Congenital Human Cytomegalovirus and the Complement System
Abstract
1. Introduction
2. Human Cytomegalovirus
3. The Human Complement System
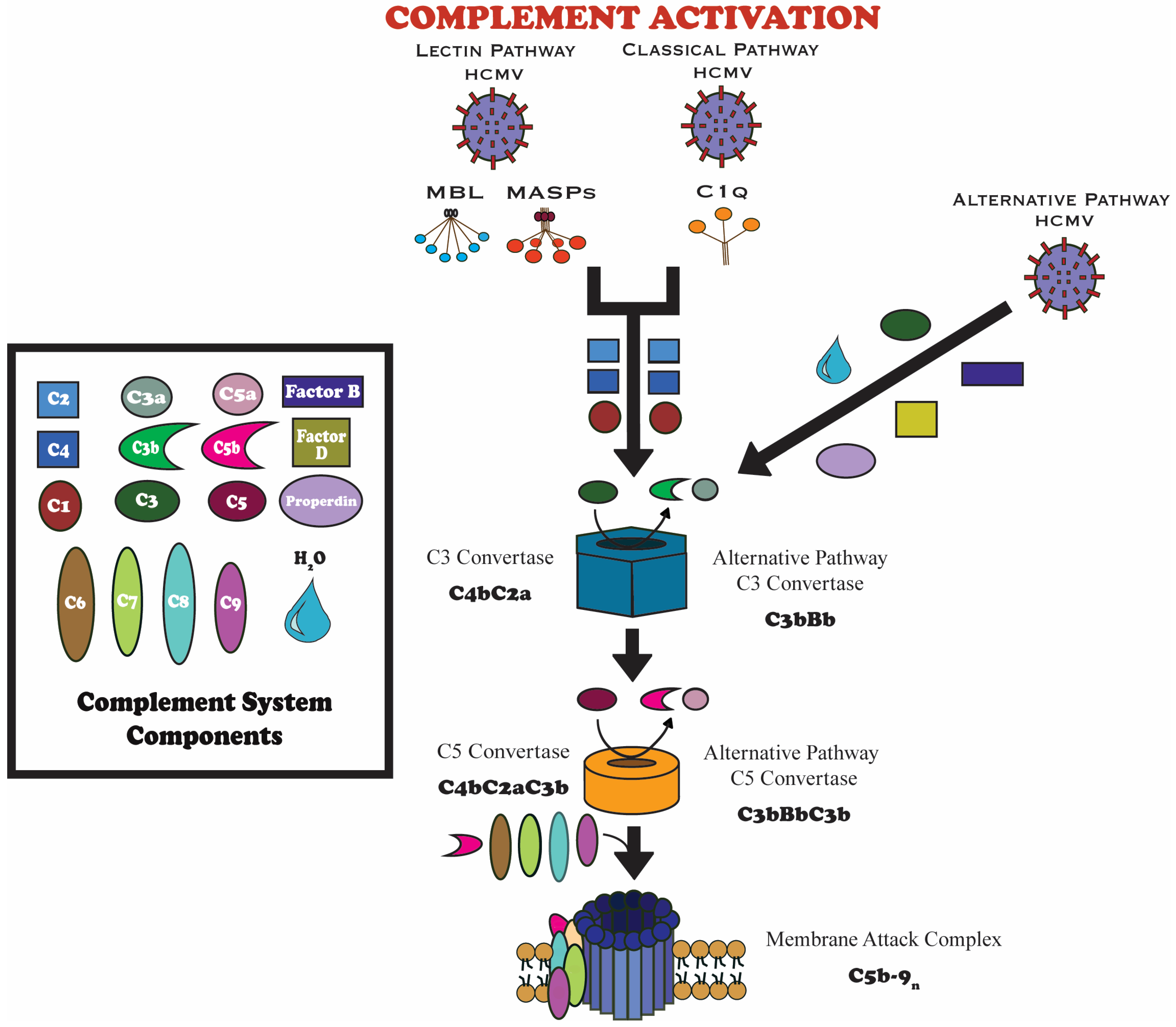
3.1. Complement System Overview
3.2. Complement Pathways
4. Role of Complement in Fetal Development and Pregnancy
4.1. Complement-Mediated Neural Development
4.2. The Complement System in Placental Biology
4.3. Balancing Immunity and Tolerance: The Role of Complement in Gestation
5. Complement Activation During Congenital HCMV Infection and Viral Modulation of Complement in Pregnancy
5.1. Evidence of Activation and Pathophysiological Consequences for the Fetus
5.2. Known Viral Strategies and Potential Fetal Compartment-Specific Modulators
6. Complement and Neurodevelopmental Outcomes
6.1. Disruption of Microglial Pruning and Synaptic Refinement
6.2. Injury, Neuroinflammation, and Long-Term Cognitive Risks
7. Clinical Implications and Therapeutic Potential
7.1. Complement as a Diagnostic and Prognostic Biomarker
7.2. Complement-Targeted Therapeutics
8. Challenges and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodrum, F.; Britt, W.; Mocarski, E.S. Cytomegalovirus. In Fields Virology: DNA Viruses, 7th ed.; Knipe, D.M., Howley, P., Eds.; Wolters Kluwer Health, Lippincott and Williams & Wilkins: Philadelphia, PA, USA, 2021; Volume 1, pp. 389–444. [Google Scholar]
- Krug, L.T.; Pellett, P.E. The Family Herpesviridae: A Brief Introduction. In Fields Virology: DNA Viruses, 7th ed.; Knipe, D.M., Howley, P., Eds.; Wolters Kluwer Health, Lippincott and Williams & Wilkins: Philadelphia, PA, USA, 2021; Volume 1, pp. 212–234. [Google Scholar]
- Salomè, S.; Corrado, F.R.; Mazzarelli, L.L.; Maruotti, G.M.; Capasso, L.; Blazquez-Gamero, D.; Raimondi, F. Congenital cytomegalovirus infection: The state of the art and future perspectives. Front. Pediatr. 2023, 11, 1276912. [Google Scholar] [CrossRef]
- Khalil, A.; Heath, P.T.; Jones, C.E.; Soe, A.; Ville, Y.G.; the Royal College of Obstetricians and Gynaecologists. Congenital Cytomegalovirus Infection: Update on Screening, Diagnosis and Treatment: Scientific Impact Paper No. 56. BJOG 2024, 132, e42–e52. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Hashida, N. Overview of Cytomegalovirus Ocular Diseases: Retinitis, Corneal Endotheliitis, and Iridocyclitis. Viruses 2024, 16, 1110. [Google Scholar] [CrossRef]
- Pass, R.F.; Anderson, B. Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. J. Pediatr. Infect. Dis. Soc. 2014, 3 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Ross, S.A.; Fowler, K.B. Congenital Cytomegalovirus Infection: Clinical Outcome. Clin. Infect. Dis. 2013, 57, S178–S181. [Google Scholar] [CrossRef]
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries with Universal Screening: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2120736. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R. Cytomegalovirus vaccines and methods of production (WO20009049138): The emerging recognition of the importance of virus neutralization at the epithelial/endothelial interface. Expert Opin. Ther. Pat. 2010, 20, 597–602. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Wang, D.; Oualim, A.; Diamond, D.J.; Kotton, C.N.; Mossman, S.; Carfi, A.; Anderson, D.; Dormitzer, P.R. The Status of Vaccine Development Against the Human Cytomegalovirus. J. Infect. Dis. 2020, 221, S113–S122. [Google Scholar] [CrossRef]
