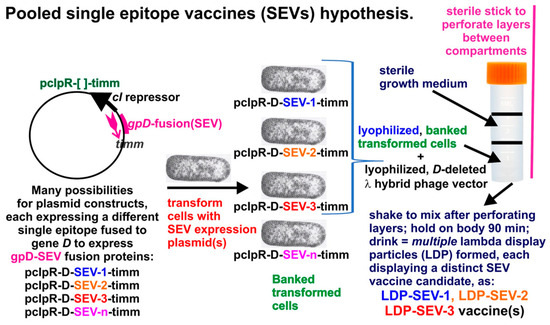
Figure 6.
Multiple fully gpD<-coated phage particles (LDP) can be produced in a single infection. This hypothesis is based on the result for simultaneously producing three phage particles, each displaying a distinct disease-specific epitope [87] of the capsid spike protein of the SARS coronavirus (shown in Figure 5, band in Tube 6). Of note, the patented, ingestible E. coli Nissle 1917 strain (EcN) [GmbH/Pharma-Zentrale GmbH, 58313 Herdecke/Germany] has been described [88] and if a λ-sensitive variant was available/obtainable, it might become a useful host strain.
In addition to our earlier studies [75] with the Porcine Circovirus capsid fusion gpD-CAP, we explored whether other complex gpD< fusion proteins could be added to the λ capsid head in the presence of gpD+. E. coli cells expressing gpD fusions to epitopes from Bovine Viral Diarrhea Virus 2 (BVDV2) E2 capsid protein were infected with λimm434cI#5, i.e., a D+ phage. The phage particles from CsCl bands shown in lanes 1 (gpD-BVDV2-E2-Ver2) and 2 (gpD-BVDV2-E2-Ver3), Figure 5, were dialysed, diluted, and run on denaturing acrylamide gels, respectively (Figure 7A, lanes 1 and 2). The phage bands are labeled as suggested in Figure 1 of reference [89]. Phage λimm434cI#5 used in these infections does not appear to encode either of the tail fiber genes tfa or stf [90]. Both the Ver2 and Ver3 display phage particles show an extra band (examine region below the band for gpV), presumed to be 248 amino acid gpD-BVDV2-E2-Ver2 (very faint band below gpV in lane 1) and 238 amino acids gpD-BVDV2-E2-Ver3 (easily identified band below gpV, lane 2). Assuming all 420 gpD binding sites are occupied on the phage particles (Figure 5, tubes 1 and 2 obtained from the infections of cells with expression plasmids), possibly 65 binding sites for gpD may be occupied by gpD-BVDV2-Ver3. The Ver3 gpD fusion display protein is 10 amino acids shorter than the Ver2 variant that included an additional (problematic?) 79–88 amino acid loop present in the BVDV2 E2 protein (legend, Figure 7). This suggests increased “recalcitrance” in capsid head binding by fusion protein gpD-BVDV-E2-Ver2 compared with gpD-BVDV-E2-Ver3, since the gel (Figure 7C) clearly shows there is a high level of gpD-BVDV-E2-Ver2 expression from the induced plasmid, implying it was available for binding to the head. The Western blot (Figure 7B) of lanes 1 and 2 (Figure 7A), employing anti-His-gpD mouse antibody (made to the His-tagged-gpD), revealed strong binding to gpD, and to higher molecular weight aggregates of gpD (never previously observed), but there was no binding by the antibody to the gpD-BVDV2-E2-Ver2 or -Ver3 fusion proteins (i.e., to the bands in Figure 7A, lanes 1 and 2) shown slightly above the 25 kD marker (Figure 7A, lane 3).
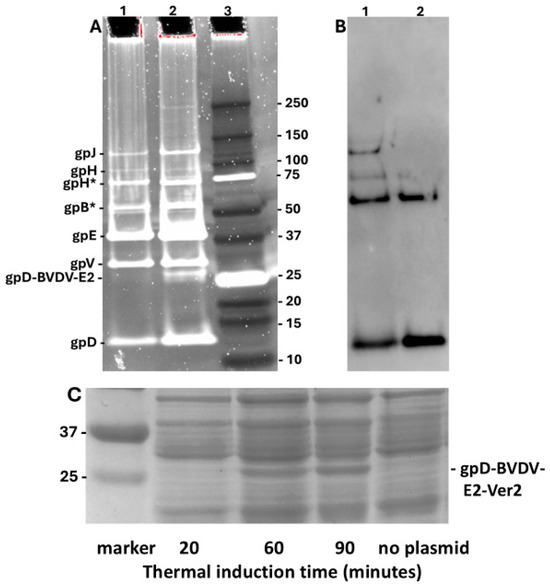
Figure 7.
Comparison of phage particles coated with gpD and gpD< (epitopes from Bovine Viral Diarrhea Virus -BVDV2 E2 protein [84,91,92,93,94,95,96] with phage particles obtained without exposure to gpD<. (A,B). Pre-cast 10–20% Tricine-SDS slab gels (Methods 2.9). Lane 1. Phage particles from band shown in Figure 5 (Tube 1): λimm434cI#5 infection of nonpermissive W3350 cells with plasmid expressing gpD-BVDV2-E2-Ver2 (see Methods). Lane 2. Phage particles from band shown in Figure 5 (Tube 2): λimm434cI#5 infection of cells with plasmid expressing gpD-BVDV2-E2-Ver3. Lanes 1, 2: Trace analysis (TotalLab software version 2.4 evaluation of UV Trans Illumination and ChemiDoc MP images of the Oriole stain) showed the density/volume of the gpD-BVDV2-E2-Ver3 band (in lane 2) was at least 6.4-fold less than the gpD band in same lane, while the density of similar (very faint) band in lane 2 for the Ver2 display phage was >40 less. Lane 3. BioRad marker set 10 kD-250kD. (B) Western blot of the gel shown in (A), corresponding to lanes 1 and 2, employing anti-His-gpD mouse antibody (see Methods). (C) Cell extracts from W3350 cells transformed with plasmid pcIpR-Dcoe-BVDV-E2-Ver2 were grown to mid log in shaking water bath at 30 °C, then shifted to 42 °C to thermally induce the expression of the gpD-BVDV-E2-Ver2 gene fusion protein from pcIpR[GOI]-timm plasmid. E. coli cell extracts from each induction time were separated on 12% SDS PAGE, identical to gel employed in Figure 3. The culture for “no plasmid” was collected after heat shift from 30 °C to 42 °C with culture shaking for 90 min. Note: the assignment of faint band for gpH and for gpH* and gpB* is a best guess.
3.4. Evaluating Biological Activity (Cellular Toxicity) of Antimicrobial Polypeptides Fused to gpD
In a previous study [72] we examined whether antimicrobial polypeptides fused to gpD could exhibit biological activity. For this purpose, we fused antimicrobial peptides, including cathelicidins from human (LL37), pig (PR39), or human α- or β-defensins to gpD, and examined whether they retained antimicrobial activity conformation when fused to gpD. We found that fusion to the NH2- terminal end of gpD nullified antimicrobial activity for all of them, whereas the same fusions to the COOH-end of gpD each retained strong antimicrobial activity (E. coli toxicity). The high toxicity of the fused α- defensin (HD5) and β-defensins (HβD3 and DEFβ126 [deleted for C-terminal 32 amino acid residues]) expressed in 594 host cells suggest that either (a) these COOH-fused defensins undergo the correct formation of three possible intramolecular disulfide bonds after translation within the cytoplasm of wild type E. coli cells that were not impaired for synthesis of either thiroredoxin or glutathione (i.e., remained competent in trxB and gor genes), or (b) that reduced versions of the gpD-linked defensins without disulfide bonds represent the highly toxigenic form of the antimicrobial peptides. There is evidence for the β-defensin HBD1 that only after the reduction of its disulfide bridges does it become a potent antimicrobial [97]. The relative toxicity of gpD-fused α- and β-defensins and cathelicidins expressed in wild-type (594) and in DsbC trxB (C3026H) cells where disulfide bridges can form within the cytoplasm was compared (Table 3. In cells (C3026H) where the gpD-fused HβD3 β-defensin and fused HD5 α-defensin could form disulfide bridges, defensin toxicity was eliminated. This did not hold for the DEFβ126 β-defensin that had been deleted for 32 residues at COOH end. Presumably, without these additional 32 amino acids, correct folding to enable disulfide bond formation between any of its 7 cysteins cannot occur. Strain C3026H had no influence on toxicity when either the pig or human cathlicidins were directly fused to the COOH end of gpD (both fusion combinations remained highly toxic), but it could suppress the toxicity of the complex cathelicidin LL37 fusion expressed from plasmid p619 (for which we have no explanation since the additions to gpD do not not contain cysteines). These data support the reported result for defensin HBD1, and suggest that the reduced versions of α- and β-defensins without disulfide bridges (but not the oxidized versions) are required for manifesting their antimicrobial activity.

