Acute HIV Infection and ART Response: Insights into T Cell Subsets, Activation, Exhaustion, and Blood and GALT HIV Reservoir
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. HIV Viral Load CD4 and CD8 T Cell Count
2.3. Blood Collection and PBMC Isolation
2.4. T Cell Activation, Exhaustion, Memory Subsets, and pTfh Cells
2.5. Th17 Cells and IL-22+ Cells
2.6. DNA Extraction and HIV DNA Quantitation
2.7. Statistics
3. Results
3.1. Cohort Characteristics
3.2. HIV Viral Load CD4 and CD8 T Cell Count
3.3. Quantification of Total HIV DNA
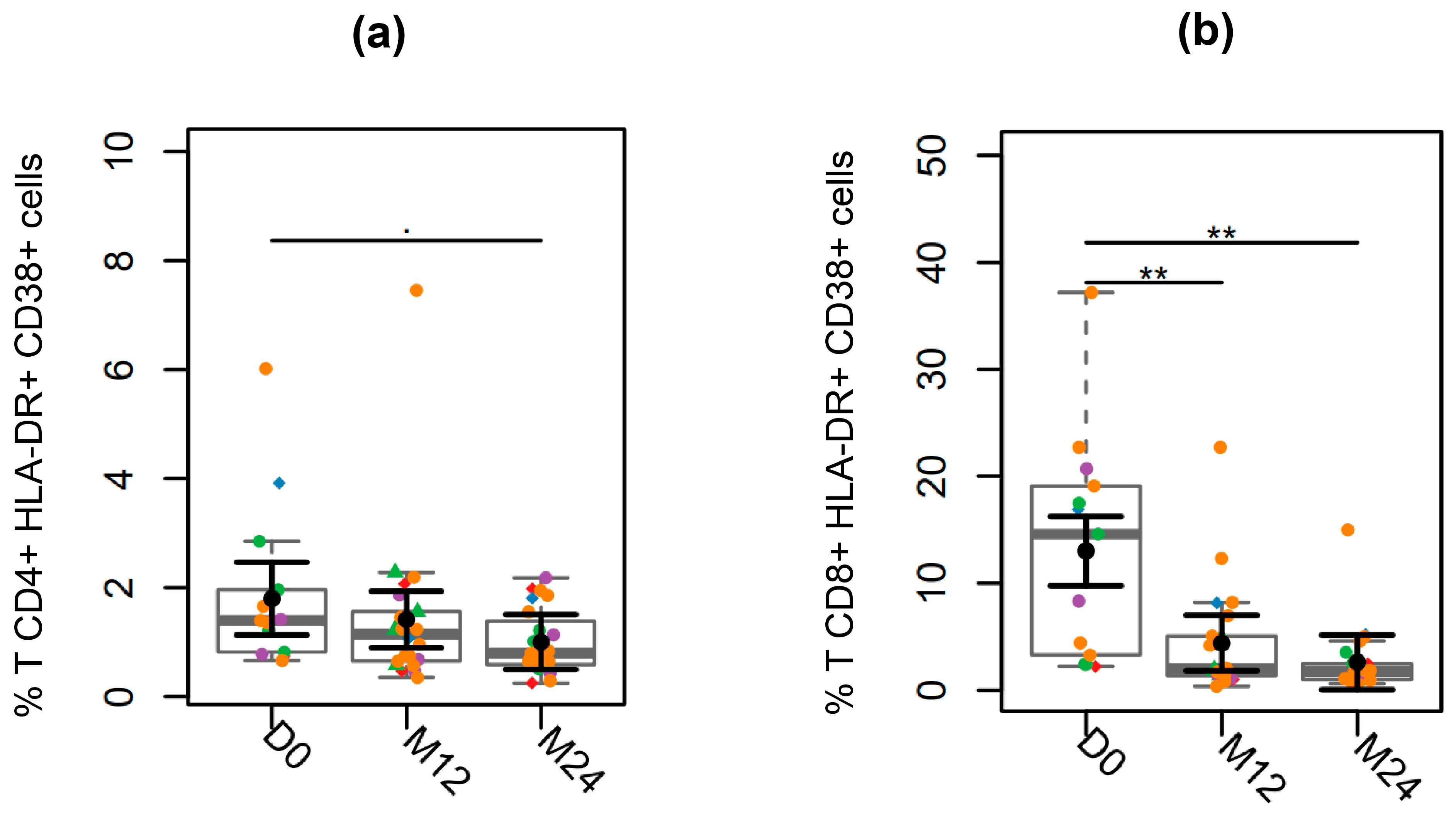
3.4. T Cell Activation
3.5. T Cell Exhaustion
3.6. CD4 and CD8 T Cells Subsets
3.7. PrEP and PEP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV; Department of Health and Human Services: Washington, DC, USA, 2024. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv (accessed on 8 August 2024).
