Cargo and Biological Properties of Extracellular Vesicles Released from Human Adenovirus Type 4-Infected Lung Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Virus Propagation and Stock Production
2.3. Viral Infection for EV Isolation
2.4. Western Blot
2.5. Transmission Electron Microscopy (TEM)
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. Flow Cytometry Analysis of EV Surface Markers
2.8. Proteome Profiling
2.9. Characterization of Small RNA Cargo
2.10. Infectivity and Neutralization Assays
2.11. Triton, Proteinase K and DNase Treatments of EV Preparations
2.12. Quantitative PCR (qPCR) Detection of Viral DNA
3. Results
3.1. Characterization of EVs from Mock- and HAdV-E4-Infected A549 Cell Supernatants
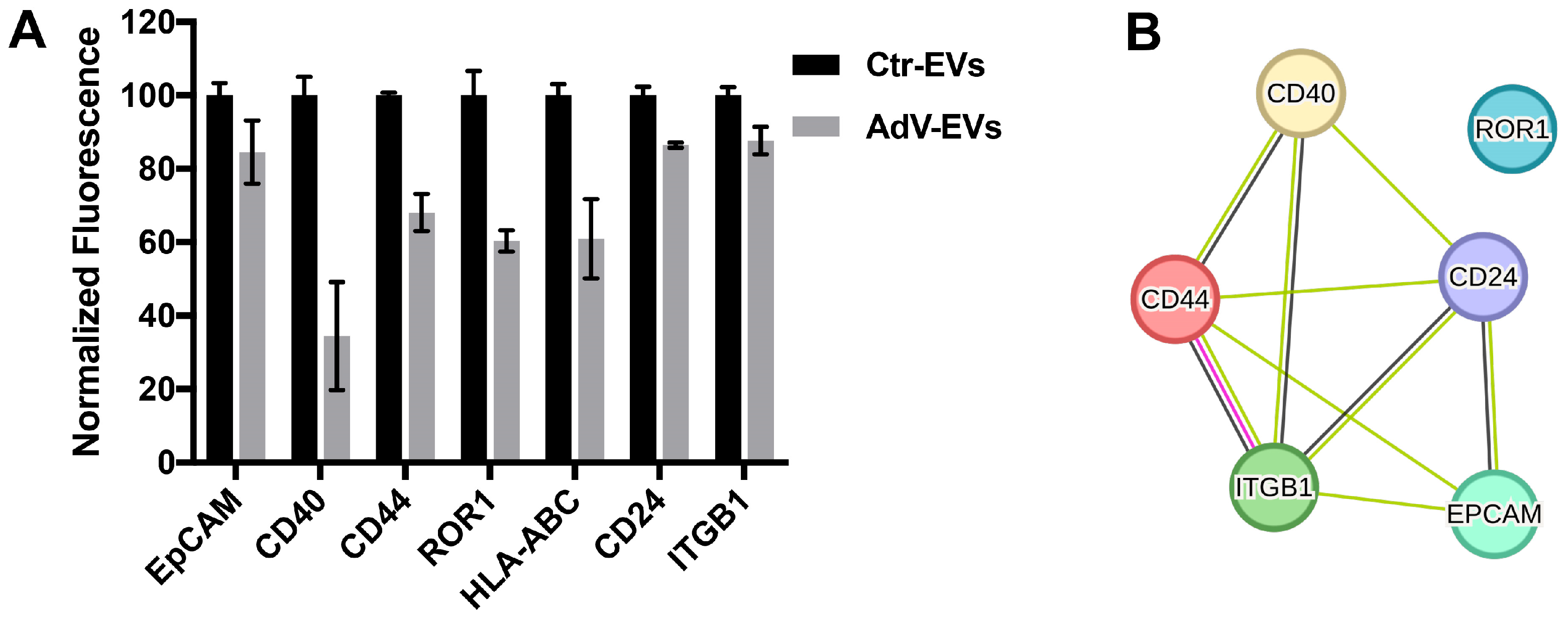
3.2. Characterization of the AdV-EVs and Ctr-EVs Surface Markers
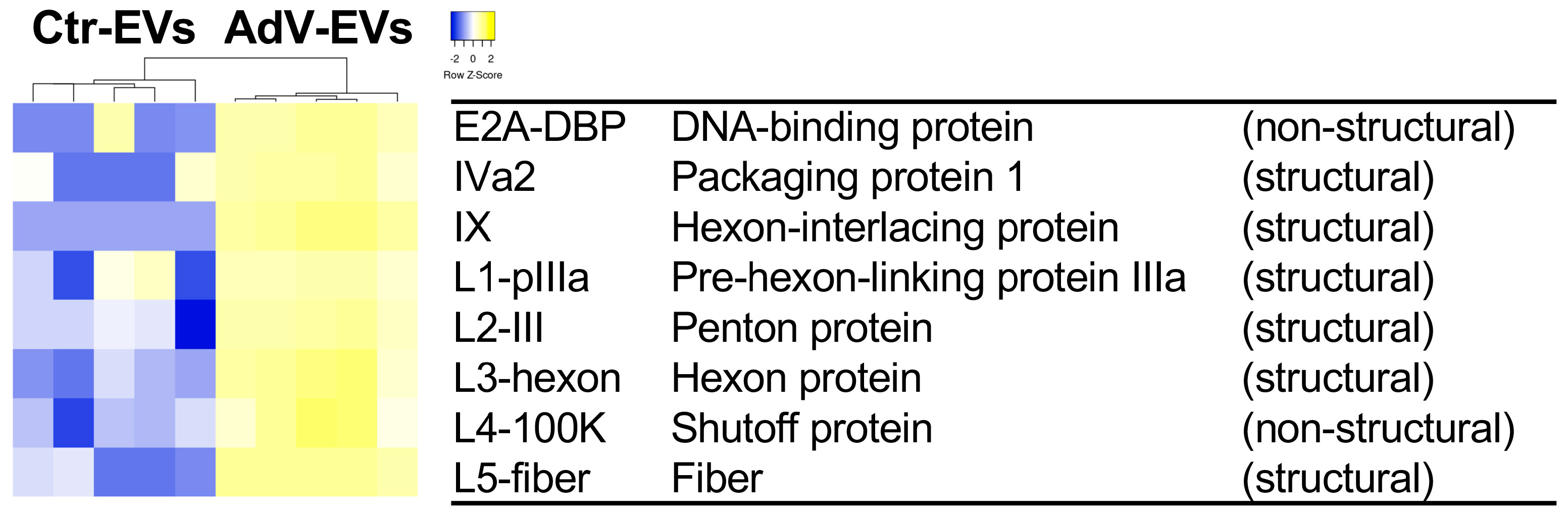
3.3. Proteomics Profiling of AdV-EVs and Ctr-EVs
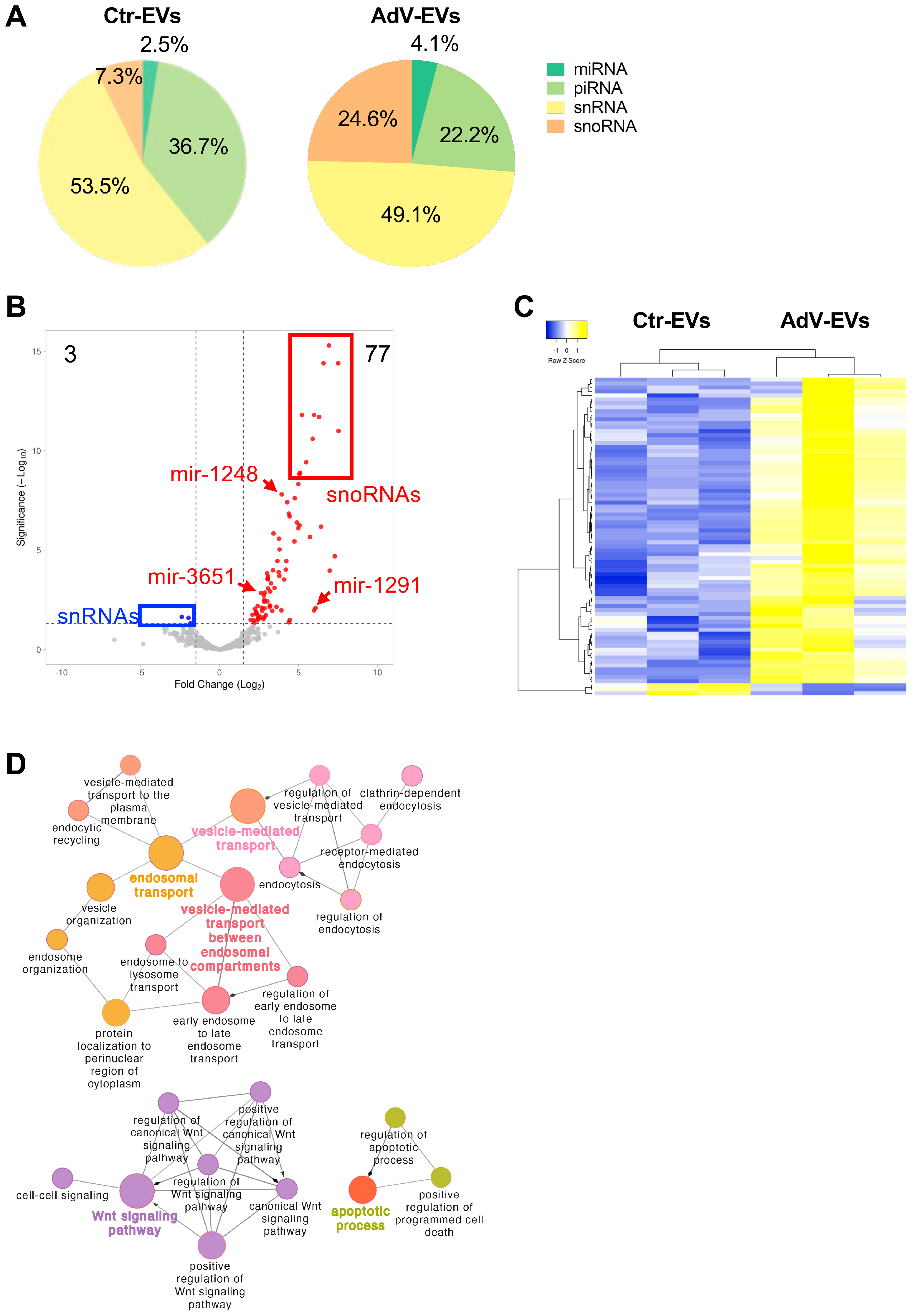
3.4. Small RNA Content of AdV-EVs and Ctr-EVs
3.5. Characterization of EV Biological Properties
3.6. Neutralization Assays
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAdV | Human adenovirus |
| EV | Extracellular vesicle |
| NTA | Nanoparticle Tracking Analysis |
| TEM | Transmission Electron Microscopy |
References
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, 10.1002/wrna.1413. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef]
- Amin, S.; Massoumi, H.; Tewari, D.; Roy, A.; Chaudhuri, M.; Jazayerli, C.; Krishan, A.; Singh, M.; Soleimani, M.; Karaca, E.E.; et al. Cell Type-Specific Extracellular Vesicles and Their Impact on Health and Disease. Int. J. Mol. Sci. 2024, 25, 2730. [Google Scholar] [CrossRef]
- Mardi, N.; Haiaty, S.; Rahbarghazi, R.; Mobarak, H.; Milani, M.; Zarebkohan, A.; Nouri, M. Exosomal transmission of viruses, a two-edged biological sword. Cell Commun. Signal 2023, 21, 19. [Google Scholar] [CrossRef]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.-H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef]
