Impact of Vaccinating Adult Women Who Are HPV-Positive or with Confirmed Cervical SIL with the 9-Valent Vaccine—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Extraction
2.3. Risk-of-Bias Assessment
3. Results
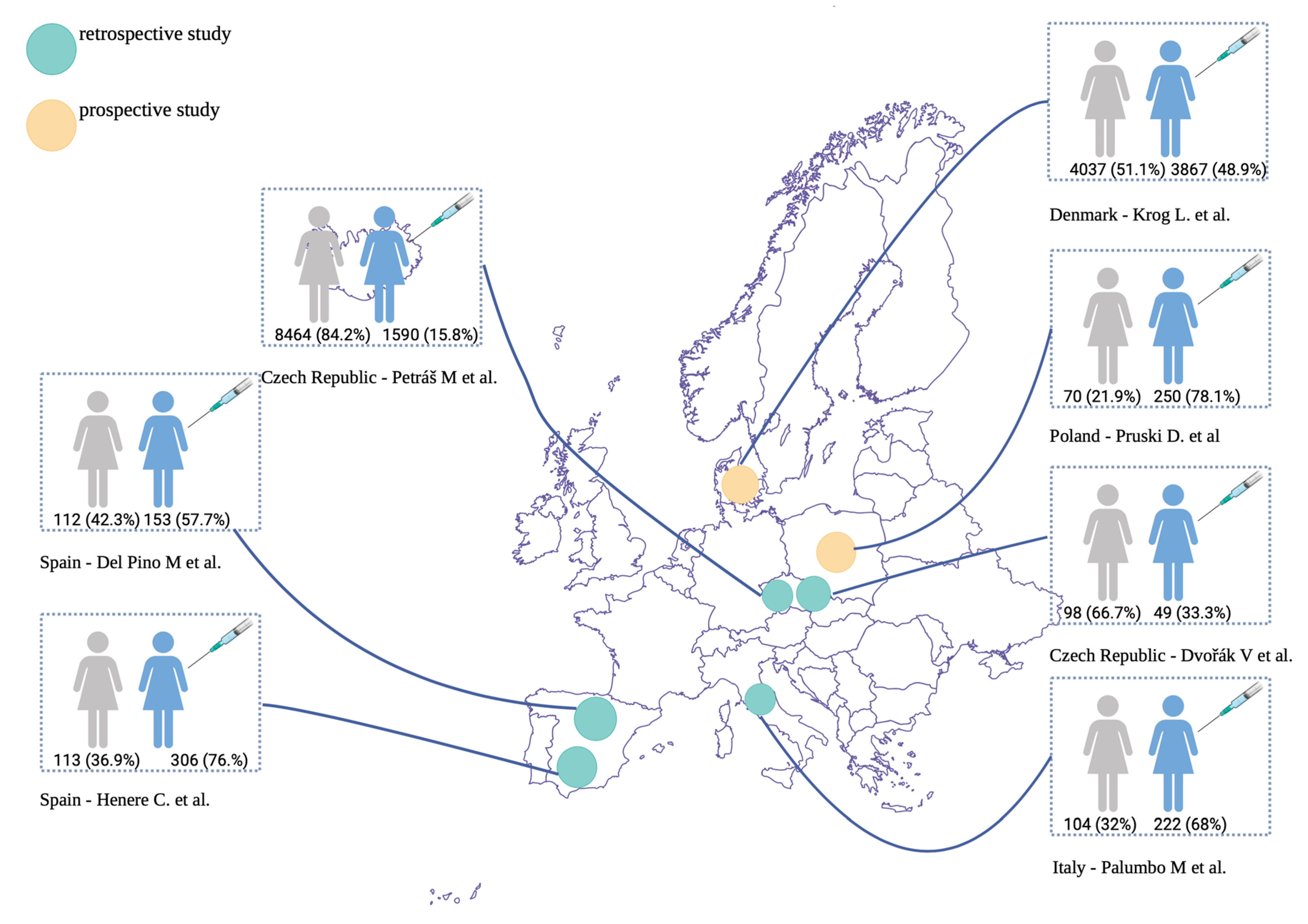
3.1. Characteristics of the Included Studies
3.2. Definition of HPV Remission/SIL Regression
3.3. Impacts of HPV Vaccination on HPV Remission/SIL Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galati, L.; Chiocca, S.; Duca, D.; Tagliabue, M.; Simoens, C.; Gheit, T.; Arbyn, M.; Tommasino, M. HPV and head and neck cancers: Towards early diagnosis and prevention. Tumour Virus Res. 2022, 14, 200245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senkomago, V.; Henley, S.J.; Thomas, C.C.; Mix, J.M.; Markowitz, L.E.; Saraiya, M. Human Papillomavirus-Attributable Cancers—United States, 2012–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 1 October 2025).