- Chun, T.-W.; Davey, R.T.; Engel, D.; Lane, H.C.; Fauci, A.S. Re-Emergence of HIV after Stopping Therapy. Nature 1999, 401, 874–875. [Google Scholar] [CrossRef]
- Ghosn, J.; Taiwo, B.; Seedat, S.; Autran, B.; Katlama, C. HIV. Lancet 2018, 392, 685–697. [Google Scholar] [CrossRef]
- Li, J.Z.; Etemad, B.; Ahmed, H.; Aga, E.; Bosch, R.J.; Mellors, J.W.; Kuritzkes, D.R.; Lederman, M.M.; Para, M.; Gandhi, R.T. The Size of the Expressed HIV Reservoir Predicts Timing of Viral Rebound after Treatment Interruption. AIDS 2016, 30, 343–353. [Google Scholar] [CrossRef]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an Inducible HIV-1 Latent Reservoir during Highly Active Antiretroviral Therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar] [CrossRef]
- Finzi, D. Identification of a Reservoir for HIV-1 in Patients on Highly Active Antiretroviral Therapy. Science 1997, 278, 1295–1300. [Google Scholar] [CrossRef]
- Wong, J.K.; Hezareh, M.; Günthard, H.F.; Havlir, D.V.; Ignacio, C.C.; Spina, C.A.; Richman, D.D. Recovery of Replication-Competent HIV despite Prolonged Suppression of Plasma Viremia. Science 1997, 278, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.M.; Aguilar-Jimenez, W.; Su, R.-C.; Rugeles, M.T. Mucosa: Key Interactions Determining Sexual Transmission of the HIV Infection. Front. Immunol. 2019, 10, 144. [Google Scholar] [CrossRef]
- Royce, R.A.; Seña, A.; Cates, W.; Cohen, M.S. Sexual Transmission of HIV. N. Engl. J. Med. 1997, 336, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal Tract as a Major Site of CD4+ T Cell Depletion and Viral Replication in SIV Infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Douek, D.C.; Hill, B.; Nishimura, Y.; Martin, M.; Roederer, M. Massive Infection and Loss of Memory CD4+ T Cells in Multiple Tissues during Acute SIV Infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; Mansfield, K.G.; Tham, I.C.; Carville, A.C.; Shvetz, D.E.; Forand, A.E.; Lackner, A.A. Dynamics of CCR5 Expression by CD4+ T Cells in Lymphoid Tissues during Simian Immunodeficiency Virus Infection. J. Virol. 2000, 74, 11001–11007. [Google Scholar] [CrossRef] [PubMed]
- Anton, P.A.; Elliott, J.; Poles, M.A.; McGowan, I.M.; Matud, J.; Hultin, L.E.; Grovit-Ferbas, K.; Mackay, C.R.; Chen, I.S.Y.; Giorgi, J.V. Enhanced Levels of Functional HIV-1 Co-Receptors on Human Mucosal T Cells Demonstrated Using Intestinal Biopsy Tissue. AIDS 2000, 14, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Poles, M.A.; Elliott, J.; Taing, P.; Anton, P.A.; Chen, I.S. A Preponderance of CCR5+ CXCR4+ Mononuclear Cells Enhances Gastrointestinal Mucosal Susceptibility to Human Immunodeficiency Virus Type 1 Infection. J. Virol. 2001, 75, 8390–8399. [Google Scholar] [CrossRef]
- Falivene, J.; Ghiglione, Y.; Laufer, N.; Socías, M.E.; Holgado, M.P.; Ruiz, M.J.; Maeto, C.; Figueroa, M.I.; Giavedoni, L.D.; Cahn, P.; et al. Th17 and Th17/Treg Ratio at Early HIV Infection Associate with Protective HIV-Specific CD8+ T-Cell Responses and Disease Progression. Sci. Rep. 2015, 5, 11511. [Google Scholar] [CrossRef]