- Huang, H.-I.; Lin, J.-Y.; Chiang, H.-C.; Huang, P.-N.; Lin, Q.-D.; Shih, S.-R. Exosomes Facilitate Transmission of Enterovirus A71 From Human Intestinal Epithelial Cells. J. Infect. Dis. 2020, 222, 456–469. [Google Scholar] [CrossRef]
- Robinson, S.M.; Tsueng, G.; Sin, J.; Mangale, V.; Rahawi, S.; McIntyre, L.L.; Williams, W.; Kha, N.; Cruz, C.; Hancock, B.M.; et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014, 10, e1004045. [Google Scholar] [CrossRef]
- Iša, P.; Pérez-Delgado, A.; Quevedo, I.R.; López, S.; Arias, C.F. Rotaviruses Associate with Distinct Types of Extracellular Vesicles. Viruses 2020, 12, 763. [Google Scholar] [CrossRef]
- Yang, J.E.; Rossignol, E.D.; Chang, D.; Zaia, J.; Forrester, I.; Raja, K.; Winbigler, H.; Nicastro, D.; Jackson, W.T.; Bullitt, E. Complexity and ultrastructure of infectious extracellular vesicles from cells infected by non-enveloped virus. Sci. Rep. 2020, 10, 7939. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Types, and Approach to Treatment. Semin. Respir. Crit. Care Med. 2021, 42, 800–821. [Google Scholar] [CrossRef] [PubMed]
- Kajon, A.E. Adenovirus infections: New insights for the clinical laboratory. J. Clin. Microbiol. 2024, 62, e0083622. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef]
- MacNeil, K.M.; Dodge, M.J.; Evans, A.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Adenoviruses in medicine: Innocuous pathogen, predator, or partner. Trends Mol. Med. 2023, 29, 4–19. [Google Scholar] [CrossRef]
- Ipinmoroti, A.O.; Crenshaw, B.J.; Pandit, R.; Kumar, S.; Sims, B.; Matthews, Q.L. Human Adenovirus Serotype 3 Infection Modulates the Biogenesis and Composition of Lung Cell-Derived Extracellular Vesicles. J. Immunol. Res. 2021, 2021, 2958394. [Google Scholar] [CrossRef]