Table 3.
Relative toxicity of gpD-fused α- and β-defensins and cathelicidins.
3.5. DNA Vaccines Delivered in Phage Lambda Particles (LDNAP)
The advantages and disadvantages of DNA vaccines, regulatory hurdles and their use against viral diseases, bacterial pathogens, cancer, and immunological responses are reviewed in [98,99,100,101]. The DNA vaccine is usually a plasmid DNA that encodes a disease-specific antigen. The antigen gene is expressed from a broadly active eukaryotic promoter, often derived from the Herpes Cytomegalovirus (CMV). The antigen gene is cloned between the eukaryotic promoter and a eukaryotic termination/polyadenylation (PA) signal. A typical method for delivering a DNA vaccine involves using a gene gun [101,102,103] containing gold particles with the DNA vaccine coated on them. Particles from the gun enter a target cell, where injected DNA enters the cell nucleus [104] and is then transcribed. The mRNA is transported out of nucleus for translation and processing of the expressed protein antigen(s) encoded by the vaccine. The objective is to target antigen presenting cells, especially dendritic cells, that can present the antigenic peptides onto host cell MHC, evoking both cell-mediated and humoral immune responses.
One key disadvantage to DNA vaccines, e.g., compared to protein vaccines, is that the vaccine DNA is degraded by ubiquitous DNase when moved as a DNA reagent into a biological milieu. Phage particles encapsulating a DNA vaccine expression system provide an important improvement over naked DNA, i.e., the DNA packed into the capsid particle (called a sheathed DNA vaccine) is protected from DNase, and particles about the size of phages like λ are readily phagocytized by dendritic cells, increasing the probability that an expressed antigenic gene product is processed by an animal’s innate immune system [105,106,107].
March and coworkers were early pioneers in evaluating mammalian expression cassettes integrated within the lambda genome for the purpose of developing DNA vaccines [108,109,110,111,112]. They were able to use whole λ particles as a DNA vaccine against Hepatitis B showing an immune response in vaccinated rabbits. In this work they linearized plasmid preCMV-HBsAg [Aldverson] and cloned it into a λgt11 DNA vector, generating phage particles for vaccination after λ in vitro packaging of the cloned expression plasmid [110]; similarly, they cloned other eukaryotic expression cassettes, as pcDNA3 or Stratagene plasmid pBK-CMV into the Stratagene cloning vector λZAP or again into λgt11.
These early attempts potentially suffered in two ways. First, all of the plasmid eukaryotic vectors incorporated into phage particles included antibiotic resistance genes that were moved via DNA vaccine construction into animal hosts. Gene transfer of antibiotic resistance into a eukaryotic host during vaccine administration seems undesirable. For example, Stratagene plasmid pBK-CMV encodes kanamycin/neomycin resistance and pcDNA3.1/myc-His(-)A (or B or C variants) encode both ampicillin resistance gene for selection in E. coli and neomycin resistance gene for selection in eukaryotic cells. Secondly, eukaryotic gene expression from the plasmids they employed was likely quite low (by a factor of greater than 20) compared to expression from the pCI-neo (Promega) cloning vector [Promega Notes Magazine 51:10 (1995)].
With these limitations in mind, we chose to design plasmids with a powerful eukaryotic expression cassette, where only the expression cassette, without other plasmid components, was moved into a phage λ genome. In short, these phage particles with cloned eukaryotic expression cassettes were designated LDNAPs. It was assumed the LDNAP phage could additionally be grown in cells expressing some gpD< fusion protein, enabling them to target ligands binding to “<“ displayed from the phage. Such particles were termed L2DP (DNA display particles).
Most of the poor eukaryotic expression plasmids included large multiple cloning sites (MCS, about 113 base pairs) and straddling bacteriophage T7 and T3 promoters (184 base pairs), with possibilities for hairpin structures that may limit gene expression. In a set of plasmids, loosely termed pSJH, focusing on pSJH-D’ herein (see Supplement Plasmid Construction Example (for Plasmid p593)), the MCS was reduced to 30 base pairs (AseI-XhoI-NotI-ClaI) and the T7 and T3 promoters were eliminated. The eukaryotic expression cassette was designed to be recombined or directly cloned (without any of the plasmid parts or selection genes), into a λ phage hybrid capable of strong plaque-forming ability, i.e., one retaining all the vector’s recombinational potential. (Note: many lambda cloning vectors are highly deleted for nonessential genes and plaque poorly). A comparison of the expression of eukaryotic genes EGFP and Luc2P from the pSJH plasmids to their expression from commercial plasmids is shown (Table 4).

Table 4.
Expression of EGFP and Luc2P, plasmid comparison a.
In providing that the pSJH vectors could enable substitutional (gene exchange) recombination (Figure 8A), i.e., replacing a dispensable portion of the λ genome with the eukaryotic expression cassette, copies of DNA from λ genome were introduced into the plasmids straddling the eukaryotic expression cassette. These included segments representing λ bases 18,968–19,328 and 23,937–24,310, color-coded as purple and orange regions, respectively (Figure 8). Their presence in possible vector phage to make LDNAP was examined by PCR (Figure 8B). Surprisingly, only four of the nine potential vector phages carried both regions, and did not include the λimm434cI#5 phage that we had used extensively in developing LDP (Figure 5 and Figure 7). An example showing recombinational uptake of a pSJH plasmid eukaryotic expression cassette straddled by the purple and orange DNA homologies into LDNAP vector is shown (Figure 9A), where the LDNAP phage plaques incorporating the eukaryotic expression cassette for EGFP can be detected by hybridization by all of the purified LDNAP plaques (Figure 9B). A diagram of the cloning sites A and B for moving a eukaryotic expression cassette from one of the plasmids into a vector phage containing purple and orange homologies into a vector phage to make an LDNAP is shown (Figure 10). Versions of the pSJH plasmids include GFP gene (replacing antibiotic resistance as a selectable marker and placed under control of the lambda pR promoter) within the eukaryotic expression cassette, that is to be moved into a λ vector. This figure shows that λ gene lom is substituted (either by recombination or cloning) when the cassette is moved into λ. LDNAP vectors made from essentially wild-type λ phage will tolerate ~6044 bp (12.5%λ) substitutions. As noted, these LDNAP vector recombinants can be modified to make L2DP, i.e., display-coated LDNAP for targeting cell receptors.
