Cellular eEF1G Inhibits Porcine Deltacoronavirus Replication by Binding Nsp12 and Disrupting Its Interaction with Viral Genomic RNA
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Plasmid Construction
2.3. Commercial and In-House Generated Antibodies
2.4. RNA Extraction and RT-PCR
2.5. Real-Time Quantitative PCR (RT-qPCR)
2.6. Western Blot
2.7. Co-Immunoprecipitation (Co-IP)
2.8. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.9. RNA Interference (RNAi)
2.10. 50% Tissue Culture Infectious Dose (TCID50) Assay
2.11. IFA
2.12. RNA Immunoprecipitation (RIP)
2.13. Strep II Pull-Down Assay
2.14. RNAscope In Situ Hybridization
2.15. Viral Adsorption and Internalization Assay
2.16. Cell Viability Assay
2.17. Statistical Analysis
3. Results
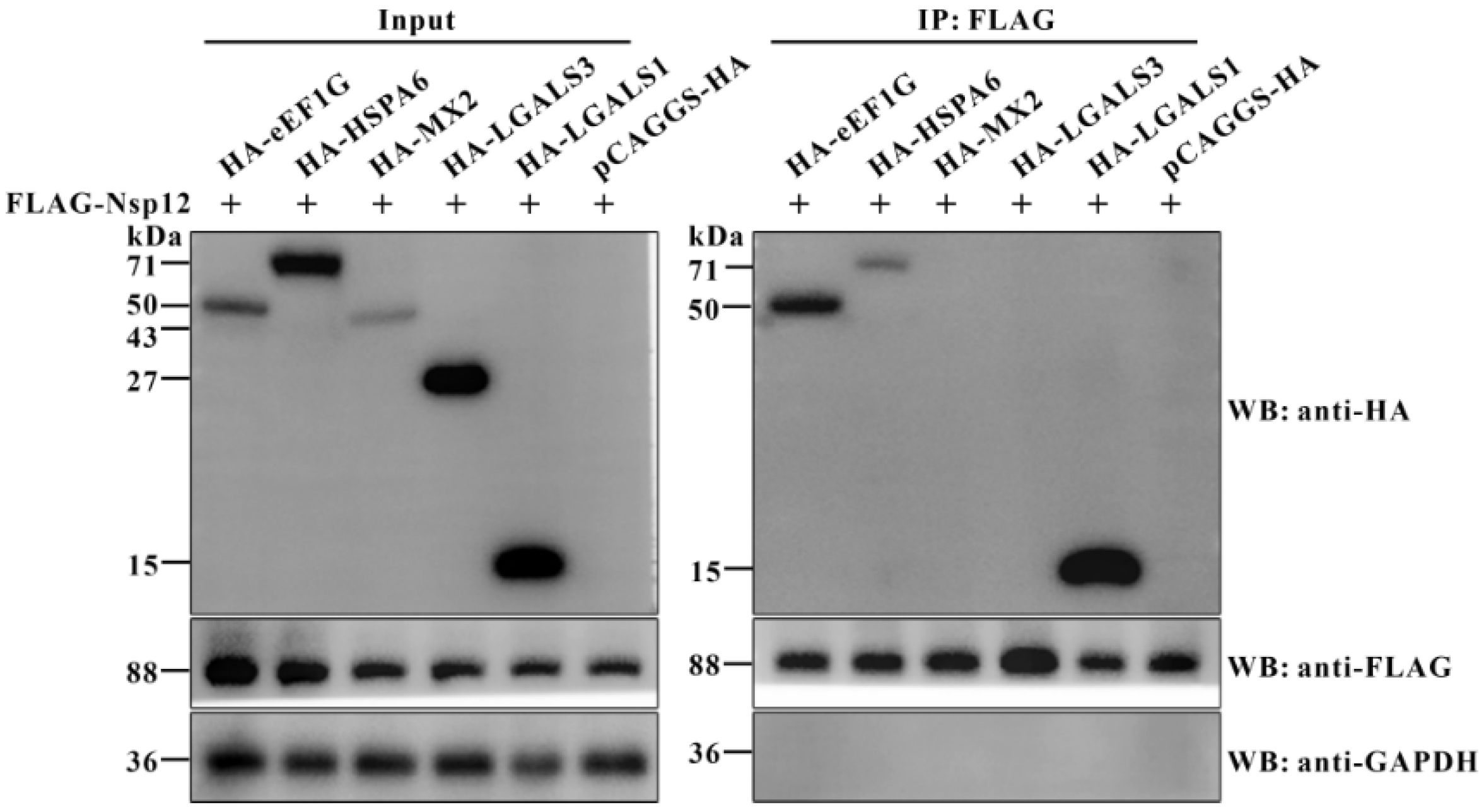
3.1. Screening and Identification of Host Proteins Interacting with PDCoV Nsp12 Protein
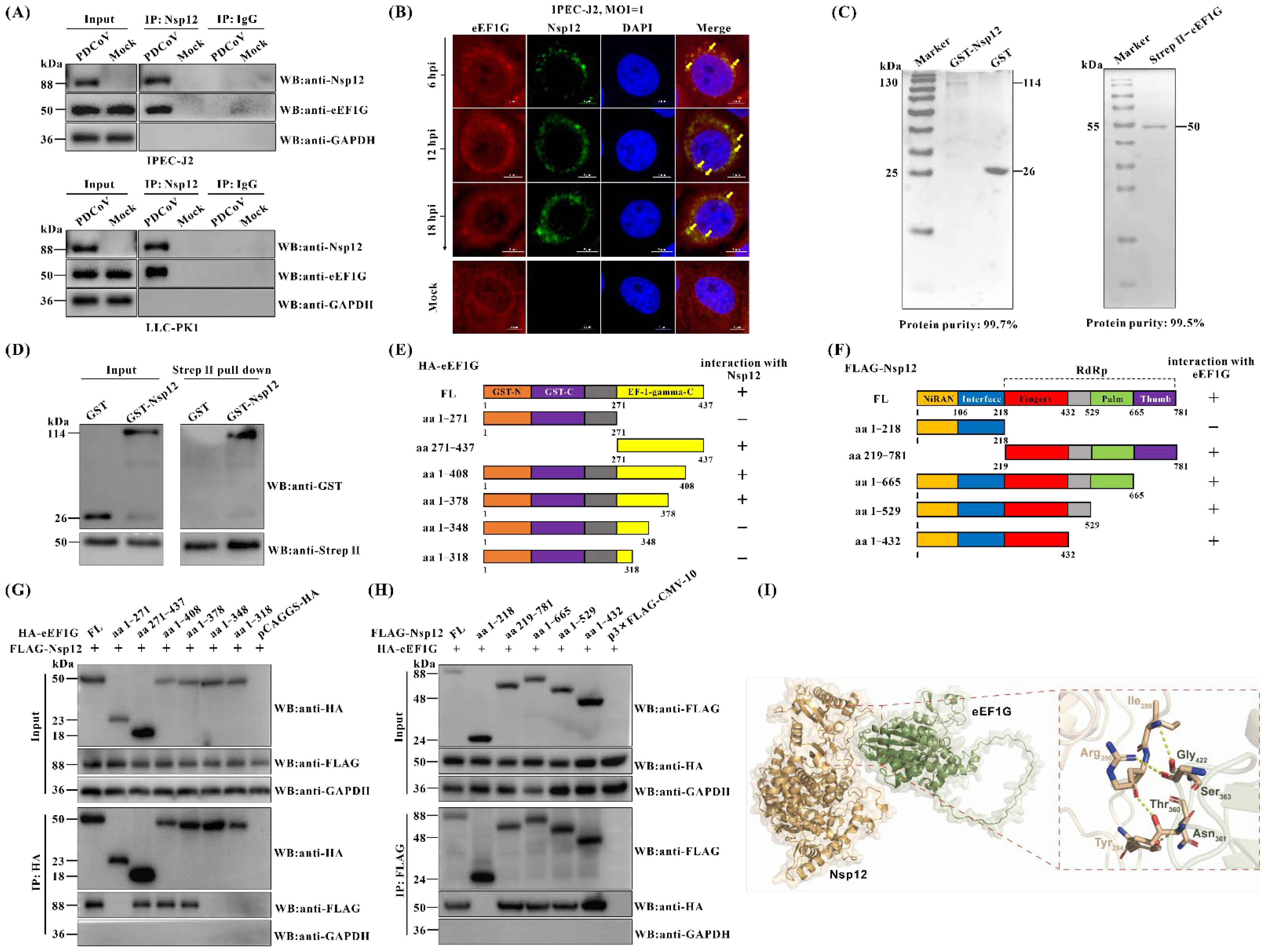
3.2. Cellular eEF1G Directly Interacts with the PDCoV Nsp12 Protein
3.3. Cellular eEF1G Acts as a Host Restriction Factor for PDCoV
3.4. eEF1G Functions to Inhibit the Synthesis of PDCoV Negative-Stranded RNA
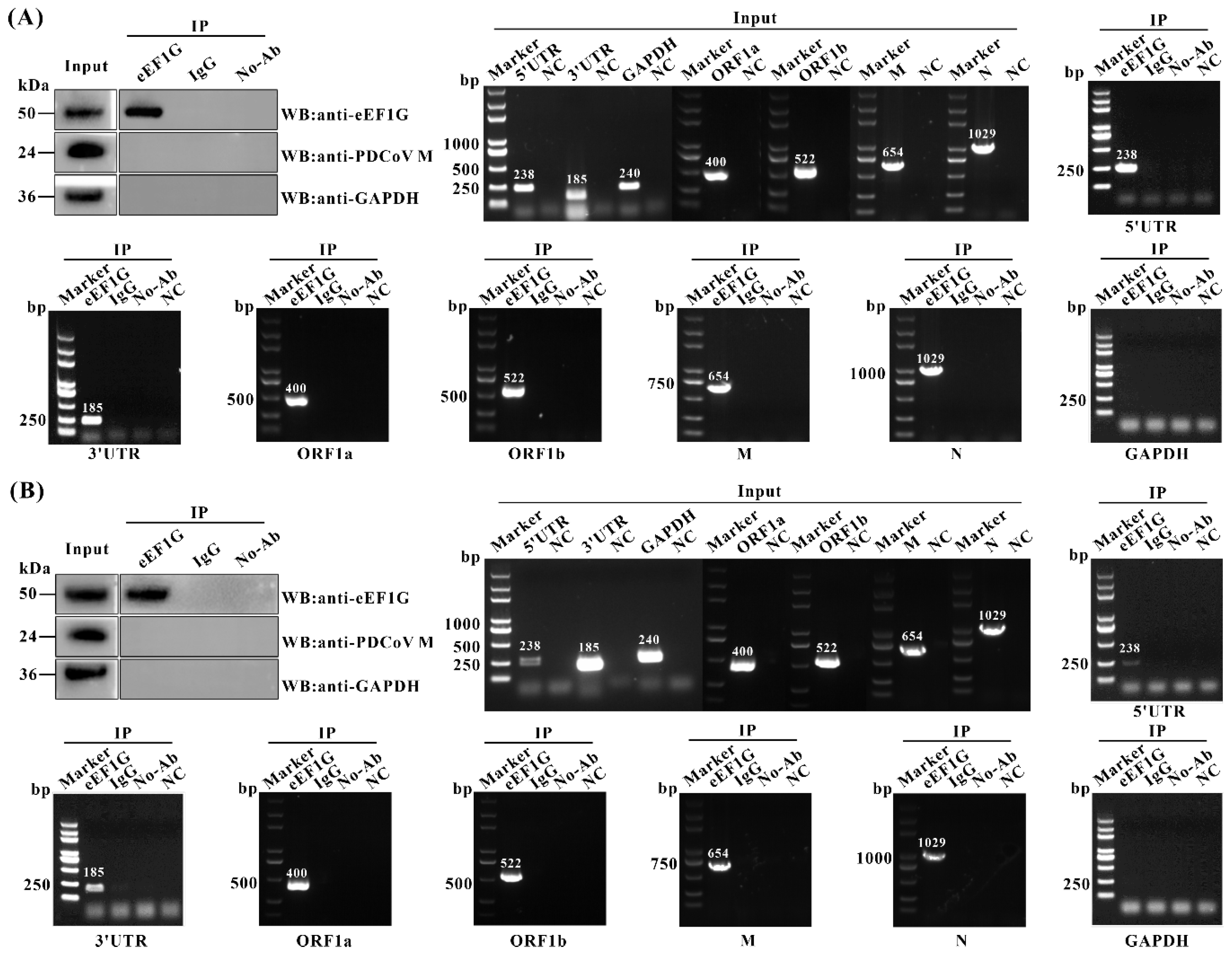
3.5. eEF1G Binds to the Genomic RNA of PDCoV During Infection
3.6. Cellular eEF1G Disrupts the Binding of Nsp12 to the PDCoV Genomic RNA During Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Yan, Q.H.; Liu, X.D.; Sun, Y.W.; Zeng, W.J.; Li, Y.W.; Zhao, F.F.; Wu, K.K.; Fan, S.Q.; Zhao, M.Q.; Chen, J.D.; et al. Swine enteric coronavirus: Diverse pathogen-host interactions. Int. J. Mol. Sci. 2022, 23, 3953. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Hu, H.; Jung, K.; Vlasova, A.N.; Chepngeno, J.; Lu, Z.Y.; Wang, Q.H.; Saif, L.J. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J. Clin. Microbiol. 2015, 53, 1537–1548. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, H.C.; Nguyen, V.G.; Moon, H.J.; Kim, H.K.; Park, S.J.; Lee, C.H.; Lee, G.E.; Park, B.K. Detection and phylogenetic analysis of porcine deltacoronavirus in Korean swine farms, 2015. Transbound. Emerg. Dis. 2016, 63, 248–252. [Google Scholar] [CrossRef]
- Suzuki, T.; Shibahara, T.; Imai, N.; Yamamoto, T.; Ohashi, S. Genetic characterization and pathogenicity of Japanese porcine deltacoronavirus. Infect. Genet. Evol. 2018, 61, 176–182. [Google Scholar] [CrossRef]
- Saeng-Chuto, K.; Lorsirigool, A.; Temeeyasen, G.; Vui, D.T.; Stott, C.J.; Madapong, A.; Tripipat, T.; Wegner, M.; Intrakamhaeng, M.; Chongcharoen, W.; et al. Different lineage of porcine deltacoronavirus in Thailand, Vietnam and Lao PDR in 2015. Transbound. Emerg. Dis. 2017, 64, 3–10. [Google Scholar] [CrossRef]
- Ajayi, T.; Dara, R.; Misener, M.; Pasma, T.; Moser, L.; Poljak, Z. Herd-level prevalence and incidence of porcine epidemic diarrhoea virus (PEDV) and porcine deltacoronavirus (PDCoV) in swine herds in Ontario, Canada. Transbound. Emerg. Dis. 2018, 65, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Z.; Wang, Q.H.; Kenney, S.P.; Jung, K.N.; Vlasova, A.N.; Saif, L.J. Porcine deltacoronaviruses: Origin, evolution, cross-species transmission and zoonotic potential. Pathogens 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, H.Y. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021, 45, 75–86. [Google Scholar] [CrossRef]
- Ye, X.; Chen, Y.J.; Zhu, X.Y.; Guo, J.H.; Xie, D.; Hou, Z.Z.; Xu, S.G.; Zhou, J.W.; Fang, L.R.; Wang, D.; et al. Cross-species transmission of deltacoronavirus and the origin of porcine deltacoronavirus. Evol. Appl. 2020, 13, 2246–2253. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Tagliamonte, M.S.; White, S.K.; Elbadry, M.A.; Alam, M.M.; Stephenson, C.J.; Bonny, T.S.; Loeb, J.C.; Telisma, T.; Chavannes, S.; et al. Independent infections of porcine deltacoronavirus among Haitian children. Nature 2021, 600, 133–137. [Google Scholar] [CrossRef]