- Planas, D.; Routy, J.-P.; Ancuta, P. New Th17-Specific Therapeutic Strategies for HIV Remission. Curr. Opin. HIV AIDS 2019, 14, 85–92. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 Are Coexpressed by Th17 Cells and Cooperatively Enhance Expression of Antimicrobial Peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Renault, C.; Veyrenche, N.; Mennechet, F.; Bedin, A.-S.; Routy, J.-P.; Van de Perre, P.; Reynes, J.; Tuaillon, E. Th17 CD4+ T-Cell as a Preferential Target for HIV Reservoirs. Front. Immunol. 2022, 13, 822576. [Google Scholar] [CrossRef]
- Maggi, L.; Santarlasci, V.; Capone, M.; Peired, A.; Frosali, F.; Crome, S.Q.; Querci, V.; Fambrini, M.; Liotta, F.; Levings, M.K.; et al. CD161 Is a Marker of All Human IL-17-Producing T-Cell Subsets and Is Induced by RORC. Eur. J. Immunol. 2010, 40, 2174–2181. [Google Scholar] [CrossRef]
- Gosselin, A.; Wiche Salinas, T.R.; Planas, D.; Wacleche, V.S.; Zhang, Y.; Fromentin, R.; Chomont, N.; Cohen, É.A.; Shacklett, B.; Mehraj, V.; et al. HIV Persists in CCR6+CD4+ T Cells from Colon and Blood during Antiretroviral Therapy. AIDS 2017, 31, 35–48. [Google Scholar] [CrossRef]
- Gong, J.; Zhan, H.; Liang, Y.; He, Q.; Cui, D. Role of Th22 Cells in Human Viral Diseases. Front. Med. 2021, 8, 708140. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, R.; Asthana, D. Increase in Frequencies of Circulating Th-17 Cells Correlates with Microbial Translocation, Immune Activation and Exhaustion in HIV-1 Infected Patients with Poor CD4 T-Cell Reconstitution. Immunobiology 2016, 221, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.N.; Cervasi, B.; Odorizzi, P.; Silverman, R.; Aberra, F.; Ginsberg, G.; Estes, J.D.; Paiardini, M.; Frank, I.; Silvestri, G. Disruption of Intestinal CD4+ T Cell Homeostasis Is a Key Marker of Systemic CD4+ T Cell Activation in HIV-Infected Individuals. J. Immunol. 2010, 185, 5169–5179. [Google Scholar] [CrossRef]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, E.I.; Macal, M.; Lewis, G.M.; Harker, J.A. Innate and Adaptive Immune Regulation During Chronic Viral Infections. Annu. Rev. Virol. 2015, 2, 573–597. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.G.; Sereti, I. Can Early Therapy Reduce Inflammation? Curr. Opin. HIV AIDS 2014, 9, 72–79. [Google Scholar] [CrossRef]
- Sneller, M.C.; Justement, J.S.; Gittens, K.R.; Petrone, M.E.; Clarridge, K.E.; Proschan, M.A.; Kwan, R.; Shi, V.; Blazkova, J.; Refsland, E.W.; et al. A Randomized Controlled Safety/Efficacy Trial of Therapeutic Vaccination in HIV-Infected Individuals Who Initiated Antiretroviral Therapy Early in Infection. Sci. Transl. Med. 2017, 9, eaan8848. [Google Scholar] [CrossRef]
- Morris, A.B.; Adams, L.E.; Ford, M.L. Influence of T Cell Coinhibitory Molecules on CD8+ Recall Responses. Front. Immunol. 2018, 9, 1810. [Google Scholar] [CrossRef]
- Rallón, N.; García, M.; García-Samaniego, J.; Cabello, A.; Álvarez, B.; Restrepo, C.; Nistal, S.; Górgolas, M.; Benito, J.M. Expression of PD-1 and Tim-3 Markers of T-Cell Exhaustion Is Associated with CD4 Dynamics during the Course of Untreated and Treated HIV Infection. PLoS ONE 2018, 13, e0193829. [Google Scholar] [CrossRef]
- Sáez-Cirión, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathog. 2013, 9, e1003211. [Google Scholar] [CrossRef]
- Williams, J.P.; Hurst, J.; Stöhr, W.; Robinson, N.; Brown, H.; Fisher, M.; Kinloch, S.; Cooper, D.; Schechter, M.; Tambussi, G.; et al. HIV-1 DNA Predicts Disease Progression and Post-Treatment Virological Control. eLife 2014, 3, e03821. [Google Scholar] [CrossRef]