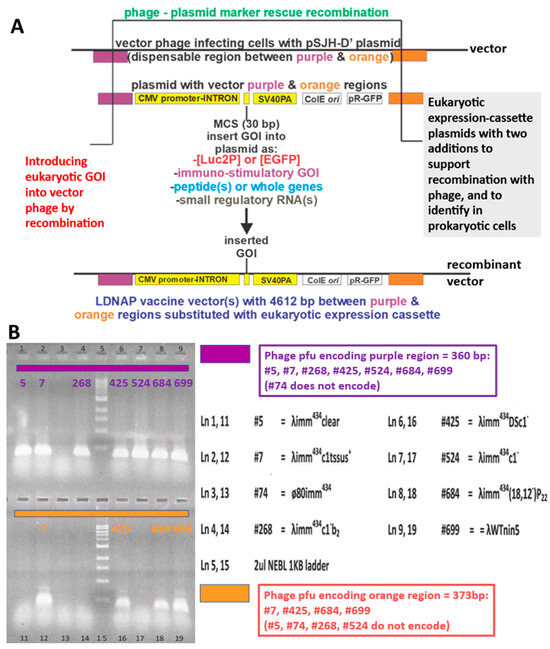
Figure 8.
(A). Marker rescue replacement recombinational uptake into the infecting phage of the segment between purple and orange regions straddling a eukaryotic expression cassette on plasmid. The infection was carried out in plasmid-transformed E. coli recD recF cells, shown to increase phage-prophage recombinational exchange by six-fold [84] above that in wild-type cells. The purple and orange regions were, respectively, amplified by primer sets L-PciI:18968+22 (5′-ata tac atg tcg taa tgt gtg tat tgc cgt tgc-3′) and R-BsiWI:19328-27 (5′-ata tcg tac gca ctg acc tgc tta ctg att tg-3′) and L-AvrII:23937+20 (5′-ata tcc tag gtt gca tgc tag atc gtg a-3′) and R-EcoRIXbaI:24310-20 (5′-ata ttc tag aac agt cat aga tgg tcg gtg g-3′), amplified from λ DNA and cloned into the plasmids in two orientations (same and opposite). (B) Plaque PCR assay (Methods) with primers for purple and orange regions to determine if these regions, 360 and 377 bp, respectively, were present in collection of λimm434 phages (each lysate # for individual phage strains is shown) seepotentially used in phage display. Shown in lanes 5 and 15 is a 1 Kb ladder (NEB N0468s):, bands lowest to highest: 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10 Kb) with electrophoresis of bands performed on acrylamide gel.
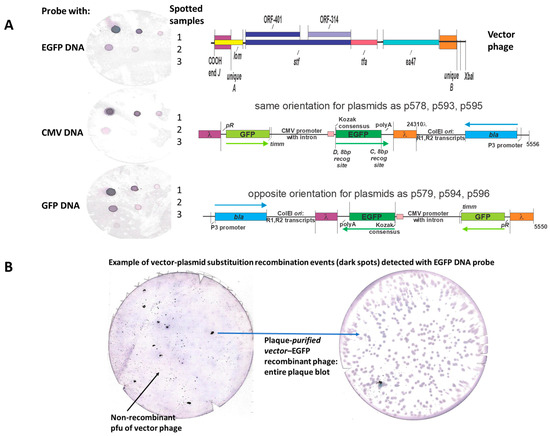
Figure 9.
(A) Possibilities for in vivo replacement recombination event between purple and orange regions between eukaryotic expression plasmids and the purple and orange sequences on vector phage (Figure 8B #7, lanes 2 and 12). (See Methods, Table 1, for the component organization of p578, p579, and p593-p596 eukaryotic expression plasmids). The phage–plasmid recombination event will substitute the EGFP cassette for phage vector genes as follows: lom [113] (mid-through COOH end, just to right of tail gene J)- orf 401-orf 314 (comprising tail fiber gene stf)-tail fiber gene tfa-and gene ea47, all being a region that is fully replaceable in lambda. The membrane blots (left of drawing) show three hybridization assays using DIG-labeled EGFP DNA, CMV DNA, or GFPuv DNA probes with spotted samples on filter membrane as follows: rows 1–3: row 1 (left to right 20, 2, and 0.2 ng plasmid p595 DNA); row 2 (20, 2, and 0.2 ng plasmid p596 DNA); row 3 Phage λimm434cI#5 lysate spots, 107, 106, and 105 pfu. (B) Left-side membrane blot: shows phage plaque recombinants (dark spots identified by DIG-labeled EFGP DNA hybridization probe) from an infection of E.coli cells transformed with plasmid p595 (Table 1) with phage vector #7 lysate (Figure 8B). Mostly shown are nonrecombinants (faint plaque outlines) that have not recombined to pick up the EGFP cassette straddled by purple and orange DNA. Right-side membrane blot: The arrow drawn from a recombinant spot points to a membrane blot of overlay agar plate where a single plaque isolate purified from some recombinant plaque region (there is no direct correlation implied by the arrow) shows that all the plaques obtained from a single plaque recombinant (left-side membrane) encoded the EGFP gene. The procedures employed are described in Methods for “nonradioactive DNA probe labeling and hybridization”.
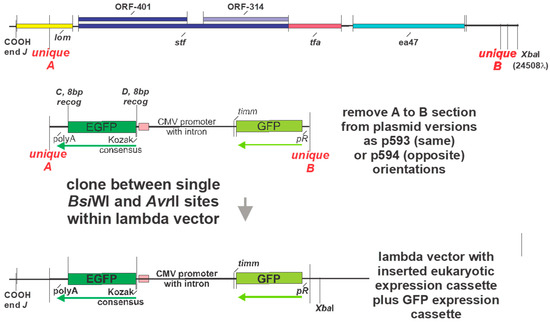
Figure 10.
Alternative cloning technique to introduce a eukaryotic expression cassette from plasmid into a vector phage genome by substituting for the lom-ea47 region on phage genome (straddled by unique single restriction endonuclease sites BsiWI (unique A) and AvrII (unique B) with the eukaryotic expression cassette present in plasmids as p593 and p594 that lack the purple and orange recombination regions shown for plasmids in Figure 8A and Figure 9A. This reaction requires in vitro λ packaging of the purified, then ligated phage arms with the BsiWI to AvrII stuffer DNA band, purified from one of the plasmid constructs.
3.6. Progress and Remaining Work to Exploit LDNAP as Vaccine Agents
Several recent reviews have examined the potential utility of using phage particles as vaccine delivery vehicles [114,115,116,117,118,119,120,121] and the cellular immune responses. We previously demonstrated (a) that LDP displaying fused gpD-CAP epitopes [74] of the Porcine Circovirus 2 (PCV2) produced both neutralizing IgG antibody (in an in vitro assay) and a T-cell-mediated cellular immune response to intradermal vaccination [75] and (b) that LDP displaying gpD-pRp disease-specific prion protein epitopes patterned to combat chronic wasting disease in cervids and formulated as a mucosal vaccine was taken up in Peyer’s patches and the draining mesenteric lymph nodes in claves, and resulted in both mucosal IgA and IgG immune responses [73]. In each of these published vaccination assays the LDP proved immunogenic, but a lot of work is required to assess whether this vaccination strategy, which does not require adjuvant, stimulates a protective immune response.
Further work is needed to prepare and evaluate sheathed DNA vaccines using the LDNAP cloning or recombination technology described herein in order to update the original studies pursued by March and collaborators. Many problems with this technology require a solution; each of them is linked to being able to follow the fate and immune responses of LDNAP administered to and expressed within dendritic cells. What is also important is learning if protective immune responses are evoked in vaccinated animals. Phage lambda includes two genes, lom and bor, that require consideration relative to using the phage as an approved human vaccine agent. The bor gene produce has the potential to confer serum resistance to an infected host cell [122] and the lom gene product [113] is involved in E. coli K12 bacterial adhesion to human buccal epithelial cells. It is unclear but unlikely either of these proteins are present on the phage capsid and may only influence the bacterial host cells by supporting them to become resistant to a mammalian immune response. The cloning or recombination procedures (Figure 9 and Figure 10) for inserting a eukaryotic cassette within lambda will remove lom.