- Schleiss, M.R.; Crooks, C.M.; Karthigeyan, K.P.; Kruc, R.M.; Otero, C.E.; Wang, H.S.; Permar, S.R.; Plotkin, S.A.; Gautam, R. Proceedings of the Conference “CMV Vaccine Development-How Close Are We?” (27–28 September 2023). Vaccines 2024, 12, 1231. [Google Scholar] [CrossRef]
- West, E.E.; Kolev, M.; Kemper, C. Complement and the Regulation of T Cell Responses. Annu. Rev. Immunol. 2018, 36, 309–338. [Google Scholar] [CrossRef]
- Lubbers, R.; van Essen, M.F.; van Kooten, C.; Trouw, L.A. Production of complement components by cells of the immune system. Clin. Exp. Immunol. 2017, 188, 183–194. [Google Scholar] [CrossRef]
- Lujan, E.; Zhang, I.; Garon, A.C.; Liu, F. The Interactions of the Complement System with Human Cytomegalovirus. Viruses 2024, 16, 1171. [Google Scholar] [CrossRef]
- Kolev, M.; Le Friec, G.; Kemper, C. Complement--tapping into new sites and effector systems. Nat. Rev. Immunol. 2014, 14, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond. Front. Immunol. 2020, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Presumey, J.; Bialas, A.R.; Carroll, M.C. Complement System in Neural Synapse Elimination in Development and Disease. Adv. Immunol. 2017, 135, 53–79. [Google Scholar] [CrossRef]
- Perry, V.H.; O’Connor, V. C1q: The perfect complement for a synaptic feast? Nat. Rev. Neurosci. 2008, 9, 807–811. [Google Scholar] [CrossRef]
- Fisher, S.; Genbacev, O.; Maidji, E.; Pereira, L. Human Cytomegalovirus Infection of Placental Cytotrophoblasts In Vitro and In Utero: Implications for Transmission and Pathogenesis. J. Virol. 2000, 74, 6808–6820. [Google Scholar] [CrossRef]
- Cannon, M.J.; Hyde, T.B.; Schmid, D.S. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol. 2011, 21, 240–255. [Google Scholar] [CrossRef]
- Krstanović, F.; Britt, W.J.; Jonjić, S.; Brizić, I. Cytomegalovirus Infection and Inflammation in Developing Brain. Viruses 2021, 13, 1078. [Google Scholar] [CrossRef]
- Forte, E.; Zhang, Z.; Thorp, E.B.; Hummel, M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay with the Host Immune Response. Front. Cell Infect. Microbiol. 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.; Sissons, P. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 2006, 87, 1763–1779. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, T.; Hò, G.; Pump, W.; Blasczyk, R.; Bade-Doeding, C. Battle Between Host Immune Cellular Responses and HCMV Immune Evasion. Int. J. Mol. Sci. 2019, 20, 3626. [Google Scholar] [CrossRef]
- Grgic, I.; Gorenec, L. Human Cytomegalovirus (HCMV) Genetic Diversity, Drug Resistance Testing and Prevalence of the Resistance Mutations: A Literature Review. Trop. Med. Infect. Dis. 2024, 9, 49. [Google Scholar] [CrossRef]
- Lynch, K.; Martson, A.G. The importance of drug exposure in the development of cytomegalovirus resistance. Int. J. Antimicrob. Agents 2025, 66, 107537. [Google Scholar] [CrossRef]
- Walti, C.S.; Khanna, N.; Avery, R.K.; Helantera, I. New Treatment Options for Refractory/Resistant CMV Infection. Transpl. Int. 2023, 36, 11785. [Google Scholar] [CrossRef] [PubMed]