- Saari, H.; Turunen, T.; Lõhmus, A.; Turunen, M.; Jalasvuori, M.; Butcher, S.J.; Ylä-Herttuala, S.; Viitala, T.; Cerullo, V.; Siljander, P.R.M.; et al. Extracellular vesicles provide a capsid-free vector for oncolytic adenoviral DNA delivery. J. Extracell. Vesicles 2020, 9, 1747206. [Google Scholar] [CrossRef]
- Brachtlova, T.; Li, J.; van der Meulen-Muileman, I.H.; Sluiter, F.; von Meijenfeldt, W.; Witte, I.; Massaar, S.; van den Oever, R.; de Vrij, J.; van Beusechem, V.W. Quantitative Virus-Associated RNA Detection to Monitor Oncolytic Adenovirus Replication. Int. J. Mol. Sci. 2024, 25, 6551. [Google Scholar] [CrossRef]
- Ran, L.; Tan, X.; Li, Y.; Zhang, H.; Ma, R.; Ji, T.; Dong, W.; Tong, T.; Liu, Y.; Chen, D.; et al. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials 2016, 89, 56–66. [Google Scholar] [CrossRef]
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release 2018, 283, 223–234. [Google Scholar] [CrossRef]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Rinner, B.; Cerullo, V.; Yliperttula, M.; Mazzaferro, V.; Ciana, P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control. Release 2019, 294, 165–175. [Google Scholar] [CrossRef]
- Bair, C.R.; Zhang, W.; Gonzalez, G.; Kamali, A.; Stylos, D.; Blanco, J.C.G.; Kajon, A.E. Human Adenovirus Type 4 Comprises Two Major Phylogroups with Distinct Replicative Fitness and Virulence Phenotypes. J. Virol. 2022, 96, e01090-21. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Graw, S.; Tang, J.; Zafar, M.K.; Byrd, A.K.; Bolden, C.; Peterson, E.C.; Byrum, S.D. proteiNorm—A User-Friendly Tool for Normalization and Analysis of TMT and Label-Free Protein Quantification. ACS Omega 2020, 5, 25625–25633. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; von Heydebreck, A.; Sultmann, H.; Poustka, A.; Vingron, M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 2002, 18 (Suppl. S1), S96–S104. [Google Scholar] [CrossRef] [PubMed]