3.7. Use of LDP as Antimicrobials to Antimicrobial-Resistant Bacteria, or a Nontargeted Version of Phage Therapy?
We have shown that human and porcine cathelicidins LL37 and PR39 linked to the COOH-terminal end of gpD are highly toxic when expressed within E. coli, and similar results were observed for reduced versions of selected human α- and β-defensins. Coated phage particles comprising LDP with gpD-fused cathelicidins or defensins are generated simply by infecting cells capable of expressing a gpD fusion protein below the lethal expression temperatures, i.e., below 41–42 °C. Alternatively, LDP can arise in expression cells where the gpD fusion is only toxigenic when administered to the outer surface of cells. We have found that D-defective λ phages are complemented efficiently (50–90+%) at infection temperatures of 37–39 °C by cultures of all the toxigenic gpD fusions with cathelicidins and defensins. Clearly, the cathelicidins and defensins evaluated retained biological activity when fused at the COOH end of gpD. LDP lysates produced via using a D-defective infecting phage can be employed to generate LDP coated with pathogen-sensitive gpD fusions. Preparation of antimicrobial agents made from LDP will require screening for display peptides that exhibit external cellular toxicity. In such a display system, none of the produced LDP would encode the toxic gpD fusion gene, nullifying horizontal gene transfer.
4. Summary, Conclusions, and Other Considerations
4.1. Summary and Conclusions
It is easily possible to produce fully coated LDP, or to produce partially coated LDP without any complex bacteriophage genetic engineering, making the system available to all, as outlined in Figure 11. These LDPs can represent vehicles with utility for stimulating an immune response to the displayed epitope(s), or could serve as antimicrobial agents when displaying an antimicrobial peptide, or be agents for any of the many possible uses suggested by Gupta et al. [25].
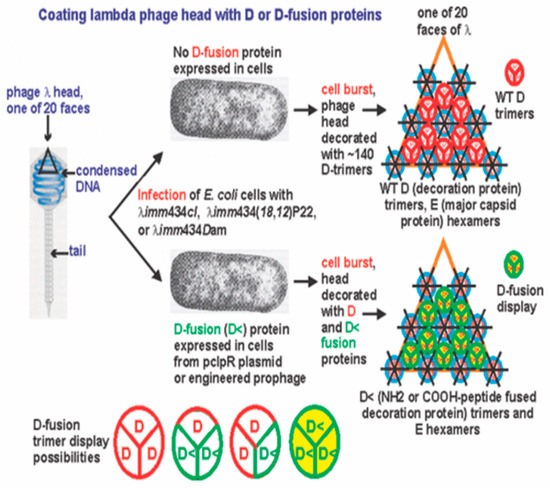
Figure 11.
Summary of gpD and gpD< fusion as trimer additions to lambda capsid head.
Infecting cells expressing gpD fusion proteins from a surrogate plasmid with a lambda phage defective in gene D (i.e., a phage incapable of expressing wild-type gpD) can result in the formation of phage capsids heads having full replacement of gpD by the gpD fusion protein. These fusion particle heads may limit the addition of phage tail structures.
Multiple single-epitope LDP vaccine reagents can be generated in a single infection lysate.
A system was introduced whereby either intracellular phage–plasmid substitution recombination, or by cloning can generate sheathed DNA vaccine particles, termed LDNAP that have the advantage of high-level eukaryotic expression cassette without incorporating plasmid resistance elements or other genes.
4.2. Other Considerations
4.2.1. Tailless Phage Particles
Careful examination of Figure 1A (three left lanes) reveals that the level of gpV is much reduced, when compared with that from a gpD competent phage (right-hand lane, Figure 1A, and lanes 1, 2 Figure 7). In earlier studies purifying phage particles coated with gpD-CAP derived from segments of the Porcine Circovirus 2 capsid, we observed the near absence of phage tails in electron micrographs of the banded phage particles [74]. At the time of this earlier publication, we suspected that the absence of tails in the EM photos showing mainly the purified capsid heads was caused by display the Circovirus capsid epitopes, or that the tails were rapidly lost from capsid heads partially coated with gpD< fusion proteins during pelleting in CsCl. Comparing the gpV bands in Figure 1A with Figure 7A suggests that gpD fusions to the lambda capsid head may interfere with the addition of pre-formed tails to the head. Perhaps, in addition, perturbing the proper level of gpD availability can negatively influence the binding of stable tails to capsid heads, reducing the expected level of a gpV band on a protein gel of the disrupted phage particles. In producing a codon-optimized version of gpD (Dcoe), fifty-five of its codons were optimized [74], suggesting that the natural gene had evolved a reduced level of expression. Some possibilities for the perturbation of gpD level include reduction via poorly efficient gpDam123 amber suppression, or an increased level of gpD (Dcoe) when expressed from surrogate plasmid. The absence of phage tails on purified lambda capsid heads coated with a foreign antigen is a preferred situation relative to the preparation of SEV phage particles, since the antigenic load represented by the tail structure is reduced.
4.2.2. gpD-gpD< Trimer Heterogeneity and the Importance of a Spacer
Figure 11 illustrates the possibility that gpD and gpD< additions onto the gpE preformed prohead scaffold may generate heterogeneous trimers with different combinations of gpD and gpD<, rather than homogeneous gpD or gpD< trimers. This requires analysis beyond the scope of this work to resolve. As noted previously, structural studies on gpD trimers revealed that both the NH2 and COOH terminal ends of gpD are buried underneath the trimer additions to the head [45]. Presumably, the gene fusions that continue from a short spacer enable the fused epitope(s) to extend from underneath the gpD trimers to the surface of the head.
4.2.3. Phage Particles as Vaccines
Phage vaccines do not require an adjuvant, are orally or nasally admissible, and can be genetically inactivated without denaturing displayed antigens, thus preventing their transmission in nature. Lambda can be stabilized without refrigeration and is stable over a wide pH range [107]. The latter characteristics are ideal for veterinary vaccines and vaccines in developing countries. Phages can act as natural adjuvants of both the cellular and humoral arms of the immune system, triggering immune response that can activate dendritic cells [123,124]. Injecting mice intravenously with 2 × 1011 phage particles gave rise to significant levels of interferon [125] which were not due to endotoxin contamination. The serum from these mice bled 18 h after injection was inhibitory to vesicular stomatitis virus in mouse L-cells. Neither the phage DNA nor phage particles without DNA produced IFN levels as high as whole phage particles. It is likely that a combination of foreign DNA containing CpG motifs and the repeating peptide motif of the phage coat can contribute to nonspecific immune stimulation [105,108]. About 711 CpG sequences are within the first 10,000 bases (48,502 bp total not scanned) on one strand, plus 106 CpGpCpG sequences and 20 CpGpCpGpC sequences per same strand of λ genome. Whether any of these sequence motifs are in the correct context to be immunostimulatory or immunoinhibitory remains unanswered at present. Of note, the uptake of lambda DNA into a eukaryotic cell nucleus has been examined [126].
4.2.4. LDNAP and L2DP
It is noted that the LDNAP phage can be propagated in bacterial cells expressing a gpD fusion protein, forming L2DP vaccine particles that could target ligands on antigen presenting cells, increasing the specificity of the sheathed DNA vaccine particle. One idea would be to coat the LDNAP with antigens that will bind dendritic cells, making what I have termed L2DP. Numerous receptors have been identified to be expressed on dendritic cells including mannose receptor (MR), DC-SIGN, scavenger receptor (SR), DEC-205, and toll-like receptors, as described in depth [127]. Lambda DNA vaccines have been prepared against different proteins [105,106,107,108,110,111]. The utility of DNA vaccines has been considered [108,128,129,130].