- He, W.T.; Ji, X.; He, W.; Dellicour, S.; Wang, S.L.; Li, G.R.; Zhang, L.T.; Gilbert, M.; Zhu, H.N.; Xing, G.; et al. Genomic epidemiology, evolution, and transmission dynamics of porcine deltacoronavirus. Mol. Biol. Evol. 2020, 37, 2641–2654. [Google Scholar] [CrossRef]
- Jung, K.; Hu, H.; Saif, L.J. Porcine deltacoronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res. 2016, 226, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Ye, G.; Si, Y.; Shen, Z.; Liu, Z.; Shi, Y.J.; Xiao, S.B.; Fu, Z.F.; Peng, G.Q. Structure of the multiple functional domains from coronavirus nonstructural protein 3. Emerg. Microbes Infect. 2021, 10, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef]
- Subissi, L.; Posthuma, C.C.; Collet, A.; Zevenhoven-Dobbe, J.C.; Gorbalenya, A.E.; Decroly, E.; Snijder, E.J.; Canard, B.; Imbert, I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc. Natl. Acad. Sci. USA 2014, 111, E3900–E3909. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.J.; Pruijssers, A.J.; Lee, H.W.; Gordon, C.J.; Tchesnokov, E.P.; Gribble, J.; George, A.S.; Hughes, T.M.; Lu, X.T.; Li, J.N.; et al. Mutations in the SARS-CoV-2 RNA-dependent RNA polymerase confer resistance to remdesivir by distinct mechanisms. Sci. Transl. Med. 2022, 14, eabo0718. [Google Scholar] [CrossRef]
- Dansako, H.; Ikeda, M.; Ariumi, Y.; Togashi, Y.; Kato, N. Hepatitis C virus NS5B triggers an MDA5-mediated innate immune response by producing dsRNA without the replication of viral genomes. FEBS J. 2023, 291, 1119–1130. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhou, Z.; Xiao, X.; Tian, Z.Q.; Dong, X.J.; Wang, C.H.; Li, L.; Ren, L.L.; Lei, X.B.; Xiang, Z.C.; et al. SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation. Cell. Mol. Immunol. 2021, 18, 945–953. [Google Scholar] [CrossRef]