- Passaes, C.; Desjardins, D.; Chapel, A.; Monceaux, V.; Lemaitre, J.; Mélard, A.; Perdomo-Celis, F.; Planchais, C.; Gourvès, M.; Dimant, N.; et al. Early Antiretroviral Therapy Favors Post-Treatment SIV Control Associated with the Expansion of Enhanced Memory CD8+ T-Cells. Nat. Commun. 2024, 15, 178. [Google Scholar] [CrossRef]
- Buranapraditkun, S.; Pissani, F.; Teigler, J.E.; Schultz, B.T.; Alter, G.; Marovich, M.; Robb, M.L.; Eller, M.A.; Martin, J.; Deeks, S.; et al. Preservation of Peripheral T Follicular Helper Cell Function in HIV Controllers. J. Virol. 2017, 91, e00497-17. [Google Scholar] [CrossRef]
- Secretaria de Vigilância Em Saúde e Ambiente, Secretaria de Ciência, Tecnologia, Inovação e Complexo Da Saúde, Ministério Da Saúde, Brasil. Protocolo Clínico e Diretrizes Terapêuticas Para Manejo Da Infecção Pelo HIV Em Adultos: Módulo 1: Tratamento. 2024. Available online: https://www.gov.br/conitec/pt-br/midias/relatorios/2023/PCDTManejodaInfecopeloHIVemAdultosMdulo1Tratamento.pdf (accessed on 17 January 2024).
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H.; et al. Dynamics of HIV Viremia and Antibody Seroconversion in Plasma Donors: Implications for Diagnosis and Staging of Primary HIV Infection. AIDS 2003, 17, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.-R.; Ghattas, G.; Brenchley, J.M.; et al. HIV Reservoir Size and Persistence Are Driven by T Cell Survival and Homeostatic Proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Hocqueloux, L.; Avettand-Fènoël, V.; Jacquot, S.; Prazuck, T.; Legac, E.; Mélard, A.; Niang, M.; Mille, C.; Le Moal, G.; Viard, J.-P.; et al. Long-Term Antiretroviral Therapy Initiated during Primary HIV-1 Infection Is Key to Achieving Both Low HIV Reservoirs and Normal T Cell Counts. J. Antimicrob. Chemother. 2013, 68, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Kassutto, S.; Maghsoudi, K.; Johnston, M.N.; Robbins, G.K.; Burgett, N.C.; Sax, P.E.; Cohen, D.; Pae, E.; Davis, B.; Zachary, K.; et al. Longitudinal Analysis of Clinical Markers Following Antiretroviral Therapy Initiated during Acute or Early HIV Type 1 Infection. Clin. Infect. Dis. 2006, 42, 1024–1031. [Google Scholar] [CrossRef]
- Hoenigl, M.; Chaillon, A.; Little, S.J. CD4/CD8 Cell Ratio in Acute HIV Infection and the Impact of Early Antiretroviral Therapy. Clin. Infect. Dis. 2016, 63, 425–426. [Google Scholar] [CrossRef]
- Routy, J.-P.; Mehraj, V. Very Early Antiretroviral Therapy Permits CD8 T Cells to Keep HIV Reservoirs at Bay. Ann. Transl. Med. 2017, 5, 434. [Google Scholar] [CrossRef]
- Colby, D.J.; Trautmann, L.; Pinyakorn, S.; Leyre, L.; Pagliuzza, A.; Kroon, E.; Rolland, M.; Takata, H.; Buranapraditkun, S.; Intasan, J.; et al. Rapid HIV RNA Rebound after Antiretroviral Treatment Interruption in Persons Durably Suppressed in Fiebig I Acute HIV Infection. Nat. Med. 2018, 24, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Sacdalan, C.P.; Pinyakorn, S.; Chomont, N.; Souza, M.; Luekasemsuk, T.; Schuetz, A.; Krebs, S.J.; Dewar, R.; Jagodzinski, L.; et al. Virological and Immunological Characteristics of HIV-Infected Individuals at the Earliest Stage of Infection. J. Virus Erad. 2016, 2, 43–48. [Google Scholar] [CrossRef]