4.2.5. Improving on Genetic System for PD
The suggestion in Figure 11 is to use phage λimm434(18,12)P22 for infections of transformed cells with ColE1-type plasmids expressing gpD< fusion proteins when preparing LDP and SEV. It would be even better to add the Dam123 mutation or introduce a deletion into D as described in plasmid p613* [72] into this phage. The rationale for substituting phage lambda replication initiation genes P and O with parallel genes from phage P22 is that each of the plasmids described in Table 1 have ColE1 replication origins. We have determined [72] that ColE1 plasmid replication establishment and maintenance within E. coli cells is extremely sensitive to gpP, and this effect (rapid plasmid loss) is not exhibited when the gene P is substituted by the P22 replication initiation genes. This would provide for longer gpD< fusion expression when preparing LDP by infection of plasmid-transformed cells with lambda vector phage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v17111406/s1, Plasmid Construction/Considerations Example.
Funding
The studies described for preparation of BVDV2 LDP was received as my portion of Bill and Melinda Gates Foundation (Grand Challenges grant: Novel bacteriophage lambda mucosal vaccine delivery system with unique safety properties). Other funding was mainly from NSERC Canada Discovery grants awarded to author between about 2005–2018 and 2020–21 sabbatical funding from University of Saskatchewan.
Data Availability Statement
Data is contained within the article or Supplementary Material. Individual DNA sequence information can be requested from the author.
Acknowledgments
The author thanks Karthic Rajamanickam for help validating plasmids p674-p676 and Connie Hayes for constructing the remaining plasmids shown in Table 1 and the Supplemental Plasmid Construction/Considerations Example.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Brussow, H.; Hendrix, R.W. Phage genomics: Small is beautiful. Cell 2002, 108, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 66. [Google Scholar] [CrossRef]
- Gonzalez-Mora, A.; Hernandez-Perez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapala, J.; Drab, M.; Jonczyk-Matysiak, E.; Lecion, D.; Kazmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M.; et al. Mammalian Host-Versus-Phage immune response determines phage fate in vivo. Sci. Rep. 2015, 5, 14802. [Google Scholar] [CrossRef]
- Jafari, N.; Abediankenari, S. Phage particles as vaccine delivery vehicles: Concepts, applications and prospects. Asian Pac. J. Cancer Prev. 2015, 16, 8019–8029. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the Immune System. Annu. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Van Belleghem, J.D.; Dabrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X.; et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, U.; Versmold, A.; Kaufmann, P.; Frank, H.G. Phage display: A molecular tool for the generation of antibodies--a review. Placenta 2000, 21 (Suppl. SA), S106–S112. [Google Scholar] [CrossRef]
- Barondess, J.J.; Beckwith, J. bor gene of phage lambda, involved in serum resistance, encodes a widely conserved outer membrane lipoprotein. J. Bacteriol. 1995, 177, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Weber-Dabrowska, B.; Zimecki, M.; Mulczyk, M. Effective phage therapy is associated with normalization of cytokine production by blood cell cultures. Arch. Immunol. Ther. Exp. 2000, 48, 31–37. [Google Scholar]
- Zimecki, M.; Weber-Dabrowska, B.; Lusiak-Szelachowska, M.; Mulczyk, M.; Boratynski, J.; Pozniak, G.; Syper, D.; Gorski, A. Bacteriophages provide regulatory signals in mitogen-induced murine splenocyte proliferation. Cell Mol. Biol. Lett. 2003, 8, 699–711. [Google Scholar]
- Kleinschmidt, W.J.; Douthart, R.J.; Murphy, E.B. Interferon production by T4 coliphage. Nature 1970, 228, 27–30. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 2013, 869718. [Google Scholar] [CrossRef]
- Gallot, D.; Seifer, I.; Lemery, D.; Bignon, Y.J. Systemic diffusion including germ cells after plasmidic in utero gene transfer in the rat. Fetal Diagn. Ther. 2002, 17, 157–162. [Google Scholar] [CrossRef]
- Glenting, J.; Wessels, S. Ensuring safety of DNA vaccines. Microb. Cell Fact. 2005, 4, 26. [Google Scholar] [CrossRef]
- Rosenthal, K.S.; Zimmerman, D.H. Vaccines: All things considered. Clin. Vaccine Immunol. 2006, 13, 821–829. [Google Scholar] [CrossRef]
- Brussow, H.; Canchaya, C.; Hardt, W.D. Phages and the evolution of bacterial pathogens: From genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 2004, 68, 560–602, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.L.; Waldor, M.K. Bacteriophage control of bacterial virulence. Infect. Immun. 2002, 70, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Broudy, T.B.; Fischetti, V.A. In vivo lysogenic conversion of Tox(-) Streptococcus pyogenes to Tox(+) with Lysogenic Streptococci or free phage. Infect. Immun. 2003, 71, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Harhala, M.; Barylski, J.; Huminska-Lisowska, K.; Lecion, D.; Wojciechowicz, J.; Lahutta, K.; Kus, M.; Kropinski, A.M.; Nowak, S.; Nowicki, G.; et al. Two novel temperate bacteriophages infecting Streptococcus pyogenes: Their genomes, morphology and stability. PLoS ONE 2018, 13, e0205995. [Google Scholar] [CrossRef]
- McShan, W.M.; McCullor, K.A.; Nguyen, S.V. The Bacteriophages of Streptococcus pyogenes. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Kurokawa, K.; Yamashita, A.; Nakata, M.; Tomiyasu, Y.; Okahashi, N.; Kawabata, S.; Yamazaki, K.; Shiba, T.; Yasunaga, T.; et al. Genome sequence of an M3 strain of Streptococcus pyogenes reveals a large-scale genomic rearrangement in invasive strains and new insights into phage evolution. Genome Res. 2003, 13, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.V.; McShan, W.M. Chromosomal islands of Streptococcus pyogenes and related streptococci: Molecular switches for survival and virulence. Front. Cell Infect. Microbiol. 2014, 4, 109. [Google Scholar] [CrossRef] [PubMed]
- Alisky, J.; Iczkowski, K.; Rapoport, A.; Troitsky, N. Bacteriophages show promise as antimicrobial agents. J. Infect. 1998, 36, 5–15. [Google Scholar] [CrossRef]
- Biswas, B.; Adhya, S.; Washart, P.; Paul, B.; Trostel, A.N.; Powell, B.; Carlton, R.; Merril, C.R. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 2002, 70, 204–210. [Google Scholar] [CrossRef]
- Carlton, R.M. Phage therapy: Past history and future prospects. Arch. Immunol. Ther. Exp. 1999, 47, 267–274. [Google Scholar]
- Stone, R. Bacteriophage therapy. Stalin’s Forgotten cure. Science 2002, 298, 728–731. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Summers, W.C. (Ed.) Felix d’Herelle and the Orgins of Molecular Biology; Yale University Press: New Haven, CT, USA, 1999; pp. 47–59. [Google Scholar]
- Merril, C.R.; Scholl, D.; Adhya, S.L. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2003, 2, 489–497. [Google Scholar] [CrossRef]
- Dabrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, K.; Switala-Jelen, K.; Opolski, A.; Weber-Dabrowska, B.; Gorski, A. Bacteriophage penetration in vertebrates. J. Appl. Microbiol. 2005, 98, 7–13. [Google Scholar] [CrossRef]
- Summers, W.C. Bacteriophage therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef]
- Djebara, S.; Maussen, C.; De Vos, D.; Merabishvili, M.; Damanet, B.; Pang, K.W.; De Leenheer, P.; Strachinaru, I.; Soentjens, P.; Pirnay, J.P. Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified. Viruses 2019, 11, 265. [Google Scholar] [CrossRef]
- Regeimbal, J.M.; Jacobs, A.C.; Corey, B.W.; Henry, M.S.; Thompson, M.G.; Pavlicek, R.L.; Quinones, J.; Hannah, R.M.; Ghebremedhin, M.; Crane, N.J.; et al. Personalized Therapeutic Cocktail of Wild Environmental Phages Rescues Mice from Acinetobacter baumannii Wound Infections. Antimicrob. Agents Chemother. 2016, 60, 5806–5816. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Hall, L.M.L.; Merabishvilli, M.; Pirnay, J.P.; Clark, J.R.; Jones, J.D. Phage Therapy for Diabetic Foot Infection: A Case Series. Clin. Ther. 2023, 45, 797–801. [Google Scholar] [CrossRef]
- Benhar, I. Biotechnological applications of phage and cell display. Biotechnol. Adv. 2001, 19, 1–33. [Google Scholar] [CrossRef]
- Gupta, A.; Oppenheim, A.B.; Chaudhary, V.K. Phage display: A molecular fashion show. In Phages. Their Role in bacterial Pathogenesis and Biotechnology; Walder, M.K., Friedman, D.I., Adhya, S.L., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 415–429. [Google Scholar]
- Wang, L.F.; Yu, M. Epitope identification and discovery using phage display libraries: Applications in vaccine development and diagnostics. Curr. Drug Targets 2004, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G. Phage display: Practicalities and prospects. Plant Mol. Biol. 2002, 50, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.J.; Tian, C.J.; Zhu, Q.S.; Zhao, M.Y.; Xin, A.G.; Nie, W.X.; Ling, S.R.; Zhu, M.W.; Wu, J.Y.; Lan, H.Y.; et al. Orally delivered foot-and-mouth disease virus capsid protomer vaccine displayed on T4 bacteriophage surface: 100% protection from potency challenge in mice. Vaccine 2008, 26, 1471–1481. [Google Scholar] [CrossRef]
- Lambkin, I.; Pinilla, C. Targeting approaches to oral drug delivery. Expert. Opin. Biol. Ther. 2002, 2, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimizadeh, W.; Rajabibazl, M. Bacteriophage vehicles for phage display: Biology, mechanism, and application. Curr. Microbiol. 2014, 69, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Li, Q.; Shivachandra, S.B.; Leppla, S.H.; Rao, V.B. Bacteriophage T4 capsid: A unique platform for efficient surface assembly of macromolecular complexes. J. Mol. Biol. 2006, 363, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.J.; Lewis, G.K.; Wingfield, P.T.; Locke, E.G.; Steven, A.C.; Black, L.W. Phage display of intact domains at high copy number: A system based on SOC, the small outer capsid protein of bacteriophage T4. Protein Sci. 1996, 5, 1833–1843. [Google Scholar] [CrossRef]
- Shivachandra, S.B.; Rao, M.; Janosi, L.; Sathaliyawala, T.; Matyas, G.R.; Alving, C.R.; Leppla, S.H.; Rao, V.B. In vitro binding of anthrax protective antigen on bacteriophage T4 capsid surface through Hoc-capsid interactions: A strategy for efficient display of large full-length proteins. Virology 2006, 345, 190–198. [Google Scholar] [CrossRef]
- Wu, J.; Tu, C.; Yu, X.; Zhang, M.; Zhang, N.; Zhao, M.; Nie, W.; Ren, Z. Bacteriophage T4 nanoparticle capsid surface SOC and HOC bipartite display with enhanced classical swine fever virus immunogenicity: A powerful immunological approach. J. Virol. Methods 2007, 139, 50–60. [Google Scholar] [CrossRef]
- Deng, X.; Wang, L.; You, X.; Dai, P.; Zeng, Y. Advances in the T7 phage display system (Review). Mol. Med. Rep. 2018, 17, 714–720. [Google Scholar] [CrossRef]
- Malys, N.; Chang, D.Y.; Baumann, R.G.; Xie, D.; Black, L.W. A bipartite bacteriophage T4 SOC and HOC randomized peptide display library: Detection and analysis of phage T4 terminase (gp17) and late sigma factor (gp55) interaction. J. Mol. Biol. 2002, 319, 289–304. [Google Scholar] [CrossRef]
- Ren, Z.; Black, L.W. Phage T4 SOC and HOC display of biologically active, full-length proteins on the viral capsid. Gene 1998, 215, 439–444. [Google Scholar] [CrossRef]
- Feiss, M.; Becker, A. DNA packaging and cutting. In Lambda II; Hendrix, R.W., Roberts, J.W., Stahl, F.W., Weisberg, R.A., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1983; pp. 305–330. [Google Scholar]
- Georgopoulos, C.; Tilly, K.; Casjens, S. Lambdoid phage head assembly. In Lambda II; Hendrix, R.W., Roberts, J.W., Stahl, F.S., Weisberg, R.A., Eds.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1983; pp. 279–304. [Google Scholar]
- Katsura, I. Structure and function of the major tail protein of bacteriophage lambda. Mutants having small major tail protein molecules in their virion. J. Mol. Biol. 1981, 146, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Lander, G.C.; Evilevitch, A.; Jeembaeva, M.; Potter, C.S.; Carragher, B.; Johnson, J.E. Bacteriophage lambda stabilization by auxiliary protein gpD: Timing, location, and mechanism of attachment determined by cryo-EM. Structure 2008, 16, 1399–1406. [Google Scholar] [CrossRef]
- Sternberg, N.; Weisberg, R. Packaging of coliphage lambda DNA. I. The role of the cohesive end site and the gene A protein. J. Mol. Biol. 1977, 117, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, N.; Weisberg, R. Packaging of coliphage lambda DNA. II. The role of the gene D protein. J. Mol. Biol. 1977, 117, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Forrer, P.; Dauter, Z.; Conway, J.F.; Cheng, N.; Cerritelli, M.E.; Steven, A.C.; Pluckthun, A.; Wlodawer, A. Novel fold and capsid-binding properties of the lambda-phage display platform protein gpD. Nat. Struct. Biol. 2000, 7, 230–237. [Google Scholar] [CrossRef]
- Dunn, I.S. Assembly of functional bacteriophage lambda virions incorporating C-terminal peptide or protein fusions with the major tail protein. J. Mol. Biol. 1995, 248, 497–506. [Google Scholar] [CrossRef]
- Pavoni, E.; Vaccaro, P.; D’Alessio, V.; De Santis, R.; Minenkova, O. Simultaneous display of two large proteins on the head and tail of bacteriophage lambda. BMC Biotechnol. 2013, 13, 79. [Google Scholar] [CrossRef]
- Ansuini, H.; Cicchini, C.; Nicosia, A.; Tripodi, M.; Cortese, R.; Luzzago, A. Biotin-tagged cDNA expression libraries displayed on lambda phage: A new tool for the selection of natural protein ligands. Nucleic Acids Res. 2002, 30, e78. [Google Scholar] [CrossRef]
- Cicchini, C.; Ansuini, H.; Amicone, L.; Alonzi, T.; Nicosia, A.; Cortese, R.; Tripodi, M.; Luzzago, A. Searching for DNA-protein interactions by lambda phage display. J. Mol. Biol. 2002, 322, 697–706. [Google Scholar] [CrossRef]
- Cortese, R.; Monaci, P.; Luzzago, A.; Santini, C.; Bartoli, F.; Cortese, I.; Fortugno, P.; Galfre, G.; Nicosia, A.; Felici, F. Selection of biologically active peptides by phage display of random peptide libraries. Curr. Opin. Biotechnol. 1996, 7, 616–621. [Google Scholar] [CrossRef]