- Strander, B.; Hällgren, J.; Sparén, P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: Population-based cohort study of long te-m incidence and mortality. BMJ 2014, 348, f7361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muthukrishnan, M.; Loux, T.; Shacham, E.; Tiro, J.A.; Arnold, L.D. Barriers to human papillomavirus (HPV) vaccination among young adults, aged 18-35. Prev. Med. Rep. 2022, 29, 101942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phongsamart, W.; Lou, P.J.; Sukarom, I.; Wu, Y.H.; Zaidi, O.; Du, F.; Simon, A.; Bernauer, M. Integrative literature review on human papillomavirus vaccination recommendations in national immunization programs in select areas in the Asia-Pacific region. Hum. Vaccin. Immunother. 2024, 20, 2362449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tse, K.Y.; Tan, A.L.; Subedi, K.; Pervin, S.; Gupta, K.; Tjokroprawiro, B.A.; Woo, Y.L.; Wilailak, S.; Ochiai, K.; Lumbiganon, P.; et al. The AOFOG recommendations on human papillomavirus vaccination in the Asia-Pacific region. J. Obs. Gynaecol. Res. 2024, 50 (Suppl. S1), 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Dvořák, V.; Petráš, M.; Dvořák, V.; Lomozová, D.; Dlouhý, P.; Králová Lesná, I.; Pilka, R. Reduced risk of CIN2+ recurrence in women immunized with a 9-valent HPV vaccine post-excision: Retrospective cohort study. Hum. Vaccines Immunother. 2024, 20, 2343552. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.; Lavitola, G.; Di Filippo, C.; Foreste, V.; Granata, M.; Imperatore, O.; Ascione, M.; Della Corte, L.; Bifulco, G. Impact of Human papillomavirus 9-valent vaccine on viral clearance after surgical treatment: A single-center retrospective observational study. Eur. J. Obs. Gynecol. Reprod. Biol. 2025, 310, 113994. [Google Scholar] [CrossRef] [PubMed]
- Petráš, M.; Lomozová, D.; Dvořák, V.; Dvořák, V., Jr.; Malinová, J.; Trnková, M.; Fišer, I.; Dlouhý, P.; Rosina, J.; Lesná, I.K. Early and long-term effects of prophylactic and post-excision human papillomavirus vaccination on recurrent high-grade cervical intraepithelial neoplasia relative to margin status: A retrospective cohort study in the Czech Republic. Lancet Reg. Health Eur. 2025, 55, 101337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- del Pino, M.; Martí, C.; Torras, I.; Henere, C.; Munmany, M.; Marimon, L.; Saco, A.; Torné, A.; Ordi, J. HPV Vaccination as Adjuvant to Conization in Women with Cervical Intraepithelial Neoplasia: A Study under Real-Life Conditions. Vaccines 2020, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Krog, L.; Lycke, K.D.; Kahlert, J.; Randrup, T.H.; Jensen, P.T.; Rositch, A.F.; Hammer, A. Risk of progression of cervical intraepithelial neoplasia grade 2 in human papillomavirus-vaccinated and unvaccinated women: A population-based cohort study. Am. J. Obs. Gynecol. 2024, 230, 430.e1–430.e11. [Google Scholar] [CrossRef] [PubMed]
- Pruski, D.; Millert-Kalińska, S.; Jach, R.; Przybylski, M. Effect of vaccination against HPV in the HPV-positive patients not covered by primary prevention on the disappearance of infection. Sci. Rep. 2025, 15, 12642. [Google Scholar] [CrossRef]
- Henere, C.; Torné, A.; Llupià, A.; Aldea, M.; Martí, C.; Glickman, A.; Saco, A.; Marimon, L.; Manzotti, C.; Rakislova, N.; et al. HPV Vaccination in Women with Cervical Intraepithelial Neoplasia Undergoing Excisional Treatment: Insights into Unsolved Questions. Vaccines 2022, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies; University of Ottawa: Ottawa, ON, Canada, 2014. [Google Scholar]