- Riis, B.; Rattan, S.I.S.; Clark, B.F.C.; Merrick, W.C. Eukaryotic protein elongation-factors. Trends Biochem. Sci. 1990, 15, 420–424. [Google Scholar] [CrossRef]
- Le Sourd, F.; Boulben, S.; Le Bouffant, R.; Cormier, P.; Morales, J.; Belle, R.; Mulner-Lorillon, O. eEF1B: At the dawn of the 21st century. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2006, 1759, 13–31. [Google Scholar] [CrossRef]
- Warren, K.; Wei, T.; Li, D.; Qin, F.; Warrilow, D.; Lin, M.-H.; Sivakumaran, H.; Apolloni, A.; Abbott, C.M.; Jones, A.; et al. Eukaryotic elongation factor 1 complex subunits are critical HIV-1 reverse transcription cofactors. Proc. Natl. Acad. Sci. USA 2012, 109, 9587–9592. [Google Scholar] [CrossRef] [PubMed]
- Sammaibashi, S.; Yamayoshi, S.; Kawaoka, Y. Strain-specific contribution of eukaryotic elongation factor 1 gamma to the translation of influenza A virus proteins. Front. Microbiol. 2018, 9, 1446. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.; Sasvari, Z.; Izotova, L.; Kinzy, T.G.; Nagy, P.D. Synergistic roles of eukaryotic translation elongation factors 1Bγ and 1A in stimulation of tombusvirus minus-strand synthesis. PLoS Pathog. 2011, 7, e1002438. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Ward, A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019, 10, 2342. [Google Scholar] [CrossRef]
- Yang, Z.S.; Lin, C.Y.; Huang, S.W.; Wang, W.H.; Urbina, A.N.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; Wang, S.F. Regulatory roles of galectins on influenza A virus and their potential as a therapeutic strategy. Biomed. Pharmacother. 2021, 139, 111713. [Google Scholar] [CrossRef]