- Niwa, M.; Maruyama, H.; Fujimoto, T.; Dohi, K.; Maruyama, I.N. Affinity selection of cDNA libraries by lambda phage surface display. Gene 2000, 256, 229–236. [Google Scholar] [CrossRef]
- Santi, E.; Capone, S.; Mennuni, C.; Lahm, A.; Tramontano, A.; Luzzago, A.; Nicosia, A. Bacteriophage lambda display of complex cDNA libraries: A new approach to functional genomics. J. Mol. Biol. 2000, 296, 497–508. [Google Scholar] [CrossRef]
- Santini, C.; Brennan, D.; Mennuni, C.; Hoess, R.H.; Nicosia, A.; Cortese, R.; Luzzago, A. Efficient display of an HCV cDNA expression library as C-terminal fusion to the capsid protein D of bacteriophage lambda. J. Mol. Biol. 1998, 282, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, A.; Dente, L.; Santonico, E.; Castagnoli, L.; Cesareni, G. Selection of ligands by panning of domain libraries displayed on phage lambda reveals new potential partners of synaptojanin 1. J. Mol. Biol. 2001, 307, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, N.; Hoess, R.H. Display of peptides and proteins on the surface of bacteriophage lambda. Proc. Natl. Acad. Sci. USA 1995, 92, 1609–1613. [Google Scholar] [CrossRef]
- Mikawa, Y.G.; Maruyama, I.N.; Brenner, S. Surface display of proteins on bacteriophage lambda heads. J. Mol. Biol. 1996, 262, 21–30. [Google Scholar] [CrossRef]
- Vilchez, S.; Jacoby, J.; Ellar, D.J. Display of biologically functional insecticidal toxin on the surface of lambda phage. Appl. Environ. Environ. Microbiol. 2004, 70, 6587–6594. [Google Scholar] [CrossRef]
- Kong, B.; Ma, W.J. Display of aggregation-prone ligand binding domain of human PPAR gamma on surface of bacteriophage lambda. Acta Pharmacol. Sin. 2006, 27, 91–99. [Google Scholar] [CrossRef]
- Beghetto, E.; Gargano, N. Lambda-display: A powerful tool for antigen discovery. Molecules 2011, 16, 3089–3105. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Onda, M.; Pastan, I.; Adhya, S.; Chaudhary, V.K. High-density functional display of proteins on bacteriophage lambda. J. Mol. Biol. 2003, 334, 241–254. [Google Scholar] [CrossRef]
- Chaudhary, V.K.; Gupta, A.; Adhya, S.; Pastan, I. Novel Lambda Phage Display System and the Process. WO2003096969A2. 2003. [Google Scholar]
- Enquist, L.; Sternberg, N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzym. Enzymol. 1979, 68, 281–298. [Google Scholar] [CrossRef]
- Hohn, B. DNA as substrate for packaging into bacteriophage lambda, in vitro. J. Mol. Biol. 1975, 98, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Hohn, B.; Murray, K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc. Natl. Acad. Sci. USA 1977, 74, 3259–3263. [Google Scholar] [CrossRef]
- Sternberg, N.; Tiemeier, D.; Enquist, L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene 1977, 1, 255–280. [Google Scholar] [CrossRef]
- Benchimol, S.; Lucko, H.; Becker, A. Bacteriophage A DNA packaging in vitro; the involvement of the A Fl gene product, single-stranded DNA, and a novel X-directed protein in the packaging reaction. J. Biol. Chem. 1982, 257, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Minenkova, O.; Pucci, A.; Pavoni, E.; De Tomassi, A.; Fortugno, P.; Gargano, N.; Cianfriglia, M.; Barca, S.; De Placido, S.; Martignetti, A.; et al. Identification of tumor-associated antigens by screening phage-displayed human cDNA libraries with sera from tumor patients. Int. J. Cancer 2003, 106, 534–544. [Google Scholar] [CrossRef]
- Pavoni, E.; Vaccaro, P.; Pucci, A.; Monteriu, G.; Beghetto, E.; Barca, S.; Dupuis, M.L.; De Pasquale Ceratti, A.; Lugini, A.; Cianfriglia, M.; et al. Identification of a panel of tumor-associated antigens from breast carcinoma cell lines, solid tumors and testis cDNA libraries displayed on lambda phage. BMC Cancer 2004, 4, 78. [Google Scholar] [CrossRef]
- Couvreur, B.; Letellier, C.; Olivier, F.; Dehan, P.; Elouahabi, A.; Vandenbranden, M.; Ruyschaert, J.M.; Hamers, C.; Pastoret, P.P.; Kerkhofs, P. Sequence-optimised E2 constructs from BVDV-1b and BVDV-2 for DNA immunisation in cattle. Vet. Res. 2007, 38, 819–834. [Google Scholar] [CrossRef]
- Zanghi, C.N.; Lankes, H.A.; Bradel-Tretheway, B.; Wegman, J.; Dewhurst, S. A simple method for displaying recalcitrant proteins on the surface of bacteriophage lambda. Nucleic Acids Res. 2005, 33, e160. [Google Scholar] [CrossRef]
- Zanghi, C.N.; Sapinoro, R.; Bradel-Tretheway, B.; Dewhurst, S. A tractable method for simultaneous modifications to the head and tail of bacteriophage lambda and its application to enhancing phage-mediated gene delivery. Nucleic Acids Res. 2007, 35, e59. [Google Scholar] [CrossRef]
- Qiu, X. Heat induced capsid disassembly and DNA release of bacteriophage lambda. PLoS ONE 2012, 7, e39793. [Google Scholar] [CrossRef]
- Hayes, S. Bacterial Virus Lambda Gpd-Fusions to Cathelicidins, alpha- and beta-Defensins, and Disease-Specific Epitopes Evaluated for Antimicrobial Toxicity and Ability to Support Phage Display. Viruses 2019, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cano, P.; Gamage, L.N.A.; Marciniuk, K.; Hayes, C.; Napper, S.; Hayes, S.; Griebel, P.J. Lambda display phage as a mucosal vaccine delivery vehicle for peptide antigens. Vaccine 2017, 35, 7256–7263. [Google Scholar] [CrossRef]
- Hayes, S.; Gamage, L.N.; Hayes, C. Dual expression system for assembling phage lambda display particle (LDP) vaccine to porcine Circovirus 2 (PCV2). Vaccine 2010, 28, 6789–6799. [Google Scholar] [CrossRef] [PubMed]
- Gamage, L.N.; Ellis, J.; Hayes, S. Immunogenicity of bacteriophage lambda particles displaying porcine Circovirus 2 (PCV2) capsid protein epitopes. Vaccine 2009, 27, 6595–6604. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Rajamanickam, K.; Hayes, C. Complementation Studies of Bacteriophage lambda O Amber Mutants by Allelic Forms of O Expressed from Plasmid, and O-P Interaction Phenotypes. Antibiotics 2018, 7, 31. [Google Scholar] [CrossRef]
- Hayes, S.; Horbay, M.A.; Hayes, C. A CI-Independent Form of Replicative Inhibition: Turn Off of Early Replication of Bacteriophage Lambda. PLoS ONE 2012, 7, e36498. [Google Scholar] [CrossRef]
- Zeghouf, M.; Li, J.; Butland, G.; Borkowska, A.; Canadien, V.; Richards, D.; Beattie, B.; Emili, A.; Greenblatt, J.F. Sequential Peptide Affinity (SPA) system for the identification of mammalian and bacterial protein complexes. J. Proteome Res. 2004, 3, 463–468. [Google Scholar] [CrossRef]
- Weigle, J. Assembly of phage lambda in vitro. Proc. Natl. Acad. Sci. USA 1966, 55, 1462–1466. [Google Scholar] [CrossRef]
- Bachmann, B.J. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12. In Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology; Neidhardt, F.C., Ingraham, J.I., Low, K.B., Magasanik, B., Schaechter, M., Umbargr, H.E., Eds.; American Society for Microbiology: Washington, DC, USA, 1987; Volume 2, pp. 1192–1219. [Google Scholar]
- Parkinson, J.S. Genetics of the left arm of the chromosome of bacteriophage lambda. Genetics 1968, 59, 311–325. [Google Scholar] [CrossRef]