- Pruski, D.; Fraszczak, J.; Iwaniec, K.; Przybylski, M.; Kedzia, W.; Gretkiewicz-Tomczyk, A.; Karowicz-Bilińska, A.; Spaczyński, M. Assessment of frequency of regression and progression of mild cervical neoplasia--LGSIL in women with positive high-risk HPV DNA test result. Ginekol. Pol. 2012, 83, 572–575. [Google Scholar] [PubMed]
- Przybylski, M.; Pruski, D.; Millert-Kalinska, S.; Zmaczynski, A.; Baran, R.; Horbaczewska, A.; Jach, R.; Zaborowska, L. Remission of HPV infection after LEEP-conization—A retrospective study. Ginekol. Pol. 2022, 93, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L. Ozone–Oxygen Therapy to Prevent HPV-Related Cancers of the Lower Gynecological Tract in Infected Patients: The Rationale for Further Developments. Cancers 2025, 17, 543. [Google Scholar] [CrossRef] [PubMed]
- Pruski, D.; Millert-Kalinska, S.; Lewek, A.; Kedzia, W. Sensitivity and specificity of HR HPV E6/E7 mRNA test in detecting cervical squamous intraepithelial lesion and cervical cancer. Ginekol. Pol. 2019, 90, 66–71. [Google Scholar] [CrossRef] [PubMed]

| Database | Number of Results | Search Strategy |
|---|---|---|
| PubMed | 393 |
|
| Scopus | 145 |
|
| Cochrane | 115 |
|
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
| Study | Dvořák V. et al. (2024) [9] | Palumbo M. et al. (2025) [10] | Petráš M. et al. (2025) [11] | Del Pino M. et al. (2020) [12] | Krog L. et al. (2024) [13] | Pruski D. et al. (2025) [14] | Henere C. et al. (2022) [15] |
|---|---|---|---|---|---|---|---|
| Main Aims of the Study | To evaluate the impact of post-excisional administration of the 9-valent HPV vaccine on the risk of CIN2+ recurrence in women. | To evaluate the effect of adjuvant 9-valent HPV vaccination following surgical excision or ablation in women with persistent low-grade (CIN1) or high-grade (CIN2–3) cervical intraepithelial neoplasia, compared with excision or ablation alone. | To estimate the effect of HPV vaccination on CIN2+ recurrence in relation to the timing of vaccination, administered either before or after cervical excisional treatment. | To compare the risk of persistent or recurrent HSIL following cervical conization between HPV-vaccinated and unvaccinated women. | To investigate whether HPV vaccination reduces the risk of progression from CIN2 to CIN3 or worse among women undergoing active surveillance, compared with unvaccinated women. | To evaluate cytological and HPV DNA outcomes following a full course of 9-valent HPV vaccination in women with HPV infection detected on cervical smear. | To evaluate the effect of vaccination timing (before or after excisional treatment) on protection against HSIL, as well as to assess the impact of vaccination on reducing post-treatment lesions in patients with persistent HPV infection following HSIL excisional treatment. |
| Study Design | Retrospective cohort study | Single-center retrospective observational study | Retrospective cohort study | Retrospective cohort study | Population-based cohort study | Prospective, ongoing, non-randomized study | Retrospective cohort study |
| Population (study group and control group) | 98 unvaccinated and 49 vaccinated women. | The vaccinated group comprised 68% (222/326) of participants, while 32% (104/326) were unvaccinated. | Of the 10,054 women enrolled, 919 were vaccinated after conization, 502 prophylactically, and 169 had an undetermined timing of vaccination. | 265 women were included in the study; 153 women (57.7%) accepted vaccination, and 112 (42.3%) refused the vaccine. | 7904 women, of whom 3867 (48.9%) were vaccinated at least 1 year before a diagnosis of CIN2. | Of 320 patients, 250 (78.1%) decided to be vaccinated, and 70 (21.9%) did not. | Vaccinated group: 306 (76.9%), of which 113 (36.9%) had the first dose before excision and 193 (63.1%) after; unvaccinated group: 92 (23.1%). |