- Chun, T.-W.; Justement, J.S.; Pandya, P.; Hallahan, C.W.; McLaughlin, M.; Liu, S.; Ehler, L.A.; Kovacs, C.; Fauci, A.S. Relationship between the Size of the Human Immunodeficiency Virus Type 1 (HIV-1) Reservoir in Peripheral Blood CD4+ T Cells and CD4+:CD8+ T Cell Ratios in Aviremic HIV-1-Infected Individuals Receiving Long-Term Highly Active Antiretroviral Therapy. J. Infect. Dis. 2002, 185, 1672–1676. [Google Scholar] [CrossRef]
- Borrow, P.; Lewicki, H.; Hahn, B.H.; Shaw, G.M.; Oldstone, M.B. Virus-Specific CD8+ Cytotoxic T-Lymphocyte Activity Associated with Control of Viremia in Primary Human Immunodeficiency Virus Type 1 Infection. J. Virol. 1994, 68, 6103–6110. [Google Scholar] [CrossRef]
- Ganesan, A.; Chattopadhyay, P.K.; Brodie, T.M.; Qin, J.; Gu, W.; Mascola, J.R.; Michael, N.L.; Follmann, D.A.; Roederer, M. Immunological and Virological Events in Early HIV Infection Predict Subsequent Rate of Progression. J. Infect. Dis. 2010, 201, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Chomont, N.; Eller, L.A.; Kroon, E.; Tovanabutra, S.; Bose, M.; Nau, M.; Fletcher, J.L.K.; Tipsuk, S.; Vandergeeten, C.; et al. HIV DNA Set Point Is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. eBioMedicine 2016, 11, 68–72. [Google Scholar] [CrossRef]
- Leyre, L.; Kroon, E.; Vandergeeten, C.; Sacdalan, C.; Colby, D.J.; Buranapraditkun, S.; Schuetz, A.; Chomchey, N.; de Souza, M.; Bakeman, W.; et al. Abundant HIV-Infected Cells in Blood and Tissues Are Rapidly Cleared upon ART Initiation during Acute HIV Infection. Sci. Transl. Med. 2020, 12, eaav3491. [Google Scholar] [CrossRef] [PubMed]
- Henrich, T.J.; Hatano, H.; Bacon, O.; Hogan, L.E.; Rutishauser, R.; Hill, A.; Kearney, M.F.; Anderson, E.M.; Buchbinder, S.P.; Cohen, S.E.; et al. HIV-1 Persistence Following Extremely Early Initiation of Antiretroviral Therapy (ART) during Acute HIV-1 Infection: An Observational Study. PLoS Med. 2017, 14, e1002417. [Google Scholar] [CrossRef]
- Finzi, D.; Blankson, J.; Siliciano, J.D.; Margolick, J.B.; Chadwick, K.; Pierson, T.; Smith, K.; Lisziewicz, J.; Lori, F.; Flexner, C.; et al. Latent Infection of CD4+ T Cells Provides a Mechanism for Lifelong Persistence of HIV-1, Even in Patients on Effective Combination Therapy. Nat. Med. 1999, 5, 512–517. [Google Scholar] [CrossRef]
- Avettand-Fenoel, V.; Prazuck, T.; Hocqueloux, L.; Melard, A.; Michau, C.; Kerdraon, R.; Agoute, E.; Rouzioux, C. HIV-DNA in Rectal Cells Is Well Correlated with HIV-DNA in Blood in Different Groups of Patients, Including Long-Term Non-Progressors. AIDS 2008, 22, 1880. [Google Scholar] [CrossRef]
- Anton, P.A.; Mitsuyasu, R.T.; Deeks, S.G.; Scadden, D.T.; Wagner, B.; Huang, C.; Macken, C.; Richman, D.D.; Christopherson, C.; Borellini, F.; et al. Multiple Measures of HIV Burden in Blood and Tissue Are Correlated with Each Other but Not with Clinical Parameters in Aviremic Subjects. AIDS 2003, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, M.; Reay, E.; Sankaran, S.; Prindiville, T.; Flamm, J.; McNeil, A.; Dandekar, S. Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue during Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration Following Highly Active Antiretroviral Therapy. J. Virol. 2003, 77, 11708–11717. [Google Scholar] [CrossRef]