- Thurman, T.J.; Washam, C.L.; Alkam, D.; Bird, J.T.; Gies, A.; Dhusia, K.; Robeson, M.S.; Byrum, S.D. proteoDA: A package for quantitative proteomics. J. Open Source Softw. 2023, 8, 5184. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Chitti, S.V.; Gummadi, S.; Kang, T.; Shahi, S.; Marzan, A.L.; Nedeva, C.; Sanwlani, R.; Bramich, K.; Stewart, S.; Petrovska, M.; et al. Vesiclepedia 2024: An extracellular vesicles and extracellular particles repository. Nucleic Acids Res. 2023, 52, D1694–D1698. [Google Scholar] [CrossRef]
- Kang, J.; Tang, Q.; He, J.; Li, L.; Yang, N.; Yu, S.; Wang, M.; Zhang, Y.; Lin, J.; Cui, T.; et al. RNAInter v4.0: RNA interactome repository with redefined confidence scoring system and improved accessibility. Nucleic Acids Res. 2022, 50, D326–D332. [Google Scholar] [CrossRef]
- Heim, A.; Ebnet, C.; Harste, G.; Pring-Åkerblom, P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 2003, 70, 228–239. [Google Scholar] [CrossRef]
- Rubinstein, E.; Thery, C.; Zimmermann, P. Tetraspanins affect membrane structures and the trafficking of molecular partners: What impact on extracellular vesicles? Biochem. Soc. Trans. 2025, 53, 371–382. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Kawabe, T.; Matsushima, M.; Hashimoto, N.; Imaizumi, K.; Hasegawa, Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 2011, 73, 69–78. [Google Scholar] [PubMed]
- O’Shea, C.C.; Choi, S.; McCormick, F.; Stokoe, D. Adenovirus overrides cellular checkpoints for protein translation. Cell Cycle 2005, 4, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Kim, Y.J.; Hong, J.; Chang, H.G.; Yoon, A.R.; Yun, C.O. ErbB3-Targeting Oncolytic Adenovirus Causes Potent Tumor Suppression by Induction of Apoptosis in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7127. [Google Scholar] [CrossRef] [PubMed]
- Albarnaz, J.D.; Weekes, M.P. Proteomic analysis of antiviral innate immunity. Curr. Opin. Virol. 2023, 58, 101291. [Google Scholar] [CrossRef]