- Boxer, L.; Dale, D.C. Neutropenia: Causes and consequences. Semin. Hematol. 2002, 39, 75–81. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Domanski, L.; Dziedziejko, V.; Kajdy, A.; Stefanska, K.; Kwiatkowski, S. The Mechanism of Drug Nephrotoxicity and the Methods for Preventing Kidney Damage. Int. J. Mol. Sci. 2021, 22, 6109. [Google Scholar] [CrossRef]
- Krauter, S.; Buscher, N.; Brauchle, E.; Ortega Iannazzo, S.; Penner, I.; Kramer, N.; Gogesch, P.; Thomas, S.; Kreutz, M.; Dejung, M.; et al. An Attenuated Strain of Human Cytomegalovirus for the Establishment of a Subviral Particle Vaccine. Vaccines 2022, 10, 1326. [Google Scholar] [CrossRef]
- Riccomagno, M.M.; Kolodkin, A.L. Sculpting neural circuits by axon and dendrite pruning. Annu. Rev. Cell Dev. Biol. 2015, 31, 779–805. [Google Scholar] [CrossRef]
- Nimmo, J.; Byrne, R.A.J.; Daskoulidou, N.; Watkins, L.M.; Carpanini, S.M.; Zelek, W.M.; Morgan, B.P. The complement system in neurodegenerative diseases. Clin. Sci. 2024, 138, 387–412. [Google Scholar] [CrossRef]
- Chighizola, C.B.; Lonati, P.A.; Trespidi, L.; Meroni, P.L.; Tedesco, F. The Complement System in the Pathophysiology of Pregnancy and in Systemic Autoimmune Rheumatic Diseases During Pregnancy. Front. Immunol. 2020, 11, 2084. [Google Scholar] [CrossRef]
- Garred, P.; Tenner, A.J.; Mollnes, T.E. Therapeutic Targeting of the Complement System: From Rare Diseases to Pandemics. Pharmacol. Rev. 2021, 73, 792–827. [Google Scholar] [CrossRef]
- Pierik, E.; Prins, J.R.; van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of Complement Activation and Placental Dysfunction: A Potential Target to Treat Preeclampsia? Front. Immunol. 2020, 10, 3098. [Google Scholar] [CrossRef]
- Balasundaram, P.; Farhana, A. Immunology at the Maternal-Fetal Interface. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lynch, A.M.; Salmon, J.E. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta 2010, 31, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Burwick, R.M.; Feinberg, B.B. Complement activation and regulation in preeclampsia and hemolysis, elevated liver enzymes, and low platelet count syndrome. Am. J. Obstet. Gynecol. 2022, 226, S1059–S1070. [Google Scholar] [CrossRef] [PubMed]
- Lillegard, K.E.; Loeks-Johnson, A.C.; Opacich, J.W.; Peterson, J.M.; Bauer, A.J.; Elmquist, B.J.; Regal, R.R.; Gilbert, J.S.; Regal, J.F. Differential effects of complement activation products C3a and C5a on cardiovascular function in hypertensive pregnant rats. J. Pharmacol. Exp. Ther. 2014, 351, 344–351. [Google Scholar] [CrossRef]
- Klos, A.; Tenner, A.J.; Johswich, K.O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef]
- Yang, L.; Semmes, E.C.; Ovies, C.; Megli, C.; Permar, S.; Gilner, J.B.; Coyne, C.B. Innate immune signaling in trophoblast and decidua organoids defines differential antiviral defenses at the maternal-fetal interface. eLife 2022, 11, e79794. [Google Scholar] [CrossRef] [PubMed]
- Regal, J.F.; Gilbert, J.S.; Burwick, R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015, 67, 56–70. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, A.K.; Kadni, T.S.; Mullick, J.; Sahu, A. Virus-Encoded Complement Regulators: Current Status. Viruses 2021, 13, 208. [Google Scholar] [CrossRef]
- Hung, S.L.; Srinivasan, S.; Friedman, H.M.; Eisenberg, R.J.; Cohen, G.H. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J. Virol. 1992, 66, 4013–4027. [Google Scholar] [CrossRef]