| Time of Vaccination | On the day of conization or later, after surgical excision. | The first dose was administered either before surgery or within 30 days post-surgery. | Women were classified as vaccinated prophylactically or post-excision if their last HPV vaccine dose was administered before or within one year of conization, respectively. | The first dose of the vaccine was scheduled after HSIL/CIN2-3 diagnosis, and it was provided either immediately before or after conization; women who had conization from July 2016 to July 2017 and had not been previously vaccinated had the first dose within 1–12 months after HSIL/CIN2-3 diagnosis. | Exposure was defined as having received ≥1 dose of a human papillomavirus vaccine at least 1 year before the CIN2 diagnosis. | The first dose before conization or a month after conization. | 113 with the first dose before excision and 193 with the first dose after excision. |
| Results | The incidence rate of repeat conization: 18 per 100,000 person-days in the unvaccinated cohort, and 2 per 100,000 person-days in the vaccinated group. | Among women treated for CIN1, a positive HPV test was observed in 38% of unvaccinated women versus 18% of vaccinated women (p = 0.0169). In women treated for CIN2–3, 18% of unvaccinated women tested positive for HPV, compared with 8% in the vaccinated group (p = 0.0353). | CIN2+ recurrence was substantially reduced with vaccination. Among women with positive cone margins, 272 of 1568 unvaccinated women (51.6 per 1000 person-years) experienced recurrence, compared with only 6 cases each among 84 prophylactically vaccinated and 119 post-excision vaccinated women. Recurrence within 6 months of conization was reduced by 80% with prophylactic vaccination and 89% with post-excision vaccination. | Persistent/recurrent HSIL was less frequent in vaccinated than in unvaccinated women (3.3% vs. 10.7%, p = 0.015). HPV vaccination was associated with a reduced risk of persistent/recurrent HSIL. Vaccine uptake increased significantly, from 35.9% to 79.1%, when publicly funded. | Vaccination before age 15 reduced the risk of progression to CIN3 or worse by 35%, vaccination between ages 15 and 20 reduced the risk by 14%, while vaccination after age 20 showed no significant effect compared with unvaccinated women. | Post-vaccination outcomes compared with controls were as follows: same genotypes, 6.4% vs. 32.9%; partially same genotypes, 5.2% vs. 8.6%; complete remission, 72.4% vs. 45.7%; new infection, 12.4% vs. 5.7%; same/partially same genotype plus new infection, 3.6% vs. 7.1%. | Vaccination before treatment was associated with a lower rate of post-treatment HSIL compared with non-vaccinated women (0.9% vs. 6.5%; p = 0.047). Among women with persistent HPV infection after treatment, vaccinated women also had a lower prevalence of post-treatment HSIL than non-vaccinated women (2.6% vs. 10.5%; p = 0.043). |