- Mellors, J.; Tipton, T.; Longet, S.; Carroll, M. Viral Evasion of the Complement System and Its Importance for Vaccines and Therapeutics. Front. Immunol. 2020, 11, 1450. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Seddar, L.; von Stromberg, K.; Ip, W.H.; Dobner, T.; Hidalgo, P. The adenovirus DNA-binding protein DBP. J. Virol. 2024, 98, e0188523. [Google Scholar] [CrossRef]
- Weitzman, M.D.; Ornelles, D.A. Inactivating intracellular antiviral responses during adenovirus infection. Oncogene 2005, 24, 7686–7696. [Google Scholar] [CrossRef]
- Murray, J.L.; Sheng, J.; Rubin, D.H. A role for H/ACA and C/D small nucleolar RNAs in viral replication. Mol. Biotechnol. 2014, 56, 429–437. [Google Scholar] [CrossRef]
- Price, A.M.; Hayer, K.E.; McIntyre, A.B.R.; Gokhale, N.S.; Abebe, J.S.; Della Fera, A.N.; Mason, C.E.; Horner, S.M.; Wilson, A.C.; Depledge, D.P.; et al. Direct RNA sequencing reveals m(6)A modifications on adenovirus RNA are necessary for efficient splicing. Nat. Commun. 2020, 11, 6016. [Google Scholar] [CrossRef]
- Hajikhezri, Z.; Kaira, Y.; Schubert, E.; Darweesh, M.; Svensson, C.; Akusjarvi, G.; Punga, T. Fragile X-Related Protein FXR1 Controls Human Adenovirus Capsid mRNA Metabolism. J. Virol. 2023, 97, e0153922. [Google Scholar] [CrossRef]
- van Zuylen, W.J.; Rawlinson, W.D.; Ford, C.E. The Wnt pathway: A key network in cell signalling dysregulated by viruses. Rev. Med. Virol. 2016, 26, 340–355. [Google Scholar] [CrossRef]
- Zhao, H.; Granberg, F.; Pettersson, U. How adenovirus strives to control cellular gene expression. Virology 2007, 363, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Punga, T.; Darweesh, M.; Akusjärvi, G. Synthesis, Structure, and Function of Human Adenovirus Small Non-Coding RNAs. Viruses 2020, 12, 1182. [Google Scholar] [CrossRef] [PubMed]