- Mehandru, S.; Poles, M.A.; Tenner-Racz, K.; Jean-Pierre, P.; Manuelli, V.; Lopez, P.; Shet, A.; Low, A.; Mohri, H.; Boden, D.; et al. Lack of Mucosal Immune Reconstitution during Prolonged Treatment of Acute and Early HIV-1 Infection. PLoS Med. 2006, 3, e484. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, M.; Sankaran, S.; George, M.D.; Reay, E.; Verhoeven, D.; Shacklett, B.L.; Flamm, J.; Wegelin, J.; Prindiville, T.; Dandekar, S. Viral Suppression and Immune Restoration in the Gastrointestinal Mucosa of Human Immunodeficiency Virus Type 1-Infected Patients Initiating Therapy during Primary or Chronic Infection. J. Virol. 2006, 80, 8236–8247. [Google Scholar] [CrossRef] [PubMed]
- George, M.D.; Reay, E.; Sankaran, S.; Dandekar, S. Early Antiretroviral Therapy for Simian Immunodeficiency Virus Infection Leads to Mucosal CD4+ T-Cell Restoration and Enhanced Gene Expression Regulating Mucosal Repair and Regeneration. J. Virol. 2005, 79, 2709–2719. [Google Scholar] [CrossRef]
- Tincati, C.; Biasin, M.; Bandera, A.; Violin, M.; Marchetti, G.; Piacentini, L.; Vago, G.L.; Balotta, C.; Moroni, M.; Franzetti, F.; et al. Early Initiation of Highly Active Antiretroviral Therapy Fails to Reverse Immunovirological Abnormalities in Gut-Associated Lymphoid Tissue Induced by Acute HIV Infection. Antivir. Ther. 2009, 14, 321–330. [Google Scholar] [CrossRef]
- Takata, H.; Buranapraditkun, S.; Kessing, C.; Fletcher, J.L.K.; Muir, R.; Tardif, V.; Cartwright, P.; Vandergeeten, C.; Bakeman, W.; Nichols, C.N.; et al. Delayed Differentiation of Potent Effector CD8 T Cells Reducing Viremia and Reservoir Seeding in Acute HIV Infection. Sci. Transl. Med. 2017, 9, eaag1809. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Lu, X.; Kuang, Y.-Q.; Kong, D.; Zhang, X.; Yang, X.; Wang, X.; Mu, T.; Wang, H.; et al. Dysregulation of Memory B Cells and Circulating T Follicular Helper Cells Is a Predictor of Poor Immune Recovery in HIV-Infected Patients on Antiretroviral Therapy. J. Med. Virol. 2023, 95, e28559. [Google Scholar] [CrossRef]
- Martin, G.E.; Pace, M.; Shearer, F.M.; Zilber, E.; Hurst, J.; Meyerowitz, J.; Thornhill, J.P.; Lwanga, J.; Brown, H.; Robinson, N.; et al. Levels of Human Immunodeficiency Virus DNA Are Determined Before ART Initiation and Linked to CD8 T-Cell Activation and Memory Expansion. J. Infect. Dis. 2020, 221, 1135–1145. [Google Scholar] [CrossRef]
- Zhang, Z.-N.; Zhu, M.-L.; Chen, Y.-H.; Fu, Y.-J.; Zhang, T.-W.; Jiang, Y.-J.; Chu, Z.-X.; Shang, H. Elevation of Tim-3 and PD-1 Expression on T Cells Appears Early in HIV Infection, and Differential Tim-3 and PD-1 Expression Patterns Can Be Induced by Common γ-Chain Cytokines. BioMed Res. Int. 2015, 2015, 916936. [Google Scholar] [CrossRef]
- Fromentin, R.; DaFonseca, S.; Costiniuk, C.T.; El-Far, M.; Procopio, F.A.; Hecht, F.M.; Hoh, R.; Deeks, S.G.; Hazuda, D.J.; Lewin, S.R.; et al. PD-1 Blockade Potentiates HIV Latency Reversal Ex Vivo in CD4+ T Cells from ART-Suppressed Individuals. Nat. Commun. 2019, 10, 814. [Google Scholar] [CrossRef]
- Banga, R.; Procopio, F.A.; Noto, A.; Pollakis, G.; Cavassini, M.; Ohmiti, K.; Corpataux, J.-M.; de Leval, L.; Pantaleo, G.; Perreau, M. PD-1+ and Follicular Helper T Cells Are Responsible for Persistent HIV-1 Transcription in Treated Aviremic Individuals. Nat. Med. 2016, 22, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.M.; Fujita, T.; Webb, G.M.; Burwitz, B.J.; Wu, H.L.; Reed, J.S.; Hammond, K.B.; Clayton, K.L.; Ishii, N.; Abdel-Mohsen, M.; et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016, 12, e1005349. [Google Scholar] [CrossRef]