- Spear, G.; Lurain, N.; Parker, C.; Ghassemi, M.; Payne, G.; Saifuddin, M. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses. Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV). J. Immunol. 1995, 155, 4376–4381. [Google Scholar] [CrossRef]
- Smith, W.; Tomasec, P.; Aicheler, R.; Loewendorf, A.; Nemčovičová, I.; Wang, E.; Stanton, R.; Macauley, M.; Norris, P.; Willen, L.; et al. Human cytomegalovirus glycoprotein UL141 targets the TRAIL death receptors to thwart host innate antiviral defenses. Cell Host Microbe 2013, 13, 324–335. [Google Scholar] [CrossRef]
- Magdalon, J.; Mansur, F.; Teles E Silva, A.; de Goes, V.; Reiner, O.; Sertié, A. Complement System in Brain Architecture and Neurodevelopmental Disorders. Front. Neurosci. 2020, 14, 23. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Hu, W.; Zou, H.; Luo, Q.; Deng, W.; Cao, S. The complement system: A potential target for the comorbidity of chronic pain and depression. Korean J. Pain 2024, 37, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, C.; Keymeulen, A.; Schiettecatte, E.; Acke, F.; Dhooge, I.; Smets, K.; Herregods, N. Brain MRI findings in newborns with congenital cytomegalovirus infection: Results from a large cohort study. Eur. Radiol. 2021, 31, 8001–8010. [Google Scholar] [CrossRef]
- Brennan, F.; Anderson, A.; Taylor, S.; Woodruff, T.; Ruitenberg, M. Complement activation in the injured central nervous system: Another dual-edged sword? J. Neuroinflamm. 2012, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Pietsch, M.; Cordero-Grande, L.; Price, A.; Hutter, J.; Xiao, J.; McCabe, L.; Rutherford, M.; Hughes, E.; Counsell, S.; et al. Development of human white matter pathways in utero over the second and third trimester. Proc. Natl. Acad. Sci. USA 2021, 118, e2023598118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Pan, J.; Mamtilahun, M.; Zhu, Y.; Wang, L.; Venkatesh, A.; Shi, R.; Tu, X.; Jin, K.; Wang, Y.; et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics 2020, 10, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Jiang, N.; Cowan, M.; Moonah, S.; Petri, W.J. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol. Med. 2018, 24, 794–804. [Google Scholar] [CrossRef]
- Veksler, V.; Leon-Rivera, R.; Fleysher, L.; Gonzalez, J.; Lopez, J.A.; Rubin, L.H.; Morgello, S.; Berman, J.W. CD14+CD16+ monocyte transmigration across the blood-brain barrier is associated with HIV-NCI despite viral suppression. JCI Insight 2024, 9, e179855. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, J.; Chang, R.; Wong, G. The Complement System in the Central Nervous System: From Neurodevelopment to Neurodegeneration. Biomolecules 2022, 12, 337. [Google Scholar] [CrossRef]
- Peakman, M.; Senaldi, G.; Liossis, G.; Gamsu, H.R.; Vergani, D. Complement activation in neonatal infection. Arch. Dis. Child. 1992, 67, 802–807. [Google Scholar] [CrossRef]
- Wong, E.K.; Kavanagh, D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl. Res. J. Lab. Clin. Med. 2015, 165, 306–320. [Google Scholar] [CrossRef]
- Hallstensen, R.F.; Bergseth, G.; Foss, S.; Jæger, S.; Gedde-Dahl, T.; Holt, J.; Christiansen, D.; Lau, C.; Brekke, O.L.; Armstrong, E.; et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 2015, 220, 452–459. [Google Scholar] [CrossRef]