- Vachon, V.K.; Conn, G.L. Adenovirus VA RNA: An essential pro-viral non-coding RNA. Virus Res. 2016, 212, 39–52. [Google Scholar] [CrossRef]
- Koski, A.; Kangasniemi, L.; Escutenaire, S.; Pesonen, S.; Cerullo, V.; Diaconu, I.; Nokisalmi, P.; Raki, M.; Rajecki, M.; Guse, K.; et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010, 18, 1874–1884. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Duan, J.; Ma, Y.; Shen, Z.; Wei, L.; Cui, X.; Zhang, J.; Xie, Y.; Liu, J. Quantitative proteomic analysis of exosome protein content changes induced by hepatitis B virus in Huh-7 cells using SILAC labeling and LC-MS/MS. J. Proteome Res. 2014, 13, 5391–5402. [Google Scholar]
- Oberholster, L.; Mathias, A.; Perriot, S.; Blaser, E.; Canales, M.; Jones, S.; Culebras, L.; Gimenez, M.; Kaynor, G.C.; Sapozhnik, A.; et al. Comprehensive proteomic analysis of JC polyomavirus-infected human astrocytes and their extracellular vesicles. Microbiol. Spectr. 2023, 11, e0275123. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Glitscher, M.; Tonnemacher, S.; Schollmeier, A.; Raupach, J.; Zahn, T.; Eberle, R.; Krijnse-Locker, J.; Basic, M.; Hildt, E. Presence of Intact Hepatitis B Virions in Exosomes. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kordbacheh, R.; Sin, J. Extracellular Vesicles: A Novel Mode of Viral Propagation Exploited by Enveloped and Non-Enveloped Viruses. Microorganisms 2024, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kodidela, S.; Tadrous, E.; Cory, T.J.; Walker, C.M.; Smith, A.M.; Mukherjee, A.; Kumar, S. Extracellular Vesicles in Viral Replication and Pathogenesis and Their Potential Role in Therapeutic Intervention. Viruses 2020, 12, 887. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201. [Google Scholar] [CrossRef]
- Waury, K.; Gogishvili, D.; Nieuwland, R.; Chatterjee, M.; Teunissen, C.E.; Abeln, S. Proteome encoded determinants of protein sorting into extracellular vesicles. J. Extracell. Biol. 2024, 3, e120. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