- Rinaldi, S.; de Armas, L.; Dominguez-Rodríguez, S.; Pallikkuth, S.; Dinh, V.; Pan, L.; Gärtner, K.; Pahwa, R.; Cotugno, N.; Rojo, P.; et al. T Cell Immune Discriminants of HIV Reservoir Size in a Pediatric Cohort of Perinatally Infected Individuals. PLoS Pathog. 2021, 17, e1009533. [Google Scholar] [CrossRef]
- Kök, A.; Hocqueloux, L.; Hocini, H.; Carrière, M.; Lefrou, L.; Guguin, A.; Tisserand, P.; Bonnabau, H.; Avettand-Fenoel, V.; Prazuck, T.; et al. Early Initiation of Combined Antiretroviral Therapy Preserves Immune Function in the Gut of HIV-Infected Patients. Mucosal Immunol. 2015, 8, 127–140. [Google Scholar] [CrossRef]
- Cohen, M.S.; Shaw, G.M.; McMichael, A.J.; Haynes, B.F. Acute HIV-1 Infection. N. Engl. J. Med. 2011, 364, 1943–1954. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Kuroda, M.J.; Santra, S.; Sasseville, V.G.; Simon, M.A.; Lifton, M.A.; Racz, P.; Tenner-Racz, K.; Dalesandro, M.; Scallon, B.J.; et al. Control of Viremia in Simian Immunodeficiency Virus Infection by CD8+ Lymphocytes. Science 1999, 283, 857–860. [Google Scholar] [CrossRef]
- Wilson, J.D.; Ogg, G.S.; Allen, R.L.; Davis, C.; Shaunak, S.; Downie, J.; Dyer, W.; Workman, C.; Sullivan, S.; McMichael, A.J.; et al. Direct Visualization of HIV-1-Specific Cytotoxic T Lymphocytes during Primary Infection. AIDS 2000, 14, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-Y.; Qu, M.-M.; Wang, X.; Wang, Z.-R.; Song, J.-W.; Yang, B.-P.; Guo, Y.-T.; Zhang, Y.; Zhang, C.; Fan, X.; et al. Characteristics of Blood Immune Cell Profile and Their Correlation with Disease Progression in Patients Infected with HIV-1. BMC Infect. Dis. 2023, 23, 893. [Google Scholar] [CrossRef] [PubMed]
- Kestens, L.; Vanham, G.; Gigase, P.; Young, G.; Hannet, I.; Vanlangendonck, F.; Hulstaert, F.; Bach, B.A. Expression of Activation Antigens, HLA-DR and CD38, on CD8 Lymphocytes during HIV-1 Infection. AIDS 1992, 6, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Crowell, T.A.; Ritz, J.; Coombs, R.W.; Zheng, L.; Eron, J.J.; Mellors, J.W.; Dragavon, J.; van Zyl, G.U.; Lama, J.R.; Ruxrungtham, K.; et al. Novel Criteria for Diagnosing Acute and Early Human Immunodeficiency Virus Infection in a Multinational Study of Early Antiretroviral Therapy Initiation. Clin. Infect. Dis. 2021, 73, e643–e651. [Google Scholar] [CrossRef]
- Ndhlovu, Z.; Kamya, P.; Mewalal, N.; Kløverpris, H.N.; Nkosi, T.; Pretorius, K.; Laher, F.; Ogunshola, F.; Chopera, D.; Shekhar, K.; et al. Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impacts Viral Set Point. Immunity 2015, 43, 591–604. [Google Scholar] [CrossRef]
- Grinsztejn, B.; Hoagland, B.; Moreira, R.I.; Kallas, E.G.; Madruga, J.V.; Goulart, S.; Leite, I.C.; Freitas, L.; Martins, L.M.S.; Torres, T.S.; et al. Retention, Engagement, and Adherence to Pre-Exposure Prophylaxis for Men Who Have Sex with Men and Transgender Women in PrEP Brasil: 48 Week Results of a Demonstration Study. Lancet HIV 2018, 5, e136–e145. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Petit, E.; Liegeon, G.; Laguno, M.; Miró, J.M. Primary HIV-1 Infection in Users of Pre-Exposure Prophylaxis. Lancet HIV 2021, 8, e166–e174. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Lee, G.Q.; Reddy, N.; Chikowore, T.J.B.; Baisley, K.; Dong, K.L.; Walker, B.D.; Yu, X.G.; Lichterfeld, M.; Ndung’u, T. Differences in HIV-1 Reservoir Size, Landscape Characteristics and Decay Dynamics in Acute and Chronic Treated HIV-1 Clade C Infection. eLife 2024, 13, RP96617. [Google Scholar] [CrossRef]