- Warwick, C.A.; Keyes, A.L.; Woodruff, T.M.; Usachev, Y.M. The complement cascade in the regulation of neuroinflammation, nociceptive sensitization, and pain. J. Biol. Chem. 2021, 297, 101085. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, F.A.; Khalili, B.; Touw, D.J.; Prins, J.R.; Olinga, P.; Leuvenink, H.G.D.; van Goor, H.; Gordijn, S.J.; Nagelkerke, A.; Mian, P. Placenta-on-a-Chip as an In Vitro Approach to Evaluate the Physiological and Structural Characteristics of the Human Placental Barrier upon Drug Exposure: A Systematic Review. J. Clin. Med. 2023, 12, 4315. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chiuppesi, F.; Chen, X.; Wang, C.; Tian, E.; Nguyen, J.; Kha, M.; Trinh, D.; Zhang, H.; Marchetto, M.; et al. Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep. Med. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Sun, S.; Xue, X.; Fu, J. Modeling development using microfluidics: Bridging gaps to foster fundamental and translational research. Curr. Opin. Genet. Dev. 2023, 82, 102097. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.; Ma, J.; Lawler, C.; Xie, W.; Stevenson, P.G.; Farrell, H.E. Recent Advancements in Understanding Primary Cytomegalovirus Infection in a Mouse Model. Viruses 2022, 14, 1934. [Google Scholar] [CrossRef] [PubMed]
- Roark, H.K.; Jenks, J.A.; Permar, S.R.; Schleiss, M.R. Animal Models of Congenital Cytomegalovirus Transmission: Implications for Vaccine Development. J. Infect. Dis. 2020, 221, S60–S73. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Sparer, T.E. What We Have Learned from Animal Models of HCMV. In Cytomegaloviruses: Methods and Protocols; Reddehase, M.J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; Volume 1119, pp. 267–288. [Google Scholar]

| Protein | Gestational Role | Citation |
|---|---|---|
| C1q | Mediator of trophoblast migration and spiral artery remodeling; essential for placental development and immune tolerance. | [16,38] |
| C3a/C5a | Potent anaphylatoxins that trigger inflammation; implicated in preeclampsia and fetal neuroinflammation; active effector cells. | [36,38] |
| CD55 (DAF) | Regulator of early C3 activation. | [16,38] |
| CD59 | Inhibitor of MAC formation and terminal pathway; highly expressed on nearly all human cells, including the placenta. | [16,38] |
| Factor Bb | Elevated levels act as an indicator for increased risk of preterm birth and reported in cases of preeclampsia. | [16,38] |
| C4b and Soluble C5b-9 | Elevated levels are reported in cases of preeclampsia. | [38] |
| C5 | Expressed on the zona pellucida during early embryonic development. | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garon, A.C.; Liu, Y.; Liu, F. Congenital Human Cytomegalovirus and the Complement System. Viruses 2025, 17, 1324. https://doi.org/10.3390/v17101324
Garon AC, Liu Y, Liu F. Congenital Human Cytomegalovirus and the Complement System. Viruses. 2025; 17(10):1324. https://doi.org/10.3390/v17101324
Chicago/Turabian StyleGaron, Andrea Canto, Yujun Liu, and Fenyong Liu. 2025. "Congenital Human Cytomegalovirus and the Complement System" Viruses 17, no. 10: 1324. https://doi.org/10.3390/v17101324
APA StyleGaron, A. C., Liu, Y., & Liu, F. (2025). Congenital Human Cytomegalovirus and the Complement System. Viruses, 17(10), 1324. https://doi.org/10.3390/v17101324