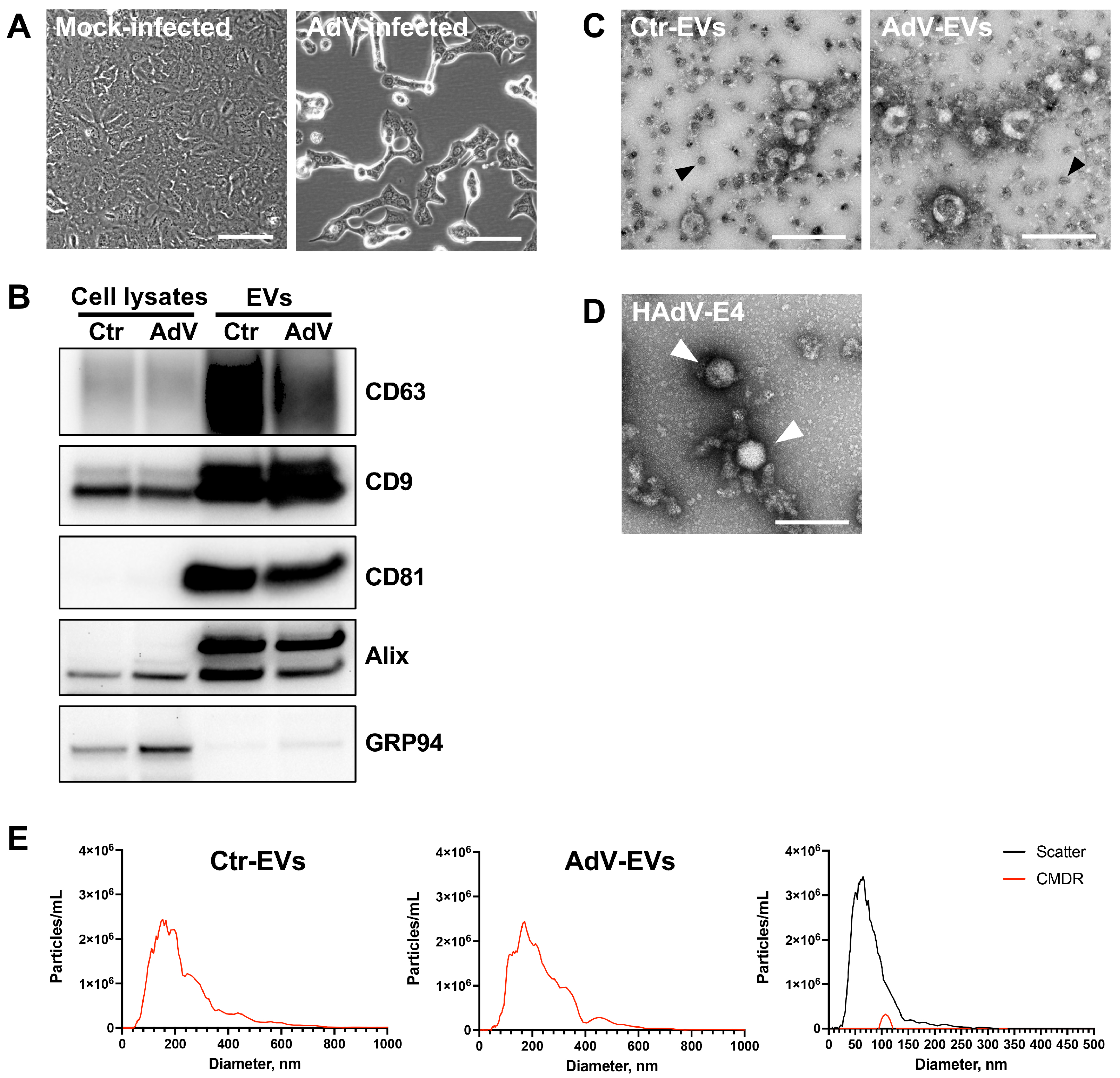




| Median Size ± SD | Concentration (Particles/mL ± SD) | |
|---|---|---|
| Ctr-EVs | 176 ± 18 nm | 1.45 × 1011 ± 5.00 × 109 |
| AdV-EVs | 165 ± 5 nm | 1.17 × 1011 ± 9.75 × 109 |
| Increased Abundance | Decreased Abundance | ||
|---|---|---|---|
| Protein Name | log2 Fold Change | Protein Name | log2 Fold Change |
| FUS | 2.58 | SLITRK6 | −3.36 |
| SLC20A1 | 2.49 | UBB;UBC | −3.10 |
| RPS6 | 2.32 | TRAF2 | −2.87 |
| RPL7 | 2.29 | KRT19 | −2.86 |
| RPL15 | 2.25 | PDGFA | −2.84 |
| COMT | 2.20 | DKK1 | −2.80 |
| RPLP0 | 2.19 | CALML5 | −2.78 |
| DTNB | 2.16 | KRT6B | −2.72 |
| RPL4 | 2.11 | RSPO3 | −2.70 |
| KLHL13 | 2.04 | IFT81 | −2.70 |
| Class | Ctr-EVs | AdV-EVs |
|---|---|---|
| miRNA (precursors) | 10 | 13 |
| miRNA (mature) | 40 | 28 |
| piRNA | 375 | 378 |
| snRNA | 124 | 107 |
| snoRNA | 264 | 280 |
| Total unique transcripts | 813 | 806 |
| Class | Higher Abundance | Lower Abundance |
|---|---|---|
| miRNA(p) | 3 | 0 |
| piRNA | 4 | 0 |
| snRNA | 0 | 3 |
| snoRNA | 70 | 0 |
| Total | 77 | 3 |
| Up-Regulated | Down-Regulated | ||
|---|---|---|---|
| Gene ID | log2 Fold Change | Gene ID | log2 Fold Change |
| SNORA41 | 7.53 | RNU6ATAC2P | −2.39 |
| SNORA74A | 7.51 | RNU6ATAC | −1.97 |
| SNORA22 | 7.30 | RNVU1-1 | −1.84 |
| SNORA22C | 6.97 | ||
| SNORA50 | 6.92 | ||
| SNORA50C | 6.58 | ||
| SNORA50A | 6.43 | ||
| SNORA78 | 6.30 | ||
| hsa-mir-1291 | 6.08 | ||
| SNORA22B | 5.99 | ||
| Serum Dilution | Percent Plaque Reduction | |
|---|---|---|
| AdV-EVs | HAdV-E4 | |
| 1:256 | 100 | 100 |
| 1:512 | 98 | 100 |
| 1:1024 | 90 | 100 |
| 1:2048 | 73 | 96 |
| 1:4196 | Not tested | 90 |
| 1:8192 | Not tested | 67 |
| Gene ID | Description |
|---|---|
| ABCA1 | Phospholipid-transporting ATPase ABCA1 |
| ANXA2 | Annexin A2 |
| APMAP | Adipocyte plasma membrane-associated protein |