- Dong, K.L.; Moodley, A.; Kwon, D.S.; Ghebremichael, M.S.; Dong, M.; Ismail, N.; Ndhlovu, Z.M.; Mabuka, J.M.; Muema, D.M.; Pretorius, K.; et al. Detection and Treatment of Fiebig Stage I HIV-1 Infection in Young at-Risk Women in South Africa: A Prospective Cohort Study. Lancet HIV 2018, 5, e35–e44. [Google Scholar] [CrossRef]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapía, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef]
- Jourdain, H.; de Gage, S.B.; Desplas, D.; Dray-Spira, R. Real-World Effectiveness of Pre-Exposure Prophylaxis in Men at High Risk of HIV Infection in France: A Nested Case-Control Study. Lancet Public Health 2022, 7, e529–e536. [Google Scholar] [CrossRef] [PubMed]





| D0 | M12 | M24 | |
|---|---|---|---|
| Age (years), median, [25th–75th] | 27 [25–36] | ||
| Gender, n (%) Cisgender men Transgender women | 23 (95.9%) 1 (4.1%) | ||
| HIV viral load (log10 copies/mL; mean [95% CI]) | 3.9 [3.5–4.3] | <40 | <40 |
| CD4 count (cells/mm3; mean [95% CI]) | 646 [540–754] | 861 [756–967] | 895 [789–1000] |
| CD4/CD8 (mean [95% CI]) | 0.75 [0.57–0.94] | 1.24 [1.06–1.42] | 1.26 [1.08–1.44] |
| Fiebig Stage (n) | |||
| I | 2 | ||
| II | 1 | ||
| III | 5 | ||
| IV | 4 | ||
| V | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, S.S.d.; Caetano, D.G.; Guimarães, M.L.; da Silva, R.K.M.; Cabral, N.; da Costa Cruz Silva, S.; Ribeiro-Alves, M.; Teixeira, S.L.M.; Georg, I.; Santos, D.V.G.d.; et al. Acute HIV Infection and ART Response: Insights into T Cell Subsets, Activation, Exhaustion, and Blood and GALT HIV Reservoir. Viruses 2025, 17, 1381. https://doi.org/10.3390/v17101381
Moura SSd, Caetano DG, Guimarães ML, da Silva RKM, Cabral N, da Costa Cruz Silva S, Ribeiro-Alves M, Teixeira SLM, Georg I, Santos DVGd, et al. Acute HIV Infection and ART Response: Insights into T Cell Subsets, Activation, Exhaustion, and Blood and GALT HIV Reservoir. Viruses. 2025; 17(10):1381. https://doi.org/10.3390/v17101381
Chicago/Turabian StyleMoura, Soraia Santana de, Diogo Gama Caetano, Monick Lindenmeyer Guimarães, Rayana Katylin Mendes da Silva, Natasha Cabral, Simone da Costa Cruz Silva, Marcelo Ribeiro-Alves, Sylvia L. M. Teixeira, Ingebourg Georg, Desirée Vieira Gomes dos Santos, and et al. 2025. "Acute HIV Infection and ART Response: Insights into T Cell Subsets, Activation, Exhaustion, and Blood and GALT HIV Reservoir" Viruses 17, no. 10: 1381. https://doi.org/10.3390/v17101381
APA StyleMoura, S. S. d., Caetano, D. G., Guimarães, M. L., da Silva, R. K. M., Cabral, N., da Costa Cruz Silva, S., Ribeiro-Alves, M., Teixeira, S. L. M., Georg, I., Santos, D. V. G. d., Nazer, S., Fraga, R. T., Hoagland, B., Villela, L., Grinsztejn, B. G. J., Veloso, V. G., Côrtes, F. H., & Cardoso, S. W. (2025). Acute HIV Infection and ART Response: Insights into T Cell Subsets, Activation, Exhaustion, and Blood and GALT HIV Reservoir. Viruses, 17(10), 1381. https://doi.org/10.3390/v17101381