- Hayes, S.; Hayes, C.; Bull, H.J.; Pelcher, L.A.; Slavcev, R.A. Acquired mutations in phage lambda genes O or P that enable constitutive expression of a cryptic lambdaN+cI[Ts]cro- prophage in E. coli cells shifted from 30 degreesC to 42 degreesC, accompanied by loss of immlambda and Rex+ phenotypes and emergence of a non-immune exclusion-state. Gene 1998, 223, 115–128. [Google Scholar]
- Hayes, S.; Hayes, C. Spontaneous lambda OR mutations suppress inhibition of bacteriophage growth by nonimmune exclusion phenotype of defective lambda prophage. J. Virol. 1986, 58, 835–842. [Google Scholar] [CrossRef]
- Hayes, S.; Asai, K.; Chu, A.M.; Hayes, C. NinR- and red-mediated phage-prophage marker rescue recombination in Escherichia coli: Recovery of a nonhomologous immlambda DNA segment by infecting lambdaimm434 phages. Genetics 2005, 170, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Erker, C.; Horbay, M.A.; Marciniuk, K.; Wang, W.; Hayes, C. Phage Lambda P Protein: Trans-Activation, Inhibition Phenotypes and their Suppression. Viruses 2013, 5, 619–653. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Hayes, S. The Bacteriophage Lambda CII Phenotypes for Complementation, Cellular Toxicity and Replication Inhibition Are Suppressed in cII-oop Constructs Expressing the Small RNA OOP. Viruses 2018, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef]
- Sonnenborn, U. Escherichia coli strain Nissle 1917-from bench to bedside and back: History of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 2016, 363, fnw212. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, R.W.; Duda, R.L. Bacteriophage lambda PaPa: Not the mother of all lambda phages. Science 1992, 258, 1145–1148. [Google Scholar] [CrossRef]
- Branza-Nichita, N.; Durantel, D.; Carrouee-Durantel, S.; Dwek, R.A.; Zitzmann, N. Antiviral effect of N-butyldeoxynojirimycin against bovine viral diarrhea virus correlates with misfolding of E2 envelope proteins and impairment of their association into E1-E2 heterodimers. J. Virol. 2001, 75, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Deregt, D.; Bolin, S.R.; van den Hurk, J.; Ridpath, J.F.; Gilbert, S.A. Mapping of a type 1-specific and a type-common epitope on the E2 (gp53) protein of bovine viral diarrhea virus with neutralization escape mutants. Virus Res. 1998, 53, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Deregt, D.; van Rijn, P.A.; Wiens, T.Y.; van den Hurk, J. Monoclonal antibodies to the E2 protein of a new genotype (type 2) of bovine viral diarrhea virus define three antigenic domains involved in neutralization. Virus Res. 1998, 57, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kohl, W.; Zimmer, G.; Greiser-Wilke, I.; Haas, L.; Moennig, V.; Herrler, G. The surface glycoprotein E2 of bovine viral diarrhoea virus contains an intracellular localization signal. J. Gen. Virol. 2004, 85, 1101–1111. [Google Scholar] [CrossRef]
- Paton, D.J.; Lowings, J.P.; Barrett, A.D. Epitope mapping of the gp53 envelope protein of bovine viral diarrhea virus. Virology 1992, 190, 763–772. [Google Scholar] [CrossRef]
- Van Gennip, H.G.P.; Miedema, G.K.W.; Moormann, R.J.M.; Van Rijn, P.A. Functionality of Chimeric E2 Glycoproteins of BVDV and CSFV in Virus Replication. Virol. Res. Treat. 2008, 1, VRT-S589. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Wu, Z.; Nuding, S.; Groscurth, S.; Marcinowski, M.; Beisner, J.; Buchner, J.; Schaller, M.; Stange, E.F.; Wehkamp, J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature 2011, 469, 419–423. [Google Scholar] [CrossRef]
- Khan, K.H. DNA vaccines: Roles against diseases. Germs 2013, 3, 26–35. [Google Scholar] [CrossRef]
- Li, B.; Ma, J.; Liu, Y.; Wen, L.; Yu, Z.; Ni, Y.; Zhang, X.; Zhou, J.; Guo, R.; Wang, X.; et al. Complete genome sequence of a highly prevalent porcine circovirus 2 isolated from piglet stool samples in China. J. Virol. 2012, 86, 4716. [Google Scholar] [CrossRef][Green Version]
- Donnelly, J.; Berry, K.; Ulmer, J.B. Technical and regulatory hurdles for DNA vaccines. Int. J. Parasitol. 2003, 33, 457–467. [Google Scholar] [CrossRef]
- Rajcani, J.; Mosko, T.; Rezuchova, I. Current developments in viral DNA vaccines: Shall they solve the unsolved? Rev. Med. Virol. 2005, 15, 303–325. [Google Scholar] [CrossRef]
- Fuller, D.H.; Simpson, L.; Cole, K.S.; Clements, J.E.; Panicali, D.L.; Montelaro, R.C.; Murphey-Corb, M.; Haynes, J.R. Gene gun-based nucleic acid immunization alone or in combination with recombinant vaccinia vectors suppresses virus burden in rhesus macaques challenged with a heterologous SIV. Immunol. Cell Biol. 1997, 75, 389–396. [Google Scholar] [CrossRef]
- Han, R.; Peng, X.; Reed, C.A.; Cladel, N.M.; Budgeon, L.R.; Pickel, M.D.; Christensen, N.D. Gene gun-mediated intracutaneous vaccination with papillomavirus E7 gene delays cancer development of papillomavirus-induced skin papillomas on rabbits. Cancer Detect. Prev. 2002, 26, 458–467. [Google Scholar] [CrossRef]
- Prayaga, S.K.; Ford, M.J.; Haynes, J.R. Manipulation of HIV-1 gp120-specific immune responses elicited via gene gun-based DNA immunization. Vaccine 1997, 15, 1349–1352. [Google Scholar] [CrossRef]
- Salman, H.; Zbaida, D.; Rabin, Y.; Chatenay, D.; Elbaum, M. Kinetics and mechanism of DNA uptake into the cell nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 7247–7252. [Google Scholar] [CrossRef]
- Clark, J.R.; March, J.B. Bacterial viruses as human vaccines? Expert. Rev. Vaccines 2004, 3, 463–476. [Google Scholar] [CrossRef]
- Clark, J.R.; March, J.B. Bacteriophages and biotechnology: Vaccines, gene therapy and antibacterials. Trends Biotechnol. 2006, 24, 212–218. [Google Scholar] [CrossRef]
- Jepson, C.D.; March, J.B. Bacteriophage lambda is a highly stable DNA vaccine delivery vehicle. Vaccine 2004, 22, 2413–2419. [Google Scholar] [CrossRef]
- Clark, J.R.; March, J.B. Bacteriophage-mediated nucleic acid immunisation. FEMS Immunol. Med. Microbiol. 2004, 40, 21–26. [Google Scholar] [CrossRef] [PubMed]
- March, J.B. Bacteriophage-Mediated Immunization. WO 02/076498. 2002. [Google Scholar]
- Casjens, S.R.; Hendrix, R.W. Locations and amounts of major structural proteins in bacteriophage lambda. J. Mol. Biol. 1974, 88, 535–545. [Google Scholar] [CrossRef] [PubMed]
- March, J.B.; Clark, J.R.; Jepson, C.D. Genetic immunisation against hepatitis B using whole bacteriophage lambda particles. Vaccine 2004, 22, 1666–1671. [Google Scholar] [CrossRef]
- March, J.B.; Jepson, C.D.; Clark, J.R.; Totsika, M.; Calcutt, M.J. Phage library screening for the rapid identification and in vivo testing of candidate genes for a DNA vaccine against Mycoplasma mycoides subsp. mycoides small colony biotype. Infect. Immun. 2006, 74, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, R.M.; Jepson, C.D.; Clark, J.R.; March, J.B.; McKeever, D.J. Efferent lymphatic cannulation of DNA vaccines. Immunology 2003, 110, 120. [Google Scholar]
- Vaca Pacheco, S.; Garcıća González, O.; Paniagua Contreras, G.L. The lom gene of bacteriophage λ is involved in Escherichia coli K12 adhesion to human buccal epithelial cells. FEMS Microbiol. Lett. 1997, 156, 129–132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).