| APOA1 | Apolipoprotein A-I |
| ATP2B1 | Plasma membrane calcium-transporting ATPase 1 |
| CD81 | CD81 antigen |
| CTNND1 | Catenin delta-1 |
| DSP | Desmoplakin |
| GNB2 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-2 |
| HP | Haptoglobin |
| IGFBP3 | Insulin-like growth factor-binding protein 3 |
| ITGA3 | Integrin alpha-3 |
| ITGB1 | Integrin beta-1 |
| JUP | Junction plakoglobin |
| KRT77 | Keratin, type II cytoskeletal 1b |
| MYO1B | Unconventional myosin-Ib |
| MYO1C | Unconventional myosin-Ic |
| PKP1 | Plakophilin-1 |
| PLEKHA7 | Pleckstrin homology domain-containing family A member 7 |
| PLG | Plasminogen |
| SLC12A2 | Solute carrier family 12 member 2 |
| SLC3A2 | Amino acid transporter heavy chain SLC3A2 |
| SMOC1 | SPARC-related modular calcium-binding protein 1 |
| STOM | Stomatin |
| TIMP3 | Metalloproteinase inhibitor 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noghero, A.; Byrum, S.; Okeoma, C.; Kajon, A.E. Cargo and Biological Properties of Extracellular Vesicles Released from Human Adenovirus Type 4-Infected Lung Epithelial Cells. Viruses 2025, 17, 1300. https://doi.org/10.3390/v17101300
Noghero A, Byrum S, Okeoma C, Kajon AE. Cargo and Biological Properties of Extracellular Vesicles Released from Human Adenovirus Type 4-Infected Lung Epithelial Cells. Viruses. 2025; 17(10):1300. https://doi.org/10.3390/v17101300
Chicago/Turabian StyleNoghero, Alessio, Stephanie Byrum, Chioma Okeoma, and Adriana E. Kajon. 2025. "Cargo and Biological Properties of Extracellular Vesicles Released from Human Adenovirus Type 4-Infected Lung Epithelial Cells" Viruses 17, no. 10: 1300. https://doi.org/10.3390/v17101300
APA StyleNoghero, A., Byrum, S., Okeoma, C., & Kajon, A. E. (2025). Cargo and Biological Properties of Extracellular Vesicles Released from Human Adenovirus Type 4-Infected Lung Epithelial Cells. Viruses, 17(10), 1300. https://doi.org/10.3390/v17101300