| Conclusions | Post-conization HPV vaccination reduced the risk of recurrence of high-grade lesions by 87% (95% CI: 19–100%). | The human papillomavirus 9-valent vaccine was associated with a significant reduction in the proportion of women with a positive HPV test. | Regardless of timing, HPV vaccination has a beneficial long-term effect in lowering the risk of CIN2+ recurrence. The greatest benefit was observed in the first 6 months post-conization, with a positive cone margin. | HPV vaccination in women undergoing conization is associated with a 4.5-fold reduction in the risk of persistent/recurrent HSIL. Vaccination policies have an important impact on vaccination compliance. | Women who were vaccinated and who were undergoing active surveillance for CIN2 had a lower risk for progression to CIN3 or worse during 28 months of follow-up, when compared with women who were not vaccinated, but only if the vaccine was administered before the age of 20 years. | Vaccination against HPV significantly affects the disappearance of the viral infection in women not vaccinated during puberty. A statistically significant disappearance of HPV infection occurs in patients, both those diagnosed with HPV and undergoing LEEP. | HPV vaccination before treatment reduces the prevalence of post-treatment HSIL, suggesting that vaccination might even benefit women with persistent HPV after treatment. |
| Timeframe | Jan 2014 to Aug 2023 | 2020–2024 | 2010–2024 | Jan 2013 to Jul 2018 | Jan 2007 to Dec 2020 | 2020–2023 | Jul 2016 to Dec 2019 |
| Country | Czech Republic | Italy | Czech Republic | Spain | Denmark | Poland | Spain |
| National Vaccination Program | 2012, for 13-year-old girls; 2015, nonavalent vaccination for 13-year-old girls; 2024, nonavalent vaccination for 11–14-yo boys and girls. | 2012, for 13-year-old girls; 2015, nonavalent vaccination for 13-year-old girls; 2024, nonavalent vaccination for 11–14-year-old boys and girls. | In July 2017, the public health system of Catalonia started funding the HPV vaccine for women undergoing treatment. | In July 2017, the public health system of Catalonia started funding the HPV vaccine for women undergoing treatment. | |||
| Grade | Very good | Very good | Very good | Very good | Very good | Very good | Very good |
| Outcome | Vaccinated Group | Unvaccinated Group | p-Value |
|---|---|---|---|
| HPV complete remission | 72.4% | 45.7% | |
| CIN2+ recurrence after conization | 3.3% | 10.7% | 0.015 |
| Progression to CIN3 | 0.9% | 6.5% | |
| Positive HPV result in LSIL | 18% | 38% | 0.0169 |
| Positive HPV result in HSIL | 8% | 18% | 0.0353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruski, D.; Millert-Kalińska, S.; Jach, R.; Żurawski, J.; Przybylski, M. Impact of Vaccinating Adult Women Who Are HPV-Positive or with Confirmed Cervical SIL with the 9-Valent Vaccine—A Systematic Review. Viruses 2025, 17, 1377. https://doi.org/10.3390/v17101377
Pruski D, Millert-Kalińska S, Jach R, Żurawski J, Przybylski M. Impact of Vaccinating Adult Women Who Are HPV-Positive or with Confirmed Cervical SIL with the 9-Valent Vaccine—A Systematic Review. Viruses. 2025; 17(10):1377. https://doi.org/10.3390/v17101377
Chicago/Turabian StylePruski, Dominik, Sonja Millert-Kalińska, Robert Jach, Jakub Żurawski, and Marcin Przybylski. 2025. "Impact of Vaccinating Adult Women Who Are HPV-Positive or with Confirmed Cervical SIL with the 9-Valent Vaccine—A Systematic Review" Viruses 17, no. 10: 1377. https://doi.org/10.3390/v17101377
APA StylePruski, D., Millert-Kalińska, S., Jach, R., Żurawski, J., & Przybylski, M. (2025). Impact of Vaccinating Adult Women Who Are HPV-Positive or with Confirmed Cervical SIL with the 9-Valent Vaccine—A Systematic Review. Viruses, 17(10), 1377. https://doi.org/10.3390/v17101377