- Su, Y.S.; Hwang, L.H.; Chen, C.J. Heat shock protein A6, a novel HSP70, is induced during enterovirus A71 infection to facilitate internal ribosomal entry site-mediated translation. Front. Microbiol. 2021, 12, 664955. [Google Scholar] [CrossRef]
- Wang, Y.X.; Niklasch, M.; Liu, T.T.; Wang, Y.; Shi, B.S.; Yuan, W.J.; Baumert, T.F.; Yuan, Z.H.; Tong, S.P.; Nassal, M.; et al. Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication. J. Hepatol. 2020, 72, 865–876. [Google Scholar] [CrossRef]
- Qin, P.; Du, E.Z.; Luo, W.T.; Yang, Y.L.; Zhang, Y.Q.; Wang, B.; Huang, Y.W. Characteristics of the life cycle of porcine deltacoronavirus (PDCoV) in vitro: Replication kinetics, cellular ultrastructure and virion morphology, and evidence of inducing autophagy. Viruses 2019, 11, 455. [Google Scholar] [CrossRef]
- te Velthuis, A.J.W. Common and unique features of viral RNA-dependent polymerases. Cell. Mol. Life Sci. 2014, 71, 4403–4420. [Google Scholar] [CrossRef] [PubMed]
- Angelini, M.M.; Akhlaghpour, M.; Neuman, B.W.; Buchmeier, M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio 2013, 4, e00524-13. [Google Scholar] [CrossRef]
- Yan, L.M.; Ge, J.; Zheng, L.T.; Zhang, Y.; Gao, Y.; Wang, T.; Huang, Y.C.; Yang, Y.X.; Gao, S.; Li, M.Y.; et al. Cryo-EM structure of an extended SARS-CoV-2 replication and transcription complex reveals an intermediate state in cap synthesis. Cell 2021, 184, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.L.; Luo, Y.; Wang, Q.; Geng, R.; Chen, Y.; Liu, M.Q.; Li, B.; Chen, J.; Wu, C.G.; Jia, J.K.; et al. Identification of TMEM53 as a novel SADS-CoV restriction factor that targets viral RNA-dependent RNA polymerase. Emerg. Microbes Infect. 2023, 12, 2249120. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, F.; Sun, H.Z.; Liu, K.P.; Chen, Z.; Yu, B.C.; Hao, H.J.; Liu, H.Z.; Ding, S.; Zhang, X.Y.; et al. Characterization of ACTN4 as a novel antiviral target against SARS-CoV-2. Signal Transduct. Target. Ther. 2024, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Slanina, H.; Madhugiri, R.; Wenk, K.; Reinke, T.; Schultheiss, K.; Schultheis, J.; Karl, N.; Linne, U.; Ziebuhr, J. Conserved characteristics of NMPylation activities of alpha- and betacoronavirus NiRAN domains. J. Virol. 2023, 97, e0046523. [Google Scholar] [CrossRef]
- Guo, S.S.; Lei, X.B.; Chang, Y.; Zhao, J.Y.; Wang, J.; Dong, X.J.; Liu, Q.; Zhang, Z.X.; Wang, L.D.; Yi, D.R.; et al. SARS-CoV-2 hijacks cellular kinase CDK2 to promote viral RNA synthesis. Signal Transduct. Target. Ther. 2022, 7, 400. [Google Scholar] [CrossRef]
- Achilonu, I.; Elebo, N.; Hlabano, B.; Owen, G.R.; Papathanasopoulos, M.; Dirr, H.W. An update on the biophysical character of the human eukaryotic elongation factor 1 beta: Perspectives from interaction with elongation factor 1 gamma. J. Mol. Recognit. 2018, 31, e2708. [Google Scholar] [CrossRef]
- Fan, Y.; Schlierf, M.; Gaspar, A.C.; Dreux, C.; Kpebe, A.; Chaney, L.; Mathieu, A.; Hitte, C.; Grémy, O.; Sarot, E.; et al. Drosophila translational elongation factor-1gamma is modified in response to DOA kinase activity and is essential for cellular viability. Genetics 2010, 184, 141–154. [Google Scholar] [CrossRef]
- Gao, P.; Chai, Y.; Song, J.; Liu, T.; Chen, P.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. Reprogramming the unfolded protein response for replication by porcine reproductive and respiratory syndrome virus. PLoS Pathog. 2019, 15, e1008169. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, A.; Guichou, J.F.; Brillet, R.; Ahnou, N.; Hernandez, E.; Pallier, C.; Pawlotsky, J.M. Inhibition of RNA binding to hepatitis C virus RNA-dependent RNA polymerase: A new mechanism for antiviral intervention. Nucleic Acids Res. 2014, 42, 9399–9409. [Google Scholar] [CrossRef]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Linoleic acid binds to SARS-CoV-2 RdRp and represses replication of seasonal human coronavirus OC43. Sci. Rep. 2022, 12, 19114. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, C.; Liu, N.; Zhang, F.; Dong, H.; Wang, S.; Chen, M.; Jiang, X.; Zhang, K.; Gu, L. SARS-CoV-2 RdRp uses NDPs as a substrate and is able to incorporate NHC into RNA from diphosphate form molnupiravir. Int. J. Biol. Macromol. 2023, 226, 946–955. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, W.; Ge, X.; Zhou, L.; Guo, X.; Han, J.; Zhang, Y.; Yang, H. Cellular eEF1G Inhibits Porcine Deltacoronavirus Replication by Binding Nsp12 and Disrupting Its Interaction with Viral Genomic RNA. Viruses 2025, 17, 1369. https://doi.org/10.3390/v17101369
Yin W, Ge X, Zhou L, Guo X, Han J, Zhang Y, Yang H. Cellular eEF1G Inhibits Porcine Deltacoronavirus Replication by Binding Nsp12 and Disrupting Its Interaction with Viral Genomic RNA. Viruses. 2025; 17(10):1369. https://doi.org/10.3390/v17101369
Chicago/Turabian StyleYin, Weijia, Xinna Ge, Lei Zhou, Xin Guo, Jun Han, Yongning Zhang, and Hanchun Yang. 2025. "Cellular eEF1G Inhibits Porcine Deltacoronavirus Replication by Binding Nsp12 and Disrupting Its Interaction with Viral Genomic RNA" Viruses 17, no. 10: 1369. https://doi.org/10.3390/v17101369
APA StyleYin, W., Ge, X., Zhou, L., Guo, X., Han, J., Zhang, Y., & Yang, H. (2025). Cellular eEF1G Inhibits Porcine Deltacoronavirus Replication by Binding Nsp12 and Disrupting Its Interaction with Viral Genomic RNA. Viruses, 17(10), 1369. https://doi.org/10.3390/v17101369